Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D3 in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression
Abstract
:1. Introduction
- His papillary and adamantinous growths measured 2.3 cm3 and 0.7 cm3, respectively;
- His visual acuities deteriorated from 20/60 the year before, to 20/100 (left); and 20/25 in 2016 to 20/80 (right);
- He suffered bitemporal vision loss; and
- His coherence tomography data showed compressive atrophy of the left optic nerve [20];
2. Mini Reviews on the Roles of DMF, Bupropion, SAMe, and Vit-D3
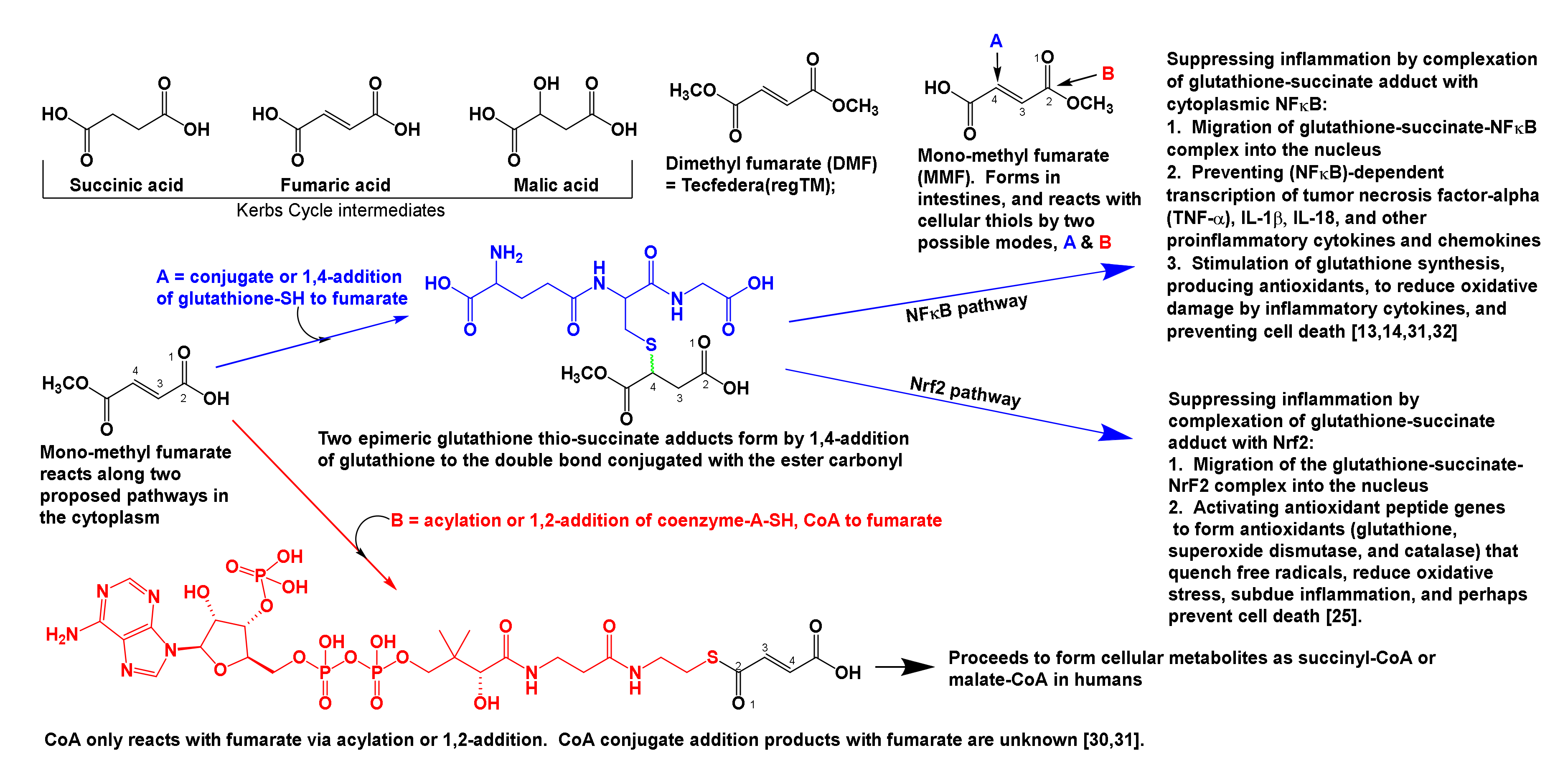
2.1. The Neuroimmune Roles of DMF in Subduing Inflammation in the CNS and the Body
- 2.1.1.
- What is DMF and what does it do?
- 2.1.2.
- DMF metabolism, and its conjugation with glutathione and nuclear factors NFκB, and NRF2 to suppress inflammation.
- 2.1.3.
- Stresses and inflammation.
- 2.1.4.
- Effect of inflammation on cells and tissues.
- 2.1.5.
- Remarks on DMF pertinent to this case.
2.1.1. What Is DMF, and What Does It Do?
2.1.2. DMF Metabolism, and Its Conjugation with Glutathione and Nuclear Factors NFκB, and Nrf2 to Suppress Inflammation
2.1.3. Stresses and Inflammation
2.1.4. Effect of Inflammation on Cells and Tissues
- Decreased accumulation of isotopic 18F-2-fluoro-2-deoxyglucose (2FDG), which indicates below normal energy metabolism (or glucose use) in the bilateral insula, left putamen, left globus pallidus, right caudate nucleus, and the cingulate gyrus, and specifies inflammation, major depression, and cognitive decline; and
2.1.5. Remarks on DMF Pertinent to This Case
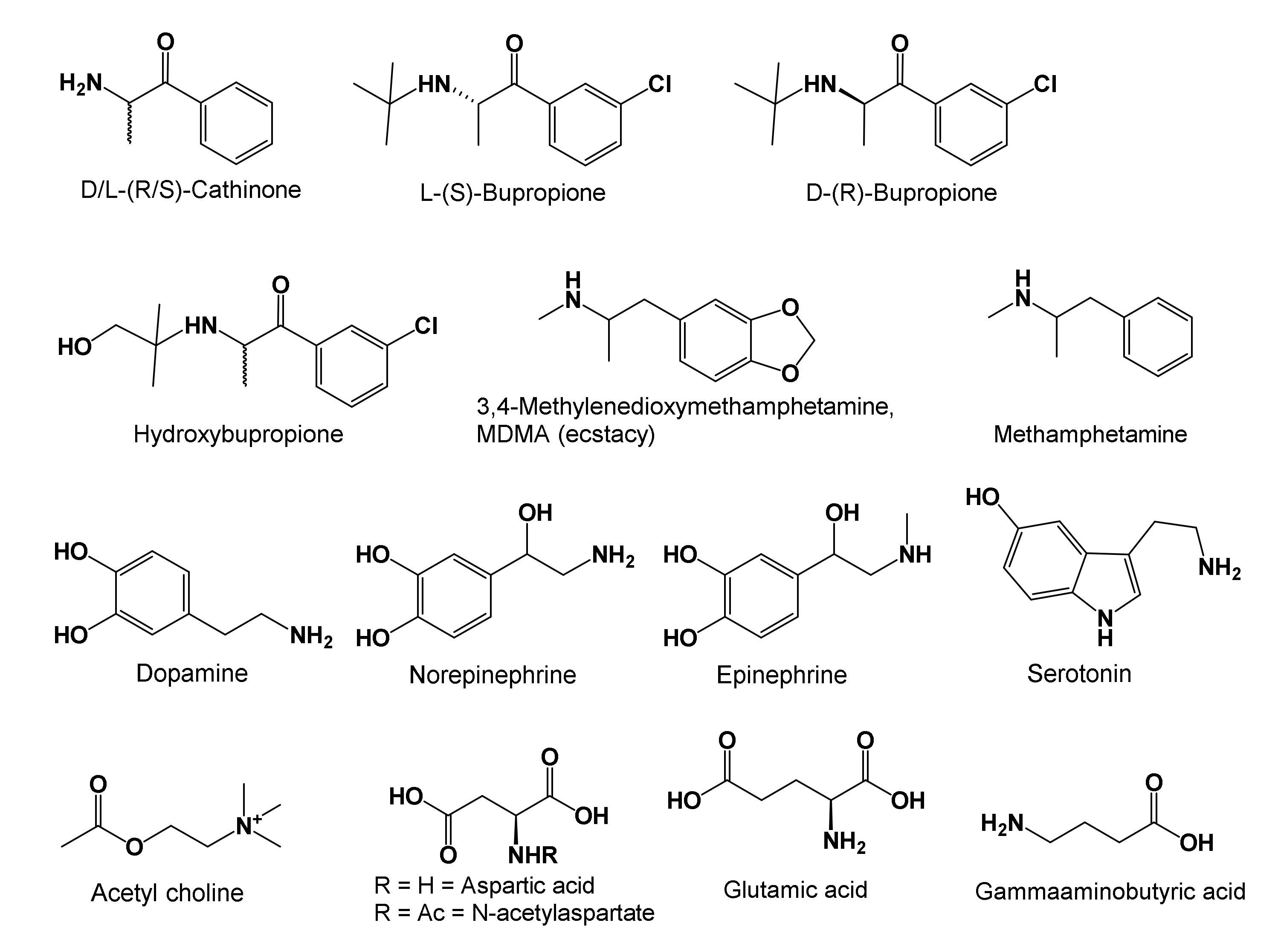
2.2. Proposed Neurochemical Roles of Bupropion in Subduing Inflammation and MD
- 2.2.1.
- Bupropion structure, metabolic activation, and what does the drug do?
- 2.2.2.
- Value added advantages of using bupropion
- 2.2.3.
- Remarks on bupropion pertinent to this case
2.2.1. Bupropion Structure, Metabolic Activation, and What Does the Drug Do?
2.2.2. Value Added Advantages of Using Bupropion

2.2.3. Remarks on Bupropion as They Pertain to This Case
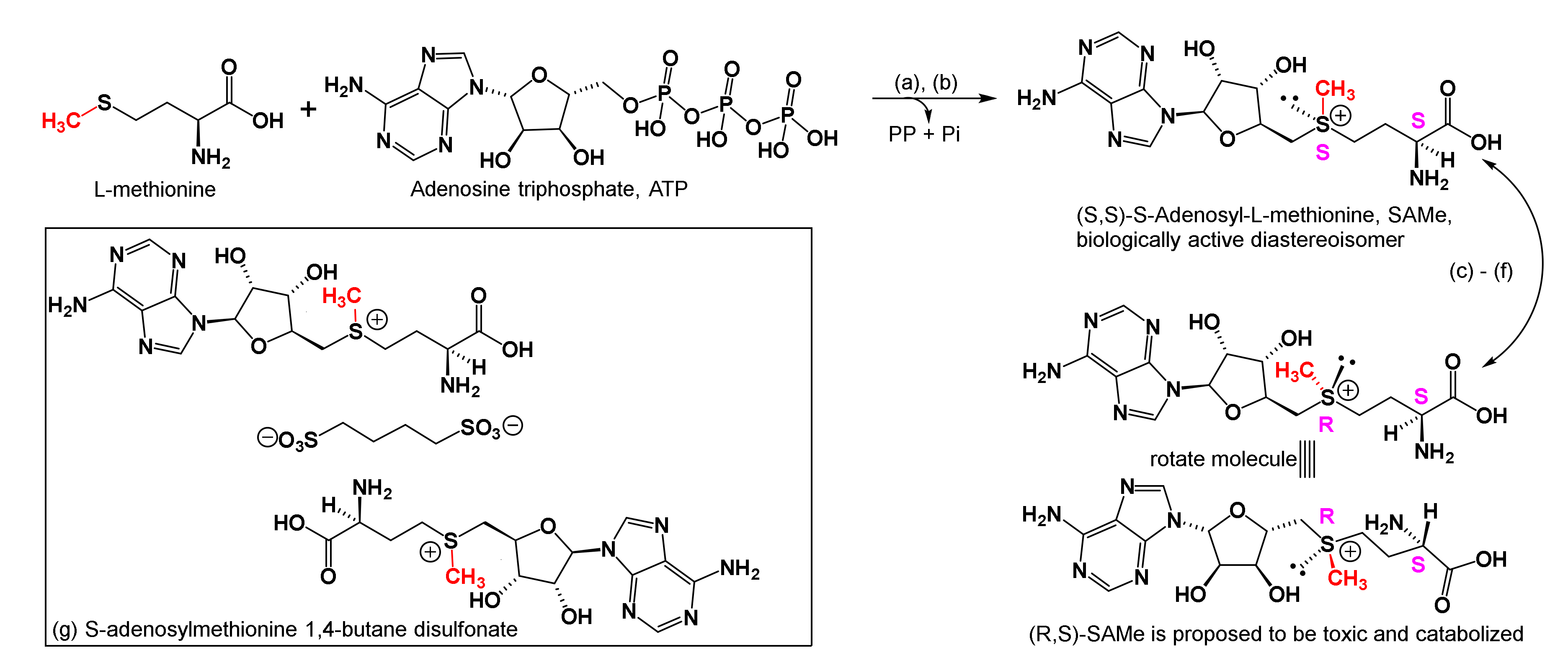
2.3. Proposed Neuroimmune Roles of SAMe in Subduing Inflammation and MD
- 2.3.1.
- SAMe structure, biosynthesis, and stability in aqueous solutions;
- 2.3.2.
- Designation, commercial production, formulation, diastereoisomeric purity, and degradation products in water;
- 2.3.3.
- Transport and concentration of SAMe in tissues in health and disease
- 2.3.4.
- One carbon transfer, transsulfuration, and physiological effects
- 2.3.5.
- Other one-carbon cofactors and physiological actions.
- 2.3.6.
- Transfer of aminopropyl groups, and physiological effects
- 2.3.7.
- Inflammation reduction and remarks on SAMe pertinent to this case
2.3.1. SAMe Structure, Biosynthesis, and Stability in Aqueous Solutions
2.3.2. Designation, Commercial Production, Formulation, Diastereoisomeric Purity, and Degradation Products in Water
2.3.3. Transport and Concentration of SAMe in Tissues in Health and Disease
2.3.4. One Carbon Transfer, Transsulfuration, and Physiological Effects

2.3.5. Other One-Carbon Cofactors and Physiological Actions
2.3.6. Transfer of Aminopropyl Groups and Physiological Effects
2.3.7. Inflammation Reduction and Remarks as They Pertain to This Case
2.4. Suggested Roles of Vitamin D3 for Reducing Inflammation and MD
- 2.4.1.
- Vit-D3 precursors and epimers
- 2.4.2.
- Bioconversions in sunlight
- 2.4.3.
- Dietary supplement, transport, bioconversions in the liver and kidneys, storage, and release
- 2.4.4.
- Diffusion through membranes and receptors for vit-D3
- 2.4.5.
- Vit-D3 concentrations in health and disease
- 2.4.6.
- Catabolism and excretion
- 2.4.7.
- Remarks on vit-D3 pertinent to this case
2.4.1. Vit-D3 Precursors and Epimers
2.4.2. Bioconversions in Sunlight
2.4.3. Dietary Supplement, Transport, Bioconversions in the Liver and Kidneys, Storage, and Release
2.4.4. Diffusion through Membranes and Receptors for Vit-D3
2.4.5. Vit-D3 Concentrations in Health and Disease
- Suicide attempters was 47 +/− 20 nmol/L (=19 +/− 8 ng/mL, n = 59 subjects);
- Non-suicidal depressed patients showed 62 +/− 27 nmol/L (=25 +/− 11 ng/mL, n = 17 subjects); and
2.4.6. Catabolism and Excretion
3. Conclusions and Hope for the Future
- DMF when orally consumed, hydrolyses to MMF, which then reacts with glutathione to form the glutathione-succinate complex. This complex suppresses the NLRP3 inflammasome, as noticed by: reduction in inflammation; decreased serum levels of inflammatory cytokines; and upregulated serum concentrations of antioxidants. Furthermore, DMF was reported to reduce excessive build-up of oxidized tryptophan metabolites (kynurenic and quinolinic acids, as well as 3-hydroxykynurenine), which, if left to accrue unchecked, can cause excitotoxicity and tissue losses in the CNS and PNS, dysregulate the HPA axis, and disrupt neurotransmitter, endocrine, and immune functioning.
- Bupropion upon oral consumption metabolizes to hydroxybupropion that lasts in serum longer than bupropion, and functions as a stimulant and antidepressant. It is known to: maintain adequate serum and CNS neurotransmitter concentrations to help alertness, learning, cognitive functioning, memory, and mood; improve endurance during strenuous activities; increase libido in depressed patients; reduce inflammation and the perception of pain; decrease the need to smoke and overeat; and balance innate and adaptive immune functions when fighting excessive stress. In addition, we discussed why bupropion as well as SAMe may not be prescribed to patients who experience mania, or tend to get excessively agitated when in pain. To facilitate readers’ understanding of the psychoneuroimmunology of bupropion, we enpassant (in passing) summarized physiology concepts of neurotransmitters in Box 1 to ease following our discussions on bupropion.
- Regarding the neuroimmune roles of SAMe in subduing inflammation and MD, we discussed its biosynthesis, chiral aspects affecting its biological activity versus toxicity, and temperature and ionic conditions affecting its stability. Thereafter, we discussed its roles in: one carbon metabolism; its own build up and catabolism in different biological cycles; the biosynthesis of methionine, glutathione, and cysteine, that, in turn, reduce free radical oxidative damage in CNS and PNS; and epigenetic reactions that are implicated in relieving inflammation and major depression. We introduced additional concept summaries in Box 2, so readers may compare the roles of SAMe with other one carbon cofactors; the fragments they transfer; and how they are described to reduce inflammation and major depression. Still further, we discussed the physiological chemistry of spermidine and spermine as they are reported to accumulate in CNS tissues of patients who completed suicide.
- Our discussions on the neuroimmune chemistry of vit-D3 involved: its biosynthesis, dosage, structural changes in pro and pre-vitamin forms that produce the active hormone and immune modulator (calcidiol); and its serum concentrations that are reported to subdue inflammation, and excessive buildup of its metabolite calcitriol which specifies on-going inflammation or neuroimmune diseases. In addition, we discussed mechanisms by which vit-D3 is reported to subdue inflammation in patients with MS, MD, asthma, type-2 diabetes, and Chron’s, bone, and other inflammatory diseases by blocking the inflammasome NLRP3 in forming and releasing inflammatory cytokines. Our discussions on vit-D3 ended by showing the successive side chain oxidations that catabolize the vitamin for excretion.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Sachinvala, N.D.; Stergiou, A.; Haines, D.E. Remitting longstanding major depression in a multiple sclerosis (MS) patient with several concurrent conditions. Part 1, Case Report. Neuropsychiatr. Dis. Treat. 2018, 14, 2545–2550. [Google Scholar] [CrossRef] [Green Version]
- Sachinvala, N.D.; Stergiou, A.; Haines, D.E.; Kocharian, A.; Lawton, A. Post-craniopharyngioma and cranial nerve-VI palsy update on a MS patient with major depression and concurrent neuroimmune conditions. Brain Sci. 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selective IgM deficiency. Genetic and Rare Diseases Information Center (GARD)—An NCATS Program. Available online: https://rarediseases.info.nih.gov/diseases/12547/selective-igm-deficiency (accessed on 25 June 2020).
- Nasr, Z.; Majed, M.; Rostami, A.; Sahraian, M.A.; Minagar, A.; Amini, A.; McGee, J.C.; Etemadifar, M. Prevalence of multiple sclerosis in Iranian emigrants: Review of the evidence. Neurol. Sci. 2016, 37, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Naser Moghadasi, A. Multiple sclerosis in parsis: A historical issue. Iran. J. Public Health 2014, 43, 387–388. [Google Scholar] [PubMed]
- Bhatia, R.; Bali, P.; Chaudhari, R.M. Epidemiology and genetic aspects of multiple sclerosis in India. Ann. Indian Acad. Neurol. 2015, 18 (Suppl. S6). [Google Scholar] [CrossRef]
- Research Gate lists 60 Peer-Reviewed Papers by this Author and the World Intellectual Property Organization lists 22 US and International Patents/Applications by NS. Available online: https://www.researchgate.net/profile/Navzer_Sachinvala2/publicationshttps://patentscope.wipo.int/search/en/; type Sachinvala (accessed on 25 June 2020).
- Beck Depression Inventory (BDI). Available online: http://www.apa.org/pi/about/publications/caregivers/practice-settings/assessment/tools/beck-depression (accessed on 25 June 2020).
- Barber, J. Hypnotic analgesia: Clinical considerations. In Hypnosis and Suggestion in the Treatment of Pain: A Clinical Guide; Barber, J., Ed.; WW Norton Company: New York, NY, USA, 1996; Chapter 5; pp. 85–118. [Google Scholar]
- Tiers, M. Chapter 17, Medical Hypnosis. In Integrative Hypnosis: A Comprehensive Course in Change; Createspace Independent Pub: New York, NY, USA, 2010; pp. 251–298. [Google Scholar]
- Saeed, S.A.; Antonacci, D.J.; Bloch, R.M. Exercise, yoga, and meditation for depressive and anxiety disorders. Am. Fam. Physician 2010, 81, 981–986. [Google Scholar]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Inflammation and Repair. In Robbins and Cotran Pathological Basis of Disease, 9th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015; Chapter 3; pp. 69–112. [Google Scholar]
- Carlström, K.E.; Ewing, E.; Granqvist, M.; Gyllenberg, A.; Aeinehband, S.; Enoksson, S.L.; Checa, A.; Badam, T.V.S.; Huang, J.; Gomez-Cabrero, D.; et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 2019, 10, 3081. [Google Scholar] [CrossRef] [Green Version]
- Diebold, M.; Sievers, C.; Bantug, G.; Sanderson, N.; Kappos, L.; Kuhle, J.; Lindberg, R.L.P.; Derfuss, T. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J. Autoimmun. 2018, 86, 39–50. [Google Scholar] [CrossRef]
- Drug Bank Bupropion. Available online: https://www.drugbank.ca/drugs/DB01156 (accessed on 25 June 2020).
- Drug Bank S-adenosylmethionine. Available online: https://www.drugbank.ca/drugs/DB00118 (accessed on 25 June 2020).
- Drug Bank vitamin D3. Available online: https://www.drugbank.ca/drugs/DB00169 (accessed on 25 June 2020).
- Craniopharyngioma by MRI. Available online: https://radiopaedia.org/articles/craniopharyngioma (accessed on 25 June 2020).
- Einstien, A.; Virani, R.A. Clinical relevance of single-voxel 1H MRS metabolites in discriminating suprasellar tumors. J. Clin. Diagn. Res. 2016, 10. [Google Scholar] [CrossRef]
- Yang, L.; Qu, Y.; Lu, W.; Liu, F. Evaluation of macular ganglion cell complex and peripapillary retinal nerve fiber layer in primary craniopharyngioma by Fourier-domain optical coherence tomography. Med. Sci. Monit. 2016, 22, 2309–2314. [Google Scholar] [CrossRef]
- Barber, S.M.; Teh, B.S.; Baskin, D.S. Fractionated stereotactic radiotherapy for pituitary adenomas: Single-center experience in 75 consecutive patients. Neurosurgery 2016, 79, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Cheah, S.K.; Matthews, T.; Teh, B.S. Hippocampal sparing whole brain radiotherapy and integrated simultaneous boost vs stereotactic radiosurgery boost: A comparative dosimetric planning study. Asian Pac. J. Cancer Prev. 2016, 17, 4233–4235. [Google Scholar] [PubMed]
- The patient’s current medications and nutritional supplements are discussed in Reference 1.
- Yazdi, M.R.; Mrowietz, U. Fumaric acid esters. Clin. Dermatol. 2008, 26, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Yizhi, L.; Jiaoxue, Q.; Zhong, W.; Wanchun, Y.; Lingyun, W.; Chengyuan, J.; Gang, C. Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-NRF2-ARE system. J. Neurosurg. 2015, 123, 915–923. [Google Scholar] [CrossRef] [Green Version]
- AHR Aryl Hydrocarbon Receptor [Homo sapiens (human)]—Gene—NCBI. Available online: http://www.ncbi.nlm.nih.gov/gene/196 (accessed on 25 June 2020).
- Hellemans, R.; Bosmans, J.L.; Abramowicz, D. Induction therapy for kidney transplant recipients: Do we still need anti-il2 receptor monoclonal antibodies? Am. J. Transplant. 2017, 17, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Nebert, D.W. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 2017, 67, 38–57. [Google Scholar] [CrossRef]
- Majumder, A.; Gopalakrishna, K.N.; Cheguru, P.; Gakhar, L.; Artemyev, N.O. Interaction of aryl hydrocarbon receptor-interacting protein-like 1 with the farnesyl moiety. J. Biol. Chem. 2013, 288, 21320–21328. [Google Scholar] [CrossRef] [Green Version]
- Pellom, S.T.; Michalek, R.D.; Crump, K.E.; Langston, P.K.; Juneau, D.G.; Grayson, J.M. Increased cell surface free thiols identify effector CD8+ T cells undergoing T cell receptor stimulation. PLoS ONE 2013, 8, e81134. [Google Scholar] [CrossRef] [Green Version]
- Seidel, P.; Roth, M.; Ge, Q.; Merfort, I.; S’ng, C.T.; Ammit, A.J. IκBα glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur. Respir. J. 2011, 38, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Al-Jaderi, Z.; Maghazachi, A.A. Utilization of dimethyl fumarate and related molecules for treatment of multiple sclerosis, cancer, and other diseases. Front. Immunol. 2016, 7, 278. [Google Scholar] [CrossRef] [Green Version]
- Dibbert, S.; Clement, B.; Skak-Nielsen, T.; Mrowietz, U.; Rostami-Yazdi, M. Detection of fumarate-glutathione adducts in the portal vein blood of rats: Evidence for rapid dimethylfumarate metabolism. Arch. Dermatol. Res. 2013, 305, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Garstkiewicz, M.; Strittmatter, G.E.; Grossi, S.; Sand, J.; Fenini, G.; Werner, S.; French, L.E.; Beer, H.-D. Opposing effects of Nrf2 and Nrf2-activating compounds on the NLRP3 inflammasome independent of Nrf2-mediated gene expression. Eur. J. Immunol. 2017, 47, 806–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, T.C.; Xu, C.; Duman, R.S. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav. Immun. 2018, 72, 2–13. [Google Scholar] [CrossRef]
- Szondy, Z.; Sarang, Z.; Kiss, B.; Garabuczi, É.; Köröskényi, K. Anti-inflammatory mechanisms triggered by apoptotic cells during their clearance. Front. Immunol. 2017, 8, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Shen, J.; Ran, Z. Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy 2020, 16, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.-D. The crosstalk between Nrf2 and inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Zhang, S.; Zong, Y.; Halim, M.; Ren, Z.; Wang, Y.; Ma, Y.; Li, B.; Ma, L.; Zhou, G.; et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J. Affect. Disord. 2019, 254, 15–25. [Google Scholar] [CrossRef]
- McConnell, T.H. Inflammation: The reaction to injury. In The Nature of Disease, Pathology for the Health Professional; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Chapter 3; pp. 34–50. [Google Scholar]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Cellular responses to stress and toxic insults: Adaptation, injury, and death; and Inflammation and repair. In Robbins and Cotran, Pathologic Basis of Disease, 9th ed.; Elsevier: Philadelphia, PA, USA, 2015; Chapters 2 and 3; pp. 31–112. [Google Scholar]
- Yadav, S.K.; Mindur, J.E.; Ito, K.; Dhib-Jalbut, S. Advances in the immunopathogenesis of multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 206–219. [Google Scholar] [CrossRef]
- Su, L.; Cai, Y.; Xu, Y.; Dutt, A.; Shi, S.; Bramon, E. Cerebral metabolism in major depressive disorder: A voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry 2014, 14, 321. [Google Scholar] [CrossRef]
- Cavert, L.E.; Kuuzer, B.; Krupp, L.B. Invisible symptoms of MS: Fatigue, depression and cognition. In Multiple Sclerosis and CNS Inflammatory Disorders; Samkoff, L.M., Goodman, A.D., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; Chapter 11; pp. 114–121. [Google Scholar]
- Albornoz, E.A.; Woodruff, T.M.; Gordon, R. Inflammasomes in CNS diseases. In Inflammasomes: Clinical and Therapeutic Implications; Cordero, M.D., Alcocer-Gómez, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 41–60. [Google Scholar]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular mechanisms of inflammasome signaling. J. Leukoc. Biol. 2018, 103, 233–257. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Innate immunity in diabetes and diabetic nephropathy. Nat. Rev. Nephrol. 2015, 12, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Mayberg, H.S. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin. Neurosci. 2014, 16, 479–490. [Google Scholar] [PubMed]
- Maller, J.J.; Thomson, R.H.S.; Rosenfeld, J.V.; Anderson, R.; Daskalakis, Z.J.; Fitzgerald, P.B. Occipital bending in depression. Brain 2014, 137, 1830–1837. [Google Scholar] [CrossRef] [Green Version]
- Maller, J.J.; Thomson, R.H.S.; Rosenfeld, J.V.; Anderson, R.; Daskalakis, Z.J.; Fitzgerald, P.B. Reply: Occipital bending in depression. Brain 2015, 138, e318. [Google Scholar] [CrossRef]
- O’Keane, V.; Dinan, T.G.; Scott, L.; Corcoran, C. Changes in hypothalamic–pituitary–adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol. Psychiatry 2005, 58, 963–968. [Google Scholar] [CrossRef]
- Bonaz, B.; Picq, C.; Sinniger, V.; Mayol, J.F.; Clarençon, D. Vagus nerve stimulation: From epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol. Motil. 2013, 25, 208–221. [Google Scholar] [CrossRef]
- Hannestad, J.; Subramanyam, K.; DellaGioia, N.; Planeta-Wilson, B.; Weinzimmer, D.; Pittman, B.; Carson, R.E. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J. Nucl. Med. 2012, 53, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Berti, V.; Mosconi, L.; Pupi, A. Brain: Normal variations and benign findings in fluorodeoxyglucose-pet/computed tomography imaging. PET Clin. 2014, 9, 129–140. [Google Scholar] [CrossRef] [Green Version]
- Mathur, D.; López-Rodas, G.; Casanova, B.; Marti, M.B. Perturbed glucose metabolism: Insights into multiple sclerosis pathogenesis. Front. Neurol. 2014, 5, 250. [Google Scholar] [CrossRef]
- Rajda, C.; Majláth, Z.; Pukoli, D.; Vécsei, L. Kynurenines and multiple sclerosis: The dialogue between the immune system and the central nervous system. Int. J. Mol. Sci. 2015, 16, 18270–18282. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Lemos, H.; Mautino, M.; Mellor, A. Altered tryptophan metabolism as a paradigm for good and bad aspects of immune privilege in chronic inflammatory diseases. Front. Immunol. 2012, 3, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wang, Y.; Wang, T.; Jiang, J.; Botting, C.H.; Liu, H.; Chen, Q.; Yang, J.; Naismith, J.H.; Zhu, X.; et al. Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule. Nat. Commun. 2016, 7, 12103. [Google Scholar] [CrossRef]
- Biogen: A Study Evaluating the Effectiveness of Tecfidera (Dimethyl Fumarate) on Multiple Sclerosis (MS) Disease Activity and Patient-Reported Outcomes (PROTEC). Available online: https://clinicaltrials.gov/ct2/show/NCT01930708 (accessed on 25 June 2020).
- Huecker, M.R.; Smiley, A.; Saadabadi, A. Bupropion; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/29262173/?from_term=bupropion+2020+review&from_pos=3 (accessed on 25 June 2020).
- Stahl, S.M. Pain and the treatment of fibromyalgia and functional somatic syndromes. In Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 3rd ed.; Cambridge University Press: Cambridge, UK, 2011; Chapter 15; pp. 773–814. [Google Scholar]
- Flockhart, D.A. Drug Interactions: Cytochrome P450 Drug Interaction Table. Indiana University School of Medicine. 2007. Available online: https://drug-interactions.medicine.iu.edu/ (accessed on 25 June 2020).
- Murdoch, C.; Aziz, H.A.; Fang, H.-Y.; Jezan, H.; Musaid, R.; Muthana, M. Khat (catha edulis) alters the phenotype and anti-microbial activity of peripheral blood mononuclear cells. J. Ethnopharmacol. 2011, 138, 780–787. [Google Scholar] [CrossRef]
- Roberts, E.; Eden Evins, A.; McNeill, A.; Robson, D. Efficacy and tolerability of pharmacotherapy for smoking cessation in adults with serious mental illness: A systematic review and network meta-analysis. Addiction 2016, 111, 599–612. [Google Scholar] [CrossRef]
- Alvi, A.; Fatima, N.; Jerah, A.A.; Rizwan, M.; Hobani, Y.H.; Sunosi, R.A.; Taha, M.M.E.H.; Habiballah, E.M.; Agarwal, P.K.; Abdulwahab, S.I. Correlation between resistin, tuberculosis and khat addiction: A study from south western province of saudi arabia. PLoS ONE 2015, 10, e0140245. [Google Scholar] [CrossRef] [PubMed]
- McGinty, E.E.; Baller, J.; Azrin, S.T.; Juliano-Bult, D.; Daumit, G.L. Interventions to address medical conditions and health-risk behaviors among persons with serious mental illness: A comprehensive review. Schizophr. Bull. 2016, 42, 96–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldinger, M.D. Psychiatric disorders and sexual dysfunction. Handb. Clin. Neurol. 2015, 130, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Heal, D.J.; Gosden, J.; Smith, S.L. Dopamine reuptake transporter (DAT) “inverse agonism”—A novel hypothesis to explain the enigmatic pharmacology of cocaine. Neuropharmacology 2014, 87, 19–40. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E.; Holtzman, D.M.; Malenka, R.C. Widely projecting systems: Monoamines, acetylcholine, and orexin. In Molecular Pharmacology: A Foundation for Clinical Neuroscience, 3rd ed.; McGraw Hill: New York, NY, USA, 2015; Chapter 6; pp. 149–183. [Google Scholar]
- Roelands, B.; Watson, P.; Cordery, P.; Decoster, S.; Debaste, E.; Maughan, R.; Meeusen, R. A dopamine/noradrenaline reuptake inhibitor improves performance in the heat, but only at the maximum therapeutic dose. Scand. J. Med. Sci. Sports 2012, 22, e93–e98. [Google Scholar] [CrossRef]
- Gualtieri, C.T.; Johnson, L.G. Bupropion normalizes cognitive performance in patients with depression. Medscape Gen. Med. 2007, 9, 22. [Google Scholar]
- Shytle, R.D.; Silver, A.A.; Lukas, R.J.; Newman, M.B.; Sheehan, D.V.; Sanberg, P.R. Nicotinic acetylcholine receptors as targets for antidepressants. Mol. Psychiatry 2002, 7, 525–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatkin, J.M.; Dullius, C.R. The management of asthmatic smokers. Asthma Res. Pract. 2016, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.S.; Vornik, L.A.; Khan, D.A.; Rush, A.J. Bupropion in the treatment of outpatients with asthma and major depressive disorder. Int. J. Psychiatry Med. 2007, 37, 23–28. [Google Scholar] [CrossRef]
- Wilkes, S. Bupropion. Drugs Today 2006, 42, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Brustolim, D.; Ribeiro-dos-Santos, R.; Kast, R.E.; Altschuler, E.L.; Soares, M.B.P. A new chapter opens in anti-inflammatory treatments: The antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int. Immunopharmacol. 2006, 6, 903–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, J.; Zychowska, M.; Makuch, W.; Rojewska, E.; Przewlocka, B. Neuronal and immunological basis of action of antidepressants in chronic pain—Clinical and experimental studies. Pharmacol. Rep. 2013, 65, 1611–1621. [Google Scholar] [CrossRef]
- Urits, I.; Peck, J.; Orhurhu, M.S.; Wolf, J.; Patel, R.; Orhurhu, V.; Kaye, A.D.; Viswanath, O. Off-label antidepressant use for treatment and management of chronic pain: Evolving understanding and comprehensive review. Curr. Pain Headache Rep. 2019, 23, 66. [Google Scholar] [CrossRef]
- Cantoni, G.L. The nature of the active methyl donor formed enzymatically from L-methionine and adenosinetriphosphate. J. Am. Chem. Soc. 1952, 74, 2942–2943. [Google Scholar] [CrossRef]
- Shelly, C.L.; José, M.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.; Barbier-Torres, L.; Fan, W.; Mato, J.M.; Lu, S.C. Methionine adenosyltransferases in liver cancer. World J. Gastroenterol. 2019, 25, 4300–4319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Klinman, J.P. High-performance liquid chromatography separation of the (S, S)—And (R, S)-forms of S-adenosyl-L-methionine. Anal. Biochem. 2015, 476, 81–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Chu, H.; Cao, G.; Du, X.; Min, X.; Wan, C. S-adenosylmethionine attenuates bile duct early warm ischemia reperfusion injury after rat liver transplantation. Mol. Immunol. 2018, 95, 83–90. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, B.; Zhu, J.; Xu, H.; Liu, P.; Wang, Z.; Li, J.; Yang, Z.; Ma, X.; Chen, S. Enhanced bacitracin production by systematically engineering S-adenosylmethionine supply modules in Bacillus licheniformis. Front. Bioeng. Biotechnol. 2020, 8, 305. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, Z.; Cai, H.; Zhou, C. Progress in the microbial production of S-adenosyl-L-methionine. World J. Microbiol. Biotechnol. 2016, 32, 153. [Google Scholar] [CrossRef]
- Desiderio, C.; Cavallaro, R.A.; De Rossi, A.; D’Anselmi, F.; Fuso, A.; Scarpa, S. Evaluation of chemical and diastereoisomeric stability of S-adenosylmethionine in aqueous solution by capillary electrophoresis. J. Pharm. Biomed. Anal. 2005, 38, 449–456. [Google Scholar] [CrossRef]
- Chishty, M.; Reichel, A.; Abbott, N.J.; Begley, D.J. S-adenosylmethionine is substrate for carrier mediated transport at the blood–brain barrier in vitro. Brain Res. 2002, 942, 46–50. [Google Scholar] [CrossRef]
- Joncquel-Chevalier Curt, M.; Voicu, P.-M.; Fontaine, M.; Dessein, A.-F.; Porchet, N.; Mention-Mulliez, K.; Dobbelaere, D.; Soto-Ares, G.; Cheillan, D.; Vamecq, J. Creatine biosynthesis and transport in health and disease. Biochimie 2015, 119, 146–165. [Google Scholar] [CrossRef]
- Oden, K.L.; Clarke, S. S-adenosyl-L-methionine synthetase from human erythrocytes: Role in the regulation of cellular S-adenosylmethionine levels. Biochemistry 1983, 22, 2978–2986. [Google Scholar] [CrossRef]
- Obeid, R.; Kostopoulos, P.; Knapp, J.-P.; Kasoha, M.; Becker, G.; Fassbender, K.; Herrmann, W. Biomarkers of folate and vitamin B12 are related in blood and cerebrospinal fluid. Clin. Chem. 2007, 53, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.D.; Smith, D.D.; Kish, S.J. Brain S-adenosylmethionine levels are severely decreased in Alzheimer’s disease. J. Neurochem. 1996, 67, 1328–1331. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M.B.; Salama, M.A.; Refaat, R.; Ali, M.A.; Khalifa, E.A.; Kamel, M.A. Evaluating the therapeutic potential of one-carbon donors in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2019, 847, 72–82. [Google Scholar] [CrossRef]
- Mahmood, N.; Cheishvili, D.; Arakelian, A.; Tanvir, I.; Khan, H.A.; Pépin, A.-S.; Szyf, M.; Rabbani, S.A. Methyl donor S-adenosylmethionine (SAM) supplementation attenuates breast cancer growth, invasion, and metastasis in vivo; therapeutic and chemopreventive applications. Oncotarget 2018, 9, 5169–5183. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Byrne, G.J.; Stough, C.; Bousman, C.; Mischoulon, D.; Murphy, J.; Macdonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; et al. Nutraceuticals for major depressive disorder- more is not merrier: An 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 2019, 245, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makins, C.; Ghosh, S.; Román-Meléndez, G.D.; Malec, P.A.; Kennedy, R.T.; Marsh, E.N.G. Does viperin function as a radical S-adenosyl-L-methionine-dependent enzyme in regulating farnesylpyrophosphate synthase expression and activity? J. Biol. Chem. 2016, 291, 26806–26815. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, A.V.; Lam, R.W.; Filteau, M.J.; Lespérance, F.; Kennedy, S.H.; Parikh, S.V.; Patten, S.B. Canadian network for mood and anxiety treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults.: V. Complementary and alternative medicine treatments. J. Affec. Disord. 2009, 117 (Suppl. S1), S54–S64. [Google Scholar] [CrossRef]
- Abeysundera, H.; Gill, R. Possible same-induced mania. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Ding, W.; Higgins, D.P.; Yadav, D.K.; Godbole, A.A.; Pukkila-Worley, R.; Walker, A.K. Stress-responsive and metabolic gene regulation are altered in low S-adenosylmethionine. PLoS Genet. 2018, 14, e1007812. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Cahill, C.M.; Huang, X.; Roffman, J.L.; Lamon-Fava, S.; Fava, M.; Mischoulon, D.; Rogers, J.T. S-adenosyl methionine and transmethylation pathways in neuropsychiatric diseases throughout life. Neurotherapeutics 2018, 15, 156–175. [Google Scholar] [CrossRef] [Green Version]
- Niciu, M.J.; Kelmendi, B.; Sanacora, G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol. Biochem. Behav. 2012, 100, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Wilcke, T.; Fuchs, E.; Funke, K.; Vlachos, A.; Müller-Dahlhaus, F.; Puts, N.A.J.; Harris, R.E.; Edden, R.A.E. GABA—From inhibition to cognition: Emerging concepts. Neuroscientist 2018, 24, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. S-adenosylmethionine (SAMe) for neuropsychiatric disorders: A clinician-oriented review of research. J. Clin. Psychiatry 2017, 78, e656–e667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tjong, E.; Mohiuddin, S.S. Biochemistry, Tetrahydrofolate; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539712/ (accessed on 25 June 2020).
- Murphy, M.M.; Guéant, J.-L. B vitamins and one carbon metabolism micronutrients in health and disease. Biochimie 2020, 173, 1–2. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-carbon metabolism in health and disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Bistas, K.G.; Tadi, P. Biotin; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32119380 (accessed on 25 June 2020).
- Li, X.; Li, N.; Huang, L.; Xu, S.; Zheng, X.; Hamsath, A.; Zhang, M.; Dai, L.; Zhang, H.; Wong, J.J.-L.; et al. Is hydrogen sulfide a concern during treatment of lung adenocarcinoma with ammonium tetrathiomolybdate? Front. Oncol. 2020, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Jabs, S.; Biton, A.; Bécavin, C.; Nahori, M.-A.; Ghozlane, A.; Pagliuso, A.; Spanò, G.; Guérineau, V.; Touboul, D.; Gianetto, Q.G.; et al. Impact of the gut microbiota on the m6A epitranscriptome of mouse cecum and liver. Nat. Commun. 2020, 11, 1344. [Google Scholar] [CrossRef]
- Tollefsbol, T.O. Advances in Epigenetic Technology. In Epigenetic Protocols, 2nd ed.; Humana Press, Springer Science: New York, NY, USA, 2011; Chapter 1; pp. 1–10. [Google Scholar]
- Li, H.-P.; Peng, C.-C.; Chung, I.C.; Huang, M.-Y.; Huang, S.-T.; Chen, C.-C.; Chang, K.-P.; Hsu, C.-L.; Chang, Y.-S. Aberrantly hypermethylated homeobox A2 derepresses metalloproteinase-9 through TBP and promotes invasion in nasopharyngeal carcinoma. Oncotarget 2014, 4, 2154–2165. [Google Scholar] [CrossRef] [Green Version]
- Nestler, E.J.; Peña, C.J.; Kundakovic, M.; Mitchell, A.; Akbarian, S. Epigenetic basis of mental illness. Neuroscientist 2016, 22, 447–463. [Google Scholar] [CrossRef]
- Saavedra, K.; Molina-Márquez, A.M.; Saavedra, N.; Zambrano, T.; Salazar, L.A. Epigenetic modifications of major depressive disorder. Int. J. Mol. Sci. 2016, 17, 1279. [Google Scholar] [CrossRef]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Sidoli, S.; Lu, C.; Coradin, M.; Wang, X.; Karch, K.R.; Ruminowicz, C.; Garcia, B.A. Metabolic labeling in middle-down proteomics allows for investigation of the dynamics of the histone code. Epigenetics Chromatin 2017, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Bae, D.-H.; Lane, D.J.R.; Jansson, P.J.; Richardson, D.R. The old and new biochemistry of polyamines. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2053–2068. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. Spermine and gene methylation: A mechanism of lifespan extension induced by polyamine-rich diet. Amino Acids 2020, 52, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Ivanov, I.P. Roles of polyamines in translation. J. Biol. Chem. 2018, 293, 18719–18729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Maity, R.; Whitelegge, J.P.; Hadjikyriacou, A.; Li, Z.; Zurita-Lopez, C.; Al-Hadid, Q.; Clark, A.T.; Bedford, M.T.; Masson, J.-Y.; et al. Mammalian protein arginine methyltransferase 7 (PRMT7) specifically targets RXR sites in lysine- and arginine-rich regions. J. Biol. Chem. 2013, 288, 37010–37025. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.G.; Fiori, L.M.; Moquin, L.; Gratton, A.; Mamer, O.; Mechawar, N.; Turecki, G. Evidence of altered polyamine concentrations in cerebral cortex of suicide completers. Neuropsychopharmacology 2010, 35, 1477–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiori, L.M.; Turecki, G. Genetic and epigenetic influences on expression of spermine synthase and spermine oxidase in suicide completers. Int. J. Neuropsychopharmacol. 2010, 13, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Fiori, L.M.; Mechawar, N.; Turecki, G. Identification and characterization of spermidine/spermine N1-acetyltransferase promoter variants in suicide completers. Biol. Psychiatry 2009, 66, 460–467. [Google Scholar] [CrossRef]
- McCarty, M.F.; DiNicolantonio, J.J. Neuroprotective potential of high-dose biotin. Med. Hypotheses 2017, 109, 145–149. [Google Scholar] [CrossRef]
- Stuehr, D.J. Arginine metabolism: Enzymology, nutrition, and clinical significance. J. Nutr. 2004, 134 (Suppl. S10), 2741S–2897S. [Google Scholar]
- Camargo, A.; Rodrigues, A.L.S. Novel targets for fast antidepressant responses: Possible role of endogenous neuromodulators. Chronic Stress 2019, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniv, A.; Antofiychuk, N.; Danylyshina, T.; Trefanenko, I.; Shuper, V. Clinical efficacy of S-adenosylmethionine in patients with non-alcoholic steatohepatitis and chronic kidney disease I-II stage. Georgian Med. News 2017, 273, 31–36. [Google Scholar]
- Shi, T.; Wu, L.; Ma, W.; Ju, L.; Bai, M.; Chen, X.; Liu, S.; Yang, X.; Shi, J. Nonalcoholic fatty liver disease: Pathogenesis and treatment in traditional chinese medicine and western medicine. Evid. Based Complement. Alternat. Med. 2020, 2020, 8749564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.; Zhou, Z.; Uriarte, S.; Wang, L.; James Kang, Y.; Chen, T.; Barve, S.; McClain, C.J. S-adenosylhomocysteine sensitizes to TNF-α hepatotoxicity in mice and liver cells: A possible etiological factor in alcoholic liver disease. Hepatology 2004, 40, 989–997. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.J.; Hill, D.B.; Song, Z.; Chawla, R.; Watson, W.H.; Chen, T.; Barve, S. S-adenosylmethionine, cytokines, and alcoholic liver disease. Alcohol 2002, 27, 185–192. [Google Scholar] [CrossRef]
- Pascale, R.M.; Peitta, G.; Simile, M.M.; Feo, F. Alterations of methionine metabolism as potential targets for the prevention and therapy of hepatocellular carcinoma. Medicina 2019, 55, 296. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e40702. [Google Scholar] [CrossRef]
- Keegan, R.-J.H.; Lu, Z.; Bogusz, J.M.; Williams, J.E.; Holick, M.F. Photobiology of vitamin D in mushrooms and its bioavailability in humans. Dermato Endocrinol. 2013, 5, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D: A D-lightful health perspective. Nutr. Rev. 2008, 66, S182–S194. [Google Scholar] [CrossRef]
- Al-Zohily, B.; Al-Menhali, A.; Gariballa, S.; Haq, A.; Shah, I. Epimers of vitamin D: A review. Int. J. Mol. Sci. 2020, 21, 470. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.; Mach, N. Role of vitamin d in the hygiene hypothesis: The interplay between vitamin d, vitamin d receptors, gut microbiota, and immune response. Front. Immunol. 2016, 7, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, E.A.; Nguyen, J.K.; Liu, J.; Keller, E.; Campbell, N.; Zhang, C.-J.; Smith, H.R.; Li, X.; Jørgensen, T.N. Low levels of vitamin D promote memory B cells in lupus. Nutrients 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezowska, M.; Coe, S.; Dawes, H. Effectiveness of vitamin D supplementation in the management of multiple sclerosis: A systematic review. Int. J. Mol. Sci. 2019, 20, 1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodamani, M.H.; Muthu, V.; Thakur, R.; Pal, A.; Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. A randomised trial of vitamin D in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Mycoses 2019, 62, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Grudet, C.; Malm, J.; Westrin, Å.; Brundin, L. Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology 2014, 50, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Umhau, J.C.; George, D.T.; Heaney, R.P.; Lewis, M.D.; Ursano, R.J.; Heilig, M.; Hibbeln, J.R.; Schwandt, M.L. Low vitamin D status and suicide: A case-control study of active duty military service members. PLoS ONE 2013, 8, e51543, correction in PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D. Dermato Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Environmental and nutritional diseases. In Robbins and Cotran, Pathologic Basis of Disease, 9th ed.; Elsevier: Philadelphia, PA, USA, 2015; Chapter 9; pp. 409–450. [Google Scholar]
- Ibid, idem, Metabolic liver disease, in Chapter 18, Liver and Gallbladder; pp. 845–847.
- Hollis, B.W.; Wagner, C.L. The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [Green Version]
- Spanier, J.A.; Nashold, F.E.; Nelson, C.D.; Praska, C.E.; Hayes, C.E. Vitamin D3-mediated resistance to a multiple sclerosis model disease depends on myeloid cell 1,25-dihydroxyvitamin D3 synthesis and correlates with increased CD4+ T cell CTLA-4 expression. J. Neuroimmunol. 2020, 338, 577105. [Google Scholar] [CrossRef]
- Hashemi, R.; Hosseini-Asl, S.S.; Arefhosseini, S.R.; Morshedi, M. The impact of vitamin D3 intake on inflammatory markers in multiple sclerosis patients and their first-degree relatives. PLoS ONE 2020, 15, e0231145. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Mimpen, M.; Oechtering, J.; Damoiseaux, J.; van den Ouweland, J.; Hupperts, R.; Kuhle, J. Vitamin D3 supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol. Scand. 2020, 141, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Inaba, M.; Fukagawa, M.; Ando, R.; Emoto, M.; Fujii, H.; Fujimori, A.; Fukui, M.; Hase, H.; Hashimoto, T.; et al. Effect of oral alfa-calcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: The J-DAVID Randomized Clinical Trial. JAMA 2018, 320, 2325–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Koyama, D.; Igarashi, R.; Maki, T.; Mizuno, H.; Furukawa, Y.; Kuro-o, M. Serum endocrine fibroblast growth factors as potential biomarkers for chronic kidney disease and various metabolic dysfunctions in aged patients. Intern. Med. 2020, 59, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Gulyás, K.; Horváth, Á.; Végh, E.; Pusztai, A.; Szentpétery, Á.; Pethö, Z.; Váncsa, A.; Bodnár, N.; Csomor, P.; Hamar, A.; et al. Effects of 1-year anti-TNF-α therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin. Rheumatol. 2020, 39, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Osorio-Landa, H.K.; Pérez-Díaz, I.; Laguna-Bárcenas, S.; López-Navarro, J.M.; Abella-Roa, M.F.; Corral-Orozco, M.; Mancilla-Ortega, J.P.; Martínez-Duarte, D.A.; Morales-Montalvo, S.I.; Múzquiz-Aguirre, S.; et al. Association of serum vitamin D levels with chronic disease and mortality. Nutr. Hosp. 2020, 37, 335–342. [Google Scholar]
- Patel, N.; Penkert, R.R.; Jones, B.G.; Sealy, R.E.; Surman, S.L.; Sun, Y.; Tang, L.; DeBeauchamp, J.; Webb, A.; Richardson, J.; et al. Baseline serum vitamin A and D levels determine benefit of oral vitamin A&D supplements to humoral immune responses following pediatric influenza vaccination. Viruses 2019, 11, 907. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-F.; Luo, B.-A.; Qin, L.-L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine 2019, 98, e17252. [Google Scholar] [CrossRef]
- Rajakumar, K.; Reis, E.C.; Holick, M.F. Dosing error with over-the-counter vitamin D supplement: A risk for vitamin D toxicity in infants. Clin. Pediatr. 2013, 52, 82–85. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.K.; Tyagi, P.; Sharma, P.; Singla, V.; Arora, V.; Bansal, N.; Kumar, A.; Arora, A. Iatrogenic hypervitaminosis D as an unusual cause of persistent vomiting: A case report. J. Med. Case Rep. 2014, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Van den Ouweland, J.; Fleuren, H.; Drabbe, M.; Vollaard, H. Pharmacokinetics and safety issues of an accidental overdose of 2,000,000 IU of vitamin D3 in two nursing home patients: A case report. BMC Pharmacol. Toxicol. 2014, 15, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ketha, H.; Wadams, H.; Lteif, A.; Singh, R.J. Iatrogenic vitamin D toxicity in an infant—A case report and review of literature. J. Steroid Biochem. Mol. Biol. 2015, 148, 14–18. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, S.K.; Mithal, A. Vitamin D toxicity resulting from overzealous correction of vitamin D deficiency. Clin. Endocrinol. 2015, 83, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, L.; Hogen, E. Vitamin D overdosage in an infant from nonprescription vitamin D drops. Am. J. Health Syst. Pharm. 2015, 72, 1262–1263. [Google Scholar] [CrossRef]
- Solidoro, P.; Bellocchia, M.; Facchini, F. The immunobiological and clinical role of vitamin D in obstructive lung diseases. Minerva Med. 2016, 107 (Suppl. S1), 12–19. [Google Scholar] [PubMed]
- Britt, R.D., Jr.; Thompson, M.A.; Freeman, M.R.; Stewart, A.L.; Pabelick, C.M.; Prakash, Y.S. Vitamin D reduces inflammation-induced contractility and remodeling of asthmatic human airway smooth muscle. Ann. Am. Thorac. Soc. 2015, 13 (Suppl. S1), S97–S98. [Google Scholar]
- Forouhi, N.G.; Menon, R.K.; Sharp, S.J.; Mannan, N.; Timms, P.M.; Martineau, A.R.; Rickard, A.P.; Boucher, B.J.; Chowdhury, T.A.; Griffiths, C.J.; et al. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2016, 18, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Arora, T.C.; Jindal, A.; Bhadoria, A.S. Efficacy of narrowband ultraviolet B phototherapy and levels of serum vitamin D3 in psoriasis: A prospective study. Indian Dermatol. Online J. 2016, 7, 87–92. [Google Scholar] [CrossRef]
- Song, L.; Papaioannou, G.; Zhao, H.; Luderer, H.F.; Miller, C.; Dall’Osso, C.; Nazarian, R.M.; Wagers, A.J.; Demay, M.B. The vitamin D receptor regulates tissue resident macrophage response to injury. Endocrinology 2016, 157, 4066–4075. [Google Scholar] [CrossRef] [Green Version]
- Reins, R.Y.; Hanlon, S.D.; Magadi, S.; McDermott, A.M. Effects of topically applied vitamin D during corneal wound healing. PLoS ONE 2016, 11, e0152889. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Kwan, P.; Ma, Z.; Iwashina, T.; Wang, J.; Shankowsky, H.A.; Tredget, E.E. Synergistic effect of vitamin D and low concentration of transforming growth factor β 1, a potential role in dermal wound healing. Burns 2016, 42, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Chen, X.; Wu, J.; Xiao, M.; Zhang, J.; Wang, B.; Fang, L.; Zhang, H.; Wang, X.; Yang, S.; et al. Vitamin D receptor inhibits NLRP3 activation by impeding its BRCC3-mediated deubiquitination. Front. Immunol. 2019, 10, 2783. [Google Scholar] [CrossRef] [PubMed]
- Jennersjö, P.; Guldbrand, H.; Björne, S.; Länne, T.; Fredrikson, M.; Lindström, T.; Wijkman, M.; Östgren, C.J.; Nystrom, F.H. A prospective observational study of all-cause mortality in relation to serum 25-OH vitamin D3 and parathyroid hormone levels in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2015, 7, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorde, R.; Waterloo, K.; Saleh, F.; Haug, E.; Svartberg, J. Neuropsychological function in relation to serum parathyroid hormone and serum 25–hydroxyvitamin D levels. The Tromsø study. J. Neurol. 2006, 253, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Raffner Basson, A.; Swart, R.; Jordaan, E.; Mazinu, M.; Watermeyer, G. Vitamin D deficiency increases the risk for moderate to severe disease activity in Crohn’s disease patients in South Africa, measured by the Harvey Bradshaw index. J. Am. Coll. Nutr. 2016, 35, 163–174. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Sandborn, W.J.; Dubois, C.; Rutgeerts, P. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin. Gastroenterol. Hepatol. 2010, 8, 357–363. [Google Scholar] [CrossRef]
- Llewellyn, D.J.; Langa, K.M.; Lang, I.A. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J. Geriatr. Psychiatry Neurol. 2009, 22, 188–195. [Google Scholar] [CrossRef]
- Khoraminya, N.; Tehrani-Doost, M.; Jazayeri, S.; Hosseini, A.; Djazayery, A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust. NZ J. Psychiatry 2013, 47, 271–275. [Google Scholar] [CrossRef]
- Shipowick, C.D.; Moore, C.B.; Corbett, C.; Bindler, R. Vitamin D and depressive symptoms in women during the winter: A pilot study. Appl. Nurs. Res. 2009, 22, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol. Rev. 1984, 64, 478–504. [Google Scholar] [CrossRef]
- Sakaki, T.; Sawada, N.; Nonaka, Y.; Ohyama, Y.; Inouye, K. Metabolic studies using recombinant Escherichia coli cells producing rat mitochondrial CYP24. Eur. J. Biochem. 1999, 262, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Dietary Reference Intake for Calcium Vitamin D; Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Food and Nutrition Board; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Haines, D.E. Neuroanatomy in Clinical Context: An. Atlas of Structures, Sections, Systems, and Syndromes, 9th ed.; Walter Kluwer Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Haines, D.E. Fundamental Neuroscience for Basic and Clinical Applications, 4th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2012. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachinvala, N.D.; Teramoto, N.; Stergiou, A. Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D3 in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression. Brain Sci. 2020, 10, 600. https://doi.org/10.3390/brainsci10090600
Sachinvala ND, Teramoto N, Stergiou A. Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D3 in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression. Brain Sciences. 2020; 10(9):600. https://doi.org/10.3390/brainsci10090600
Chicago/Turabian StyleSachinvala, Navzer D., Naozumi Teramoto, and Angeline Stergiou. 2020. "Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D3 in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression" Brain Sciences 10, no. 9: 600. https://doi.org/10.3390/brainsci10090600
APA StyleSachinvala, N. D., Teramoto, N., & Stergiou, A. (2020). Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D3 in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression. Brain Sciences, 10(9), 600. https://doi.org/10.3390/brainsci10090600




