Widespread Reductions of Spontaneous Neurophysiological Activity in Leber’s Disease—An Application of EEG Source Current Density Reconstruction
Abstract
:1. Introduction
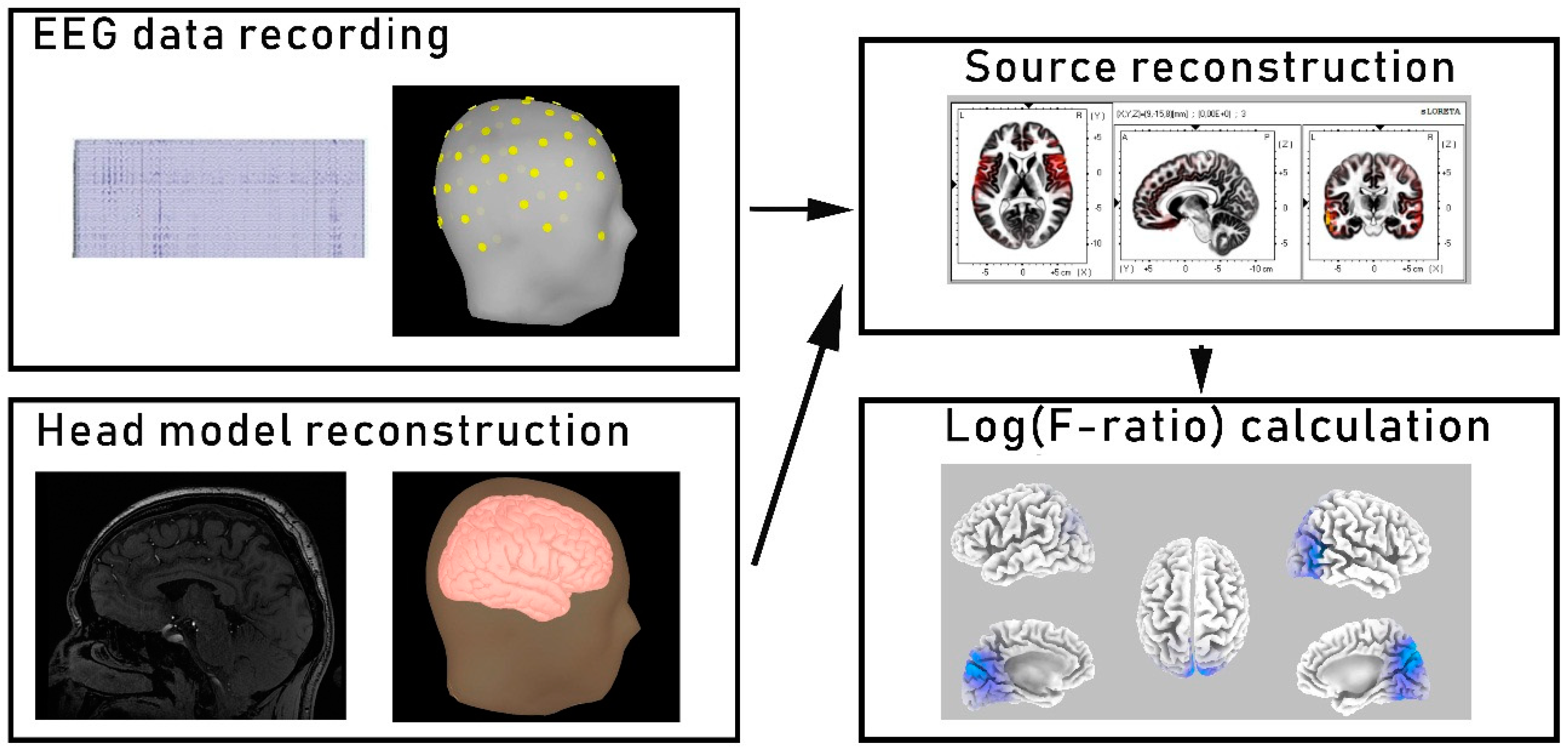
2. Methods
2.1. Participants
2.2. EEG Recording
2.3. EEG Source Localization and Functional Connectivity Analysis
2.4. Optical Coherence Tomography (OCT) Acquisition
2.5. Statistical Analysis
3. Results
3.1. Participants
3.2. Current Source Density
3.3. Associations between the Clinical Features of LHON Patients and Current Source Density Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, N.J.; Lott, M.T.; Wallace, D.C. The clinical characteristics of pedigrees of Leber’s hereditary optic neuropathy with the 11778 mutation. Am. J. Ophthalmol. 1991, 111, 750–762. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Votruba, M.; Burté, F.; La Morgia, C.; Barboni, P.; Carelli, V. A neurodegenerative perspective on mitochondrial optic neuropathies. Acta Neuropathol. 2016, 132, 789–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tońska, K.; Kodroń, A.; Bartnik, E. Genotype-phenotype correlations in Leber hereditary optic neuropathy. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 1119–1123. [Google Scholar] [CrossRef] [Green Version]
- Giordano, C.; Iommarini, L.; Giordano, L.; Maresca, A.; Pisano, A.; Valentino, M.L.; Caporali, L.; Liguori, R.; Deceglie, S.; Roberti, M.; et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber’s hereditary optic neuropathy. Brain 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziccardi, L.; Sadun, F.; De Negri, A.M.; Barboni, P.; Savini, G.; Borrelli, E.; Morgia, C.L.; Carelli, V.; Parisi, V. Retinal function and neural conduction along the visual pathways in affected and unaffected carriers with Leber’s hereditary optic neuropathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6893. [Google Scholar] [CrossRef] [Green Version]
- Barragán-Campos, H.M.; Vallée, J.N.; Lô, D.; Barrera-Ramírez, C.F.; Argote-Greene, M.; Sánchez-Guerrero, J.; Estañol, B.; Guillevin, R.; Chiras, J. Brain magnetic resonance imaging findings in patients with mitochondrial cytopathies. Arch. Neurol. 2005. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fan, K.; Zhang, Y.; Chen, Y.; Tian, Q.; Shi, D. Quantitative assessment of optic nerve in patients with Leber’s hereditary optic neuropathy using reduced field-of-view diffusion tensor imaging. Eur. J. Radiol. 2017. [Google Scholar] [CrossRef]
- Barcella, V.; Rocca, M.A.; Bianchi-Marzoli, S.; Milesi, J.; Melzi, L.; Falini, A.; Pierro, L.; Filippi, M. Evidence for retrochiasmatic tissue loss in Leber’s hereditary optic neuropathy. Hum. Brain Mapp. 2010. [Google Scholar] [CrossRef]
- Milesi, J.; Rocca, M.A.; Bianchi-Marzoli, S.; Petrolini, M.; Pagani, E.; Falini, A.; Comi, G.; Filippi, M. Patterns of white matter diffusivity abnormalities in Leber’s hereditary optic neuropathy: A tract-based spatial statistics study. J. Neurol. 2012. [Google Scholar] [CrossRef]
- Jonak, K.; Krukow, P.; Symms, M.; Maciejewski, R.; Grochowski, C. Neuroanatomical changes in Leber’s hereditary optic neuropathy: Clinical application of 7T MRI submillimeter morphometry. Brain Sci. 2020, 10, 359. [Google Scholar] [CrossRef]
- Rocca, M.A.; Valsasina, P.; Pagani, E.; Bianchi-Marzoli, S.; Milesi, J.; Falini, A.; Comi, G.; Filippi, M. Extra-visual functional and structural connection abnormalities in Leber’s hereditary optic neuropathy. PLoS ONE 2011, 6, e17081. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, O.C.; Mateus, C.; Reis, A.; Grazina, M.M.; Castelo-Branco, M. Long term cortical plasticity in visual retinotopic areas in humans with silent retinal ganglion cell loss. Neuroimage 2013. [Google Scholar] [CrossRef] [PubMed]
- Jonak, K.; Krukow, P.; Jonak, K.E.; Grochowski, C.; Karakuła-Juchnowicz, H. Quantitative and qualitative comparison of EEG-based neural network organization in two schizophrenia groups differing in the duration of illness and disease burden: Graph analysis with application of the minimum spanning tree. Clin. EEG Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Krukow, P.; Jonak, K.; Karpiński, R.; Karakuła-Juchnowicz, H. Abnormalities in hubs location and nodes centrality predict cognitive slowing and increased performance variability in first-episode schizophrenia patients. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes da Silva, F. EEG and MEG: Relevance to neuroscience. Neuron 2013, 80, 1112–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhang, Y.; Lu, L.; Yang, H. Therapeutic effects of idebenone on Leber hereditary optic neuropathy. Curr. Eye Res. 2020. [Google Scholar] [CrossRef]
- Jarc-Vidmar, M.; Tajnik, M.; Brecelj, J.; Fakin, A.; Sustar, M.; Naji, M.; Stirn-Kranjc, B.; Glavač, D.; Hawlina, M. Clinical and electrophysiology findings in Slovene patients with Leber hereditary optic neuropathy. Doc. Ophthalmol. 2015. [Google Scholar] [CrossRef]
- Oguchi, Y. Past, present, and future in Leber’s hereditary optic neuropathy. J. Jpn. Ophthalmol. Soc. 2001, 105, 809–827. [Google Scholar] [CrossRef]
- Morgia, C.L.; Achilli, A.; Iommarini, L.; Barboni, P.; Pala, M.; Olivieri, A.; Zanna, C.; Vidoni, S.; Tonon, C.; Lodi, R.; et al. Rare mtDNA variants in Leber hereditary optic neuropathy families with recurrence of myoclonus. Neurology 2008. [Google Scholar] [CrossRef]
- Niehusmann, P.; Surges, R.; von Wrede, R.D.; Elger, C.E.; Wellmer, J.; Reimann, J.; Urbach, H.; Vielhaber, S.; Bien, C.G.; Kunz, W.S. Mitochondrial dysfunction due to Leber’s hereditary optic neuropathy as a cause of visual loss during assessment for epilepsy surgery. Epilepsy Behav. 2011. [Google Scholar] [CrossRef]
- Tu, Y. Machine learning. In EEG Signal Processing and Feature Extraction; Springer Nature: London, UK, 2019; ISBN 9789811391132. [Google Scholar]
- Sanei, S.; Chambers, J.A. EEG Signal Processing; Wiley-Interscience: Hoboken, NJ, USA, 2007; ISBN 9780470025819. [Google Scholar]
- Jonak, K.; Syta, A.; Karakuła-Juchnowicz, H.; Krukow, P. The clinical application of EEG-signals recurrence analysis as a measure of functional connectivity: Comparative case study of patients with various neuropsychiatric disorders. Brain Sci. 2020, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Mohanchandra, K.; Saha, S. Machine learning methods as a test bed for EEG analysis in BCI paradigms. In Handbook of Research on Applied Cybernetics and Systems Science; Saha, S., Mandal, A., Narasimhamurthy, A., Sarasvathi, V., Sangam, S., Eds.; IGI Global: Hershey, PA, USA, 2017; pp. 186–206. ISBN 9781522524984. [Google Scholar]
- Ihlen, E.A.F.; Støen, R.; Boswell, L.; de Regnier, R.-A.; Fjørtoft, T.; Gaebler-Spira, D.; Labori, C.; Loennecken, M.C.; Msall, M.E.; Möinichen, U.I.; et al. Machine learning of infant spontaneous movements for the early prediction of cerebral palsy: A multi-site cohort study. J. Clin. Med. 2019, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imani, M.; Ghoreishi, S.F. Bayesian optimization objective-based experimental design. In Proceedings of the 2020 American Control Conference (ACC), Denver, CO, USA, 1–3 July 2020; pp. 3405–3411. [Google Scholar]
- Imani, M.; Ghoreishi, S.F.; Braga-Neto, U.M. Bayesian control of large MDPs with unknown dynamics in data-poor environments. In Advances in Neural Information Processing Systems 31; Bengio, S., Wallach, H., Larochelle, H., Grauman, K., Cesa-Bianchi, N., Garnett, R., Eds.; Curran Associates, Inc.: New York, NY, USA, 2018; pp. 8146–8156. [Google Scholar]
- Rutkove, S.B. Introduction to volume conduction. In The Clinical Neurophysiology Primer; Humana Press: Totowa, NJ, USA, 2007; ISBN 9780896039964. [Google Scholar]
- Pascual-Marqui, R.D.; Lehmann, D.; Koenig, T.; Kochi, K.; Merlo, M.C.G.; Hell, D.; Koukkou, M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. Neuroimaging 1999. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar]
- Krukow, P.; Jonak, K.; Grochowski, C.; Plechawska-Wójcik, M.; Karakuła-Juchnowicz, H. Resting-state hyperconnectivity within the default mode network impedes the ability to initiate cognitive performance in first-episode schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109959. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D. Theory of the EEG inverse problem. Quant. EEG Anal. Methods Clin. Appl. 2009, 5, 121–140. [Google Scholar]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002. [Google Scholar] [CrossRef] [Green Version]
- Lizio, R.; Vecchio, F.; Frisoni, G.B.; Ferri, R.; Rodriguez, G.; Babiloni, C. Electroencephalographic rhythms in Alzheimer’s disease. Int. J. Alzheimers. Dis. 2011, 2011, 927573. [Google Scholar] [CrossRef] [Green Version]
- Hanslmayr, S.; Backes, H.; Straub, S.; Popov, T.; Langguth, B.; Hajak, G.; Bäuml, K.H.T.; Landgrebe, M. Enhanced resting-state oscillations in schizophrenia are associated with decreased synchronization during inattentional blindness. Hum. Brain Mapp. 2013. [Google Scholar] [CrossRef]
- Kaiser, A.K.; Gnjezda, M.T.; Knasmüller, S.; Aichhorn, W. Electroencephalogram alpha asymmetry in patients with depressive disorders: Current perspectives. Neuropsychiatr. Dis. Treat. 2018, 14, 1493–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novikova, L.A. Blindness and the Electrical Activity of the Brain: Electroencephalographic Studies of the Effects of Sensory Impairment; Amer Foundation for the Blind: Arlington, VA, USA, 1974. [Google Scholar]
- Rösler, F.; Röder, B.; Heil, M.; Hennighausen, E. Topographic differences of slow event-related brain potentials in blind and sighted adult human subjects during haptic mental rotation. Cogn. Brain Res. 1993. [Google Scholar] [CrossRef]
- Röder, B.; Rösler, F.; Hennighausen, E. Different cortical activation patterns in blind and sighted humans during encoding and transformation of haptic images. Psychophysiology 1997. [Google Scholar] [CrossRef]
- Bértolo, H.; Paiva, T.; Pessoa, L.; Mestre, T.; Marques, R.; Santos, R. Visual dream content, graphical representation and EEG alpha activity in congenitally blind subjects. Cogn. Brain Res. 2003. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Wei, S.; Qiu, H.; Gong, Y.; Li, H.; Dai, Y.; Jiang, Z.; Liu, Z. Characterization of retinal nerve fiber layer thickness changes associated with Leber’s hereditary optic neuropathy by optical coherence tomography. Exp. Ther. Med. 2014, 7, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.O.; John, E.R.; Jelic, V. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, J.M.; McCarley, R.W. Gamma band oscillations: A key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr. Opin. Psychiatry 2016, 29, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Merker, B.H. Cortical gamma oscillations: Details of their genesis preclude a role in cognition. Front. Comput. Neurosci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.O.; Chinnery, P.F. Mitochondria and cortical gamma oscillations: Food for thought? Brain 2011, 134, 330–332. [Google Scholar] [CrossRef]
- Atallah, B.V.; Scanziani, M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron 2009. [Google Scholar] [CrossRef] [Green Version]
- Kann, O.; Huchzermeyer, C.; Kovács, R.; Wirtz, S.; Schuelke, M. Gamma oscillations in the hippocampus require high complex i gene expression and strong functional performance of mitochondria. Brain 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wipf, D.; Nagarajan, S. A unified Bayesian framework for MEG/EEG source imaging. Neuroimage 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Clinical/Demographical Data: | LHON (N = 20) M (SD) | HC (N = 20) M (SD) | t or χ2 | p |
|---|---|---|---|---|
| Age (years) | 34.8 (12.18) | 32.35 (6.46) | 0.79 | 0.43 |
| Education (years) | 15.2 (1.96) | 15.8 (1.98) | −1.02 | 0.31 |
| Sex (% male) | 90 | 90 | 0.17 | 0.99 |
| Mitochondrial mutation 11778G > A (%) | 100 | |||
| Duration of illness (months) | 145.2 (132.75) | |||
| Left RNFL averaged thickness (µm) | 62.11 (2.83) | |||
| Right RNFL averaged thickness (µm) | 61.77 (3.13) |
| Frequency Band | Lobe | Area | LHON (N = 20) M | HC (N = 20) M | t | p |
|---|---|---|---|---|---|---|
| Alpha | Occipital | Right Secondary Visual Cortex (BA 19) | 23.43 | 61.13 | −3.59 | 0.001 |
| Right Cuneus | 18.66 | 52.20 | −3.01 | 0.005 | ||
| Left Cuneus | 19.12 | 44.46 | −2.61 | 0.01 | ||
| Right Middle Occipital Gyrus | 21.21 | 77.12 | −3.86 | <0.001 | ||
| Left Middle Occipital Gyrus | 34.11 | 71.43 | −2.68 | 0.01 | ||
| Temporal | Right Middle Temporal Gyrus | 29.15 | 62.11 | −2.66 | 0.01 | |
| Parietal | Right Precuneus | 26.12 | 57.88 | −2.53 | 0.03 | |
| Gamma | Parietal | Right Superior Parietal Lobule | 0.021 | 0.075 | −4.11 | <0.001 |
| Right Inferior Parietal Lobule | 0.018 | 0.071 | −4.01 | <0.001 | ||
| Right Angular Gyrus | 0.022 | 0.067 | −4.03 | <0.001 | ||
| Left Precuneus | 0.011 | 0.034 | −2.97 | 0.008 | ||
| Right Precuneus | 0.013 | 0.049 | −4.10 | <0.001 | ||
| Occipital | Right Cuneus | 0.031 | 0.048 | −2.48 | 0.04 | |
| Right Middle Occipital Gyrus | 0.014 | 0.041 | −2.77 | 0.01 | ||
| Right Superior Occipital Gyrus | 0.017 | 0.053 | −2.92 | 0.007 | ||
| Temporal | Right Middle Temporal Gyrus | 0.033 | 0.073 | −2.87 | 0.008 | |
| Frontal | Right Superior Frontal Gyrus | 0.044 | 0.065 | −2.34 | 0.05 | |
| Right Inferior Frontal Gyrus | 0.039 | 0.068 | −2.41 | 0.05 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonak, K. Widespread Reductions of Spontaneous Neurophysiological Activity in Leber’s Disease—An Application of EEG Source Current Density Reconstruction. Brain Sci. 2020, 10, 622. https://doi.org/10.3390/brainsci10090622
Jonak K. Widespread Reductions of Spontaneous Neurophysiological Activity in Leber’s Disease—An Application of EEG Source Current Density Reconstruction. Brain Sciences. 2020; 10(9):622. https://doi.org/10.3390/brainsci10090622
Chicago/Turabian StyleJonak, Kamil. 2020. "Widespread Reductions of Spontaneous Neurophysiological Activity in Leber’s Disease—An Application of EEG Source Current Density Reconstruction" Brain Sciences 10, no. 9: 622. https://doi.org/10.3390/brainsci10090622




