Prefrontal Cortex Involvement during Dual-Task Stair Climbing in Healthy Older Adults: An fNIRS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equipment
2.3. Experimental Design
2.4. Cognitive and Motor Test Battery
2.5. Data Processing
2.6. Statistical Analyses
3. Results
3.1. fNIRS Hemodynamic Response
3.2. Cognitive and Motor Performance
4. Discussion
4.1. Hemodynamic Response: Single and Dual-Task
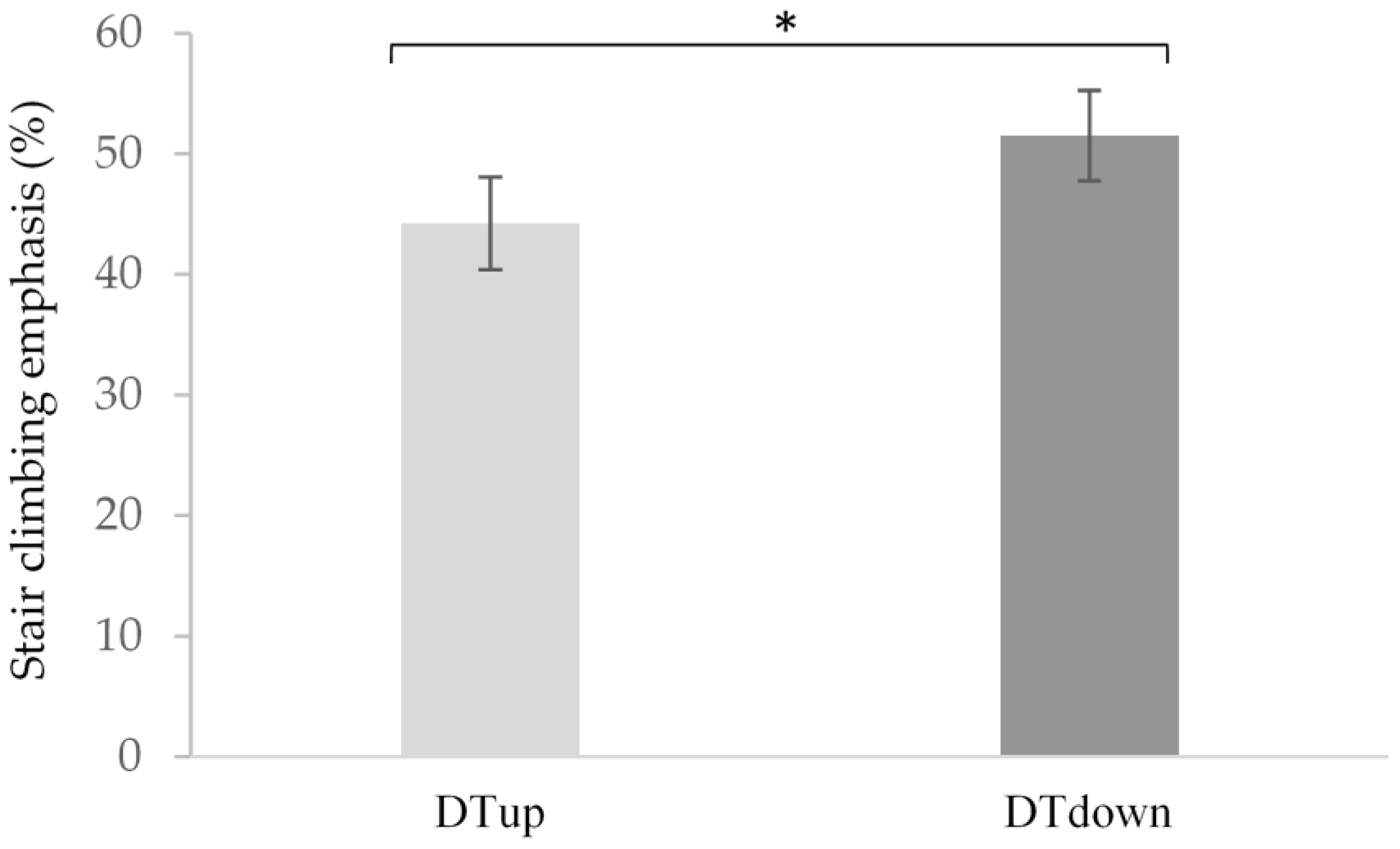
4.2. Hemodynamic Response: Ascent and Descent
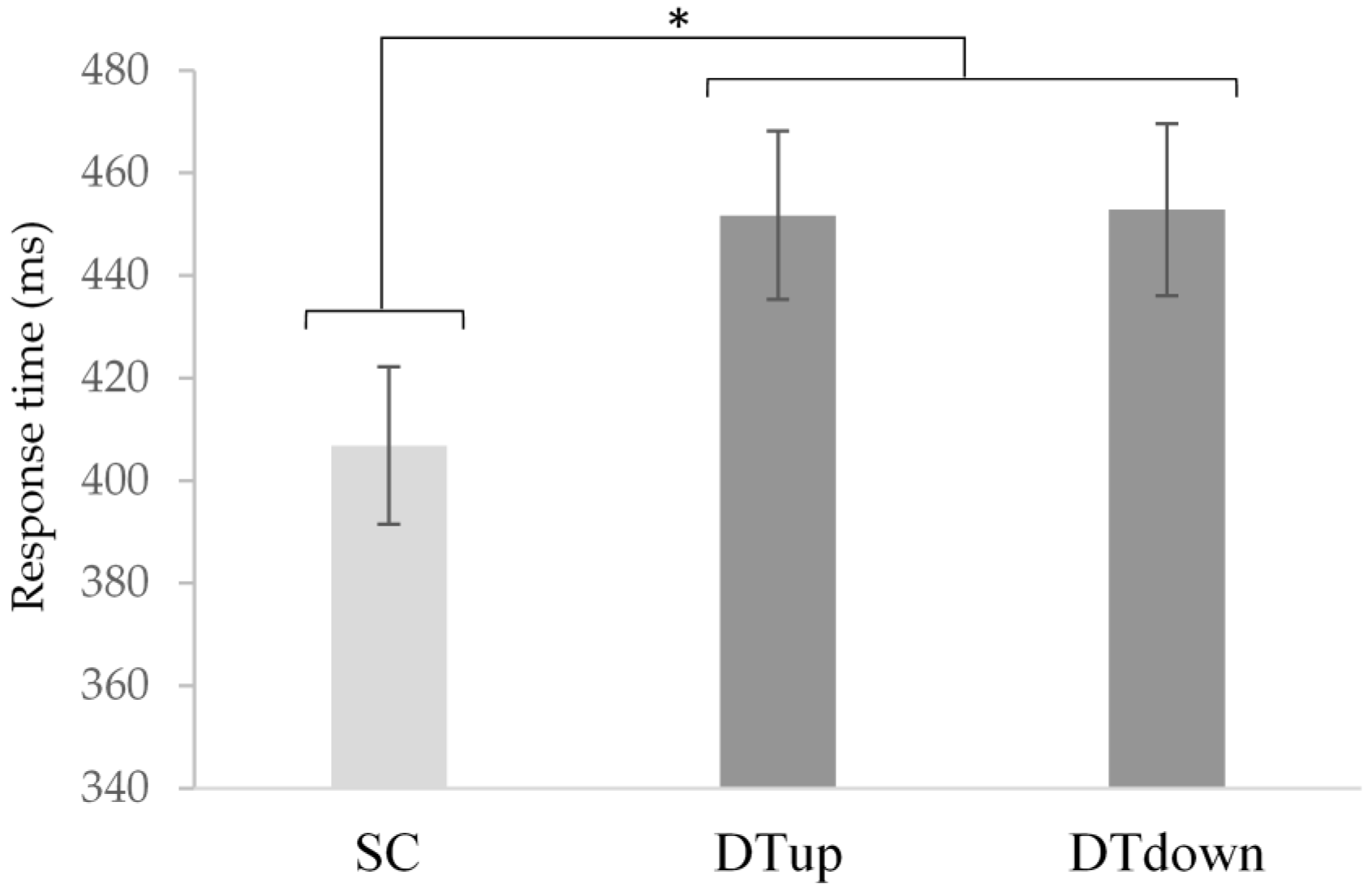
4.3. Cognitive Performance: Vocal Response Time and Accuracy
4.4. Motor Performance: Gait Speed
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobs, J.V. A Review of Stairway Falls and Stair Negotiation: Lessons Learned and Future Needs to Reduce Injury. Gait Posture 2016, 49, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beurskens, R.; Bock, O. Age-Related Deficits of Dual-Task Walking: A Review. Neural Plast. 2012, 2012, 131608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raz, N.; Lindenberger, U.; Rodrigue, K.M.; Kennedy, K.M.; Head, D.; Williamson, A.; Dahle, C.; Gerstorf, D.; Acker, J.D. Regional Brain Changes in Aging Healthy Adults: General Trends, Individual Differences and Modifiers. Cereb. Cortex 2005, 15, 1676–1689. [Google Scholar] [CrossRef] [PubMed]
- Li, K.Z.H.; Bherer, L.; Mirelman, A.; Maidan, I.; Hausdorff, J.M. Cognitive Involvement in Balance, Gait and Dual-Tasking in Aging: A Focused Review From a Neuroscience of Aging Perspective. Front. Neurol. 2018, 9, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogev, G.; Hausdorff, J.M.; Giladi, N. The Role of Executive Function and Attention in Gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 329–472. [Google Scholar] [CrossRef] [Green Version]
- Hausdorff, J.M.; Schweiger, A.; Herman, T.; Yogev-Seligmann, G.; Giladi, N. Dual Task Decrements in Gait among Healthy Older Adults: Contributing Factors. J. Gerontol. A. Biol. Sci. Med. Sci. 2008, 63, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Woollacott, M.; Shumway-Cook, A. Attention and the Control of Posture and Gait: A Review of an Emerging Area of Research. Gait Posture 2002, 16, 1–14. [Google Scholar] [CrossRef]
- Madehkhaksar, F.; Egges, A. Effect of Dual Task Type on Gait and Dynamic Stability during Stair Negotiation at Different Inclinations. Gait Posture 2016, 43, 114–119. [Google Scholar] [CrossRef]
- Tiedemann, A.C.; Sherrington, C.; Lord, S.R. Physical and Psychological Factors Associated With Stair Negotiation Performance in Older People. J. Gerontol. A. Biol. Sci. Med. Sci. 2007, 62, 1259–1265. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chou, L.-S. Balance Control during Stair Negotiation in Older Adults. J. Biomech. 2007, 40, 2530–2536. [Google Scholar] [CrossRef]
- Miyasike-daSilva, V.; Allard, F.; McIlroy, W.E. Where Do We Look When We Walk on Stairs? Gaze Behaviour on Stairs, Transitions, and Handrails. Exp. Brain Res. 2011, 209, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Startzell, J.K.; Owens, D.A.; Mulfinger, L.M.; Cavanagh, P.R. Stair Negotiation in Older People: A Review. J. Am. Geriatr. Soc. 2000, 48, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Hamel, K.A.; Cavanagh, P.R. Stair Performance in People Aged 75 and Older. J. Am. Geriatr. Soc. 2004, 52, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Bürki, C.N.; Bridenbaugh, S.A.; Reinhardt, J.; Stippich, C.; Kressig, R.W.; Blatow, M. Imaging Gait Analysis: An FMRI Dual Task Study. Brain Behav. 2017, 7, e00724. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.A.; Dupuy, O.; Pouliot, P.; Lesage, F.; Bherer, L. Comparable Cerebral Oxygenation Patterns in Younger and Older Adults during Dual-Task Walking with Increasing Load. Front. Aging Neurosci. 2016, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-Hall Series in Experimental Psychology; Prentice-Hall: Englewood Cliffs, NJ, USA, 1973; ISBN 978-0-13-050518-7. [Google Scholar]
- Ojha, H.A.; Kern, R.W.; Lin, C.-H.J.; Winstein, C.J. Age Affects the Attentional Demands of Stair Ambulation: Evidence From a Dual-Task Approach. Phys. Ther. 2009, 89, 1080–1088. [Google Scholar] [CrossRef]
- Lajoie, Y.; Teasdale, N.; Bard, C.; Fleury, M. Upright Standing and Gait: Are There Changes in Attentional Requirements Related to Normal Aging? Exp. Aging Res. 1996, 22, 185–198. [Google Scholar] [CrossRef]
- Stacoff, A.; Diezi, C.; Luder, G.; Stüssi, E.; Kramers-de Quervain, I.A. Ground Reaction Forces on Stairs: Effects of Stair Inclination and Age. Gait Posture 2005, 21, 24–38. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, W.; Song, Q.; Gu, H.; Mao, D. Performance of Older Adults under Dual Task during Stair Descent. J. Exerc. Sci. Fit. 2018, 16, 99–105. [Google Scholar] [CrossRef]
- Rosso, A.L.; Cenciarini, M.; Sparto, P.J.; Loughlin, P.J.; Furman, J.M.; Huppert, T.J. Neuroimaging of an Attention Demanding Dual-Task during Dynamic Postural Control. Gait Posture 2017, 57, 193–198. [Google Scholar] [CrossRef]
- Srygley, J.M.; Mirelman, A.; Herman, T.; Giladi, N.; Hausdorff, J.M. When Does Walking Alter Thinking? Age and Task Associated Findings. Brain Res. 2009, 1253, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeij, A.; van Beek, A.H.E.A.; Olde Rikkert, M.G.M.; Claassen, J.A.H.R.; Kessels, R.P.C. Effects of Aging on Cerebral Oxygenation during Working-Memory Performance: A Functional Near-Infrared Spectroscopy Study. PLoS ONE 2012, 7, e46210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelicioni, P.H.S.; Tijsma, M.; Lord, S.R.; Menant, J. Prefrontal Cortical Activation Measured by FNIRS during Walking: Effects of Age, Disease and Secondary Task. PeerJ 2019, 7, e6833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinti, P.; Aichelburg, C.; Gilbert, S.; Hamilton, A.; Hirsch, J.; Burgess, P.; Tachtsidis, I. A Review on the Use of Wearable Functional Near-Infrared Spectroscopy in Naturalistic Environments. Jpn. Psychol. Res. 2018, 60, 347–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorond, F.A.; Kiely, D.K.; Galica, A.; Moscufo, N.; Serrador, J.M.; Iloputaife, I.; Egorova, S.; Dell’Oglio, E.; Meier, D.S.; Newton, E.; et al. Neurovascular Coupling Is Impaired in Slow Walkers: The MOBILIZE Boston Study. Ann. Neurol. 2011, 70, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Al-Yahya, E.; Dawes, H.; Smith, L.; Dennis, A.; Howells, K.; Cockburn, J. Cognitive Motor Interference While Walking: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2011, 35, 715–728. [Google Scholar] [CrossRef]
- Chen, M.; Pillemer, S.; England, S.; Izzetoglu, M.; Mahoney, J.R.; Holtzer, R. Neural Correlates of Obstacle Negotiation in Older Adults: An FNIRS Study. Gait Posture 2017, 58, 130–135. [Google Scholar] [CrossRef]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Wang, C.; England, S.; Verghese, J. Online Fronto-Cortical Control of Simple and Attention-Demanding Locomotion in Humans. NeuroImage 2015, 112, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Mirelman, A.; Maidan, I.; Bernad-Elazari, H.; Shustack, S.; Giladi, N.; Hausdorff, J.M. Effects of Aging on Prefrontal Brain Activation during Challenging Walking Conditions. Brain Cogn. 2017, 115, 41–46. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Park, D.C. How Does It STAC Up? Revisiting the Scaffolding Theory of Aging and Cognition. Neuropsychol. Rev. 2014, 24, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the International 10-20 EEG System for Positioning of Transcranial Magnetic Stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Arafsha, F.; Hanna, C.; Aboualmagd, A.; Fraser, S.; El Saddik, A. Instrumented Wireless SmartInsole System for Mobile Gait Analysis: A Validation Pilot Study with Tekscan Strideway. J. Sens. Actuator Netw. 2018, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Arafsha, F.; Laamarti, F.; Saddik, A.E. Development of a Wireless CPS for Gait Parameters Measurement and Analysis. In Proceedings of the 2018 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Houston, TX, USA, 14–17 May 2018; pp. 1–5. [Google Scholar]
- Badawi, H.; Laamarti, F.; Arafsha, F.; El Saddik, A. Standardizing a Shoe Insole Based on ISO/IEEE 11073 Personal Health Device (X73-PHD) Standards. In Proceedings of the Information Technology and Systems; Rocha, Á, Ferrás, C., Paredes, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 764–778. [Google Scholar]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Thiers, A.; Hamacher, D.; Schega, L. Functional Near-Infrared Spectroscopy in Movement Science: A Systematic Review on Cortical Activity in Postural and Walking Tasks. Neurophotonics 2017, 4, 41403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, L.A.; McKenzie, N.C.; Doan, J.B. Age-Dependent Differences in the Attentional Demands of Obstacle Negotiation. J. Gerontol. A. Biol. Sci. Med. Sci. 2005, 60, 924–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogev-Seligmann, G.; Rotem-Galili, Y.; Mirelman, A.; Dickstein, R.; Giladi, N.; Hausdorff, J.M. How Does Explicit Prioritization Alter Walking During Dual-Task Performance? Effects of Age and Sex on Gait Speed and Variability. Phys. Ther. 2010, 90, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale-Revised (WAIS-R); Psychological Corporation: New York, NY, USA, 1981. [Google Scholar]
- Strauss, E.; Sherman, E.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed.; Oxford University Press: New York, NY, USA, 2006; ISBN 0-19-515957-8. [Google Scholar]
- Delbaere, K.; Close, J.C.T.; Mikolaizak, A.S.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. The Falls Efficacy Scale International (FES-I). A Comprehensive Longitudinal Validation Study. Age Ageing 2010, 39, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Yesavage, J.; Sheikh, A. 9/Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, M. General Equation for the Differential Pathlength Factor of the Frontal Human Head Depending on Wavelength and Age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef] [Green Version]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Verghese, J. FNIRS Study of Walking and Walking While Talking in Young and Old Individuals. J. Gerontol. A. Biol. Sci. Med. Sci. 2011, 66A, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Verghese, J.; Wang, C.; Ayers, E.; Izzetoglu, M.; Holtzer, R. Brain Activation in High-Functioning Older Adults and Falls: Prospective Cohort Study. Neurology 2017, 88, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wervey, R.A.; Harris, G.F.; Wertsch, J.J. Plantar Pressure Characteristics during Stair Climbing and Descent. In Proceedings of the 19th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, “Magnificent Milestones and Emerging Opportunities in Medical Engineering” (Cat. No.97CH36136), Chicago, IL, USA, 30 October–2 November 1997; Volume 4, pp. 1746–1748. [Google Scholar]
- Holtzer, R.; Schoen, C.; Demetriou, E.; Mahoney, J.R.; Izzetoglu, M.; Wang, C.; Verghese, J. Stress and Gender Effects on Prefrontal Cortex Oxygenation Levels Assessed during Single and Dual-Task Walking Conditions. Eur. J. Neurosci. 2017, 45, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Marusic, U.; Taube, W.; Morrison, S.A.; Biasutti, L.; Grassi, B.; De Pauw, K.; Meeusen, R.; Pisot, R.; Ruffieux, J. Aging Effects on Prefrontal Cortex Oxygenation in a Posture-Cognition Dual-Task: An FNIRS Pilot Study. Eur. Rev. Aging Phys. Act. 2019, 16, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillardin, F.; Baudry, S. Influence of Working Memory and Executive Function on Stair Ascent and Descent in Young and Older Adults. Exp. Gerontol. 2018, 106, 74–79. [Google Scholar] [CrossRef]
- Maidan, I.; Nieuwhof, F.; Bernad-Elazari, H.; Reelick, M.F.; Bloem, B.R.; Giladi, N.; Deutsch, J.E.; Hausdorff, J.M.; Claassen, J.A.H.; Mirelman, A. The Role of the Frontal Lobe in Complex Walking Among Patients With Parkinson’s Disease and Healthy Older Adults: An FNIRS Study. Neurorehabil. Neural Repair 2016, 30, 963–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain Activity during Walking: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.; Shiffrin, R.M. Controlled and Automatic Human Information Processing: I. Detection, Search, and Attention. Psychol. Rev. 1977, 84, 1. [Google Scholar] [CrossRef]
- Novak, A.C.; Komisar, V.; Maki, B.E.; Fernie, G.R. Age-Related Differences in Dynamic Balance Control during Stair Descent and Effect of Varying Step Geometry. Appl. Ergon. 2016, 52, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Oh-Park, M.; Wang, C.; Verghese, J. Stair Negotiation Time in Community Dwelling Older Adults: Normative Values and Association with Functional Decline. Arch. Phys. Med. Rehabil. 2011, 92, 2006–2011. [Google Scholar] [CrossRef] [Green Version]
- Holtzer, R.; Kraut, R.; Izzetoglu, M.; Ye, K. The Effect of Fear of Falling on Prefrontal Cortex Activation and Efficiency during Walking in Older Adults. GeroScience 2019, 41, 89–100. [Google Scholar] [CrossRef]
- Decker, L.M.; Cignetti, F.; Stergiou, N. Wearing a Safety Harness during Treadmill Walking Influences Lower Extremity Kinematics Mainly through Changes in Ankle Regularity and Local Stability. J. NeuroEngineering Rehabil. 2012, 9, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, S.M.; Novak, A.C.; Brouwer, B.; Costigan, P.A. Relationship between Stair Ambulation with and without a Handrail and Centre of Pressure Velocities during Stair Ascent and Descent. Gait Posture 2011, 34, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Reeves, N.D.; Spanjaard, M.; Mohagheghi, A.A.; Baltzopoulos, V.; Maganaris, C.N. Influence of Light Handrail Use on the Biomechanics of Stair Negotiation in Old Age. Gait Posture 2008, 28, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Forstmann, B.U.; Tittgemeyer, M.; Wagenmakers, E.-J.; Derrfuss, J.; Imperati, D.; Brown, S. The Speed-Accuracy Tradeoff in the Elderly Brain: A Structural Model-Based Approach. J. Neurosci. 2011, 31, 17242–17249. [Google Scholar] [CrossRef]
- Czernochowski, D.; NESSLER, D.; FRIEDMAN, D. On Why Not to Rush Older Adults—Relying on Reactive Cognitive Control Can Effectively Reduce Errors at the Expense of Slowed Responses. Psychophysiology 2010, 47, 637–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shumway-Cook, A.; Woollacott, M.; Kerns, K.A.; Baldwin, M. The Effects of Two Types of Cognitive Tasks on Postural Stability in Older Adults With and Without a History of Falls. J. Gerontol. A. Biol. Sci. Med. Sci. 1997, 52A, M232–M240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.Z.H.; Lindenberger, U.; Freund, A.M.; Baltes, P.B. Walking While Memorizing: Age-Related Differences in Compensatory Behavior. Psychol. Sci. 2001, 12, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-H.; Galea, E.R.; Hong, W.-H. Individual Stair Ascent and Descent Walk Speeds Measured in a Korean High-Rise Building. Fire Technol. 2014, 50, 267–295. [Google Scholar] [CrossRef]
- Harada, T.; Miyai, I.; Suzuki, M.; Kubota, K. Gait Capacity Affects Cortical Activation Patterns Related to Speed Control in the Elderly. Exp. Brain Res. 2009, 193, 445–454. [Google Scholar] [CrossRef]
- Suzuki, M.; Miyai, I.; Ono, T.; Kubota, K. Activities in the Frontal Cortex and Gait Performance Are Modulated by Preparation. An FNIRS Study. NeuroImage 2008, 39, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, K.L.M.; Roelofsen, E.G.J.; Duysens, J.; Keijsers, N.L.W. Cortical Control of Normal Gait and Precision Stepping: An FNIRS Study. NeuroImage 2014, 85, 415–422. [Google Scholar] [CrossRef] [PubMed]


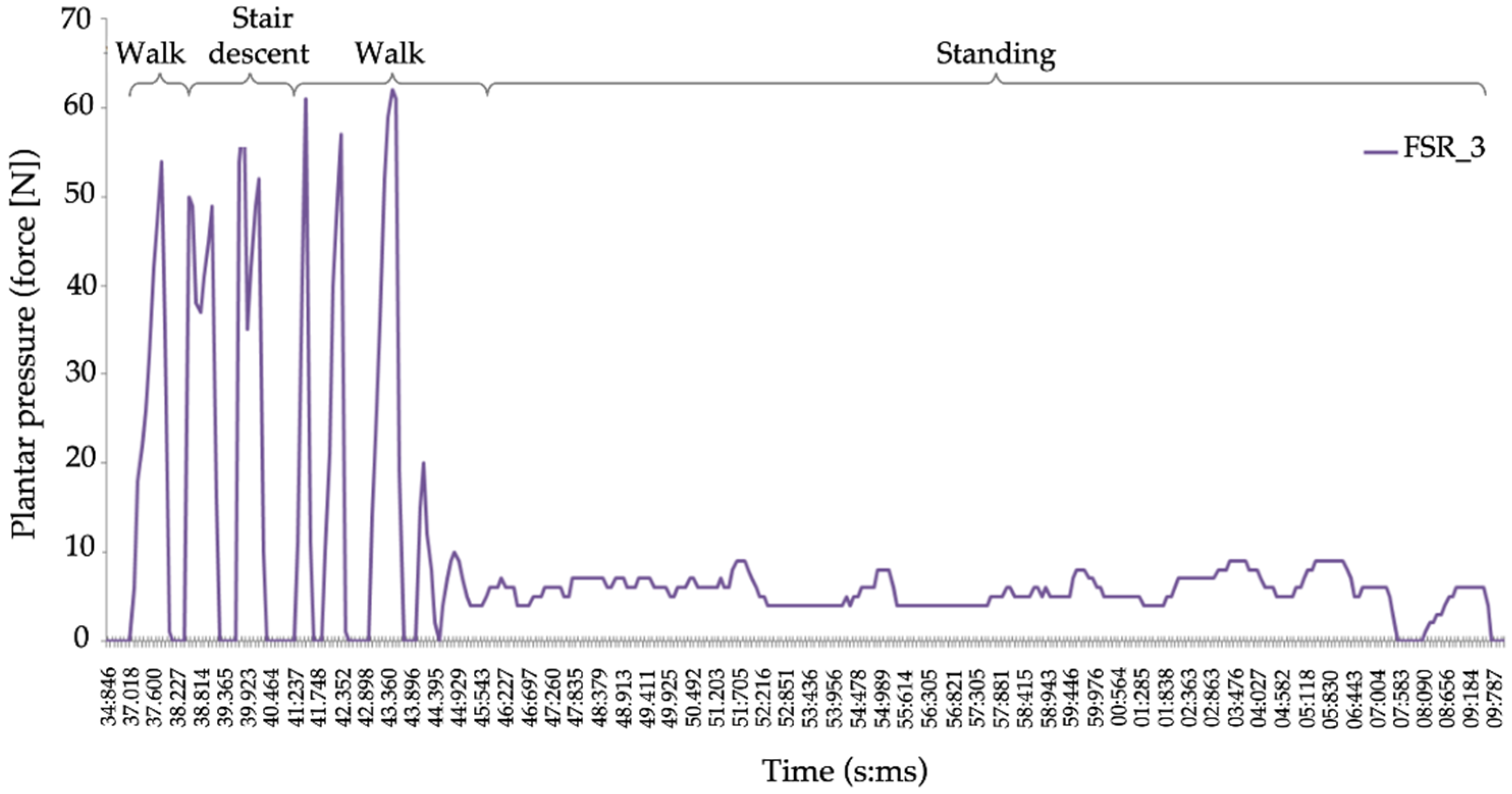

| Variable | Sample (n = 20) Mean (SD) |
|---|---|
| Age (years) | 72.7 (6.9) |
| Gender (F:M) | 14:6 |
| Education (years) | 17.3 (2.4) |
| GDS (/30) | 3.3 (3.2) |
| FES-I (/64) | 23.4 (7.6) |
| Fear going up or down stairs 1 (/4) | 1.7 (0.7) |
| MoCA (/30) | 27.3 (1.4) |
| Digit forward (/16) | 10.4 (1.6) |
| Digit backward (/14) | 7.4 (1.7) |
| Digit Symbol (/93) | 44.5 (13.4) |
| TMT A (s) | 39.3 (14.8) |
| TMT B (s) | 82.3 (34.9) |
| Variable | SC | SMup | SMdown | DTup | DTdown |
|---|---|---|---|---|---|
| ∆HbO2 (µM) | −0.04 (0.23) | −0.01 (0.31) | −0.21 (0.33) | 0.11 (0.35) | 0.04 (0.31) |
| ∆HbR (µM) | −0.02 (0.08) | −0.02 (0.12) | 0.01 (0.12) | 0.02 (0.10) | 0.01 (0.13) |
| Response time (ms) | 406.9 (68.9) | . | . | 451.8 (73.7) | 452.9 (75.1) |
| Accuracy (%) | 100.0 (0.0) | . | . | 99.3 (3.1) | 98.6 (3.7) |
| Gait speed (m/s) | . | 0.62 (0.12) | 0.63 (0.12) | 0.60 (0.12) | 0.62 (0.13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzman, T.; Aboualmagd, A.; Badawi, H.; Tobón-Vallejo, D.; Kim, H.; Dahroug, L.; Laamarti, F.; El Saddik, A.; Fraser, S. Prefrontal Cortex Involvement during Dual-Task Stair Climbing in Healthy Older Adults: An fNIRS Study. Brain Sci. 2021, 11, 71. https://doi.org/10.3390/brainsci11010071
Salzman T, Aboualmagd A, Badawi H, Tobón-Vallejo D, Kim H, Dahroug L, Laamarti F, El Saddik A, Fraser S. Prefrontal Cortex Involvement during Dual-Task Stair Climbing in Healthy Older Adults: An fNIRS Study. Brain Sciences. 2021; 11(1):71. https://doi.org/10.3390/brainsci11010071
Chicago/Turabian StyleSalzman, Talia, Ahmed Aboualmagd, Hawazin Badawi, Diana Tobón-Vallejo, Hyejun Kim, Lama Dahroug, Fedwa Laamarti, Abdulmotaleb El Saddik, and Sarah Fraser. 2021. "Prefrontal Cortex Involvement during Dual-Task Stair Climbing in Healthy Older Adults: An fNIRS Study" Brain Sciences 11, no. 1: 71. https://doi.org/10.3390/brainsci11010071







