Modulation of Functional Connectivity in Response to Mirror Visual Feedback in Stroke Survivors: An MEG Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
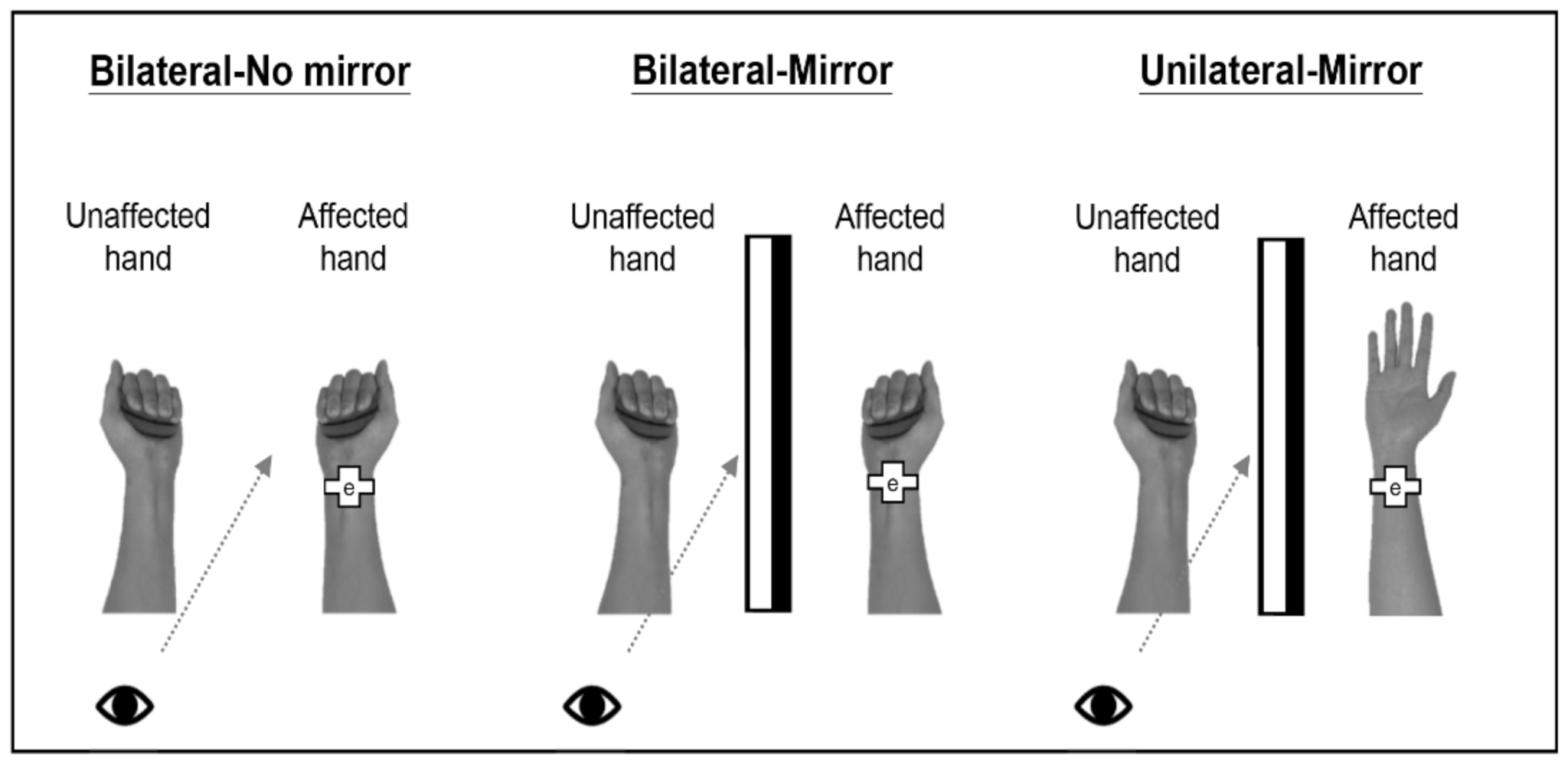
2.2. Experimental Tasks
2.3. MEG Recordings
2.4. MEG Signal Processing and Functional Connectivity Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luke, C.; Dodd, K.J.; Brock, K. Outcomes of the Bobath concept on upper limb recovery following stroke. Clin. Rehabil. 2004, 18, 888–898. [Google Scholar] [CrossRef]
- Dohle, C.; Pullen, J.; Nakaten, A.; Kust, J.; Rietz, C.; Karbe, H. Mirror therapy promotes recovery from severe hemiparesis: A randomized controlled trial. Neurorehabil. Neural Repair 2009, 23, 209–217. [Google Scholar] [CrossRef]
- Ezendam, D.; Bongers, R.M.; Jannink, M.J. Systematic review of the effectiveness of mirror therapy in upper extremity function. Disabil. Rehabil. 2009, 31, 2135–2149. [Google Scholar] [CrossRef] [PubMed]
- Colomer, C.; Noé, E.; Llorens, R. Mirror therapy in chronic stroke survivors with severely impaired upper limb function: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 271–278. [Google Scholar] [PubMed]
- Gandhi, D.B.; Sterba, A.; Khatter, H.; Pandian, J.D. Mirror Therapy in Stroke Rehabilitation: Current Perspectives. Ther. Clin. Risk Manag. 2020, 16, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschuler, E.L.; Wisdom, S.B.; Stone, L.; Foster, C.; Galasko, D.; Llewellyn, D.M.; Ramachandran, V.S. Rehabilitation of hemiparesis after stroke with a mirror. Lancet 1999, 353, 2035–2036. [Google Scholar] [CrossRef]
- Selles, R.W.; Michielsen, M.E.; Bussmann, J.B.; Stam, H.J.; Hurkmans, H.L.; Heijnen, I.; de Groot, D.; Ribbers, G.M. Effects of a mirror-induced visual illusion on a reaching task in stroke patients: Implications for mirror therapy training. Neurorehabil. Neural Repair 2014, 28, 652–659. [Google Scholar] [CrossRef]
- Garry, M.I.; Loftus, A.; Summers, J.J. Mirror, mirror on the wall: Viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp. Brain Res. 2005, 163, 118–122. [Google Scholar] [CrossRef]
- Kang, Y.J.; Ku, J.; Kim, H.J.; Park, H.K. Facilitation of corticospinal excitability according to motor imagery and mirror therapy in healthy subjects and stroke patients. Ann. Rehabil. Med. 2011, 35, 747–758. [Google Scholar] [CrossRef]
- Nojima, I.; Mima, T.; Koganemaru, S.; Thabit, M.N.; Fukuyama, H.; Kawamata, T. Human motor plasticity induced by mirror visual feedback. J. Neurosci. 2012, 32, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Novaes, M.M.; Palhano-Fontes, F.; Peres, A.; Mazzetto-Betti, K.; Pelicioni, M.; Andrade, K.C.; Dos Santos, A.C.; Pontes-Neto, O.; Araujo, D. Neurofunctional changes after a single mirror therapy intervention in chronic ischemic stroke. Int. J. Neurosci. 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumru, H.; Albu, S.; Pelayo, R.; Rothwell, J.; Opisso, E.; Leon, D.; Soler, D.; Tormos, J.M. Motor Cortex Plasticity during Unilateral Finger Movement with Mirror Visual Feedback. Neural Plast. 2016, 2016, 6087896. [Google Scholar] [CrossRef] [PubMed]
- Tai, R.Y.; Zhu, J.D.; Cheng, C.H.; Tseng, Y.J.; Chen, C.C.; Hsieh, Y.W. Cortical neural activity evoked by bilateral and unilateral mirror therapy after stroke. Clin. Neurophysiol. 2020, 131, 2333–2340. [Google Scholar] [CrossRef]
- Lewis, G.N.; Perreault, E.J. Side of lesion influences interhemispheric inhibition in subjects with post-stroke hemiparesis. Clin. Neurophysiol. 2007, 118, 2656–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartur, G.; Pratt, H.; Frenkel-Toledo, S.; Soroker, N. Neurophysiological effects of mirror visual feedback in stroke patients with unilateral hemispheric damage. Brain Res. 2018, 1700, 170–180. [Google Scholar] [CrossRef]
- Rossiter, H.E.; Borrelli, M.R.; Borchert, R.J.; Bradbury, D.; Ward, N.S. Cortical mechanisms of mirror therapy after stroke. Neurorehabil. Neural Repair 2015, 29, 444–452. [Google Scholar] [CrossRef]
- Deconinck, F.J.; Smorenburg, A.R.; Benham, A.; Ledebt, A.; Feltham, M.G.; Savelsbergh, G.J. Reflections on mirror therapy: A systematic review of the effect of mirror visual feedback on the brain. Neurorehabil. Neural Repair 2015, 29, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, S.; Adamovich, S.V.; Tunik, E. Mirrored feedback in chronic stroke: Recruitment and effective connectivity of ipsilesional sensorimotor networks. Neurorehabil. Neural Repair 2014, 28, 344–354. [Google Scholar] [CrossRef] [Green Version]
- Matthys, K.; Smits, M.; Van der Geest, J.N.; Van der Lugt, A.; Seurinck, R.; Stam, H.J.; Selles, R.W. Mirror-induced visual illusion of hand movements: A functional magnetic resonance imaging study. Arch. Phys. Med. Rehabil. 2009, 90, 675–681. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Smits, M.; Ribbers, G.M.; Stam, H.J.; van der Geest, J.N.; Bussmann, J.B.; Selles, R.W. The neuronal correlates of mirror therapy: An fMRI study on mirror induced visual illusions in patients with stroke. J. Neurol. Neurosurg. Psychiatry 2011, 82, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fritzsch, C.; Bernarding, J.; Krause, T.; Mauritz, K.H.; Brunetti, M.; Dohle, C. Cerebral activation evoked by the mirror illusion of the hand in stroke patients compared to normal subjects. NeuroRehabilitation 2013, 33, 593–603. [Google Scholar] [CrossRef]
- Rjosk, V.; Lepsien, J.; Kaminski, E.; Hoff, M.; Sehm, B.; Steele, C.J.; Villringer, A.; Ragert, P. Neural Correlates of Mirror Visual Feedback-Induced Performance Improvements: A Resting-State fMRI Study. Front. Hum. Neurosci. 2017, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Wenderoth, N.; Debaere, F.; Sunaert, S.; Swinnen, S.P. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur. J. Neurosci. 2005, 22, 235–246. [Google Scholar] [CrossRef]
- Hagmann, P.; Cammoun, L.; Gigandet, X.; Meuli, R.; Honey, C.J.; Wedeen, V.J.; Sporns, O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008, 6, e159. [Google Scholar] [CrossRef]
- Leech, R.; Braga, R.; Sharp, D.J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 2012, 32, 215–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoffelen, J.M.; Gross, J. Source connectivity analysis with MEG and EEG. Hum. Brain Mapp 2009, 30, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, W.; Matsubayashi, J.; Deguchi, Y.; Minami, C.; Kinai, T.; Nakamura, M.; Nagamine, T.; Matsuhashi, M.; Mima, T.; Fukuyama, H.; et al. A mirror reflection of a hand modulates stimulus-induced 20-Hz activity. Neuroimage 2009, 46, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, W.; Matsubayashi, J.; Furuya, M.; Matsuhashi, M.; Mima, T.; Fukuyama, H.; Mitani, A. Asymmetric activation of the primary motor cortex during observation of a mirror reflection of a hand. PLoS ONE 2011, 6, e28226. [Google Scholar] [CrossRef] [Green Version]
- Wasaka, T.; Kakigi, R. The effect of unpredicted visual feedback on activation in the secondary somatosensory cortex during movement execution. BMC Neurosci. 2012, 13, 138. [Google Scholar] [CrossRef] [Green Version]
- Wasaka, T.; Kakigi, R. Conflict caused by visual feedback modulates activation in somatosensory areas during movement execution. Neuroimage 2012, 59, 1501–1507. [Google Scholar] [CrossRef]
- Hadoush, H.; Mano, H.; Sunagawa, T.; Nakanishi, K.; Ochi, M. Optimization of mirror therapy to excite ipsilateral primary motor cortex. NeuroRehabilitation 2013, 32, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Lin, S.H.; Wu, C.Y.; Liao, Y.H.; Chang, K.C.; Hsieh, Y.W. Mirror Illusion Modulates M1 Activities and Functional Connectivity Patterns of Perceptual-Attention Circuits During Bimanual Movements: A Magnetoencephalography Study. Front. Neurosci. 2019, 13, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.H.; Tan, L.; Yu, J.T. Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2014, 2, 80. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Hari, R.; Forss, N.; Avikainen, S.; Kirveskari, E.; Salenius, S.; Rizzolatti, G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. USA 1998, 95, 15061–15065. [Google Scholar] [CrossRef] [Green Version]
- Jarvelainen, J.; Schurmann, M.; Avikainen, S.; Hari, R. Stronger reactivity of the human primary motor cortex during observation of live rather than video motor acts. Neuroreport 2001, 12, 3493–3495. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Rossi, S.; Tecchio, F.; Pasqualetti, P.; Ulivelli, M.; Pizzella, V.; Romani, G.L.; Passero, S.; Battistini, N.; Rossini, P.M. Somatosensory processing during movement observation in humans. Clin. Neurophysiol. 2002, 113, 16–24. [Google Scholar] [CrossRef]
- Zhu, J.D.; Cheng, C.H.; Tseng, Y.J.; Chou, C.C.; Chen, C.C.; Hsieh, Y.W.; Liao, Y.H. Modulation of motor cortical activities by action observation and execution in patients with stroke: An MEG study. Neural Plast. 2019, 2019, 8481371. [Google Scholar] [CrossRef]
- Cheng, C.H.; Sun, H.H.; Weng, J.Q.; Tseng, Y.J. Differential motor cortex excitability during observation of normal and abnormal goal-directed movement patterns. Neurosci. Res. 2017, 123, 36–42. [Google Scholar] [CrossRef]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Mosher, J.C.; Leahy, R.M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999, 44, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Tseng, Y.J.; Chen, R.S.; Lin, Y.Y. Reduced functional connectivity of somatosensory network in writer’s cramp patients. Brain Behav. 2016, 6, e00433. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Kilavik, B.E.; Zaepffel, M.; Brovelli, A.; MacKay, W.A.; Riehle, A. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 2013, 245, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, J.R.; Frost, J.A.; Hammeke, T.A.; Bellgowan, P.S.; Rao, S.M.; Cox, R.W. Conceptual processing during the conscious resting state. A functional MRI study. J. Cogn. Neurosci. 1999, 11, 80–95. [Google Scholar] [CrossRef]
- Caplan, L.; Chedru, F.; Lhermitte, F.; Mayman, C. Transient global amnesia and migraine. Neurology 1981, 31, 1167–1170. [Google Scholar] [CrossRef]
- Hampson, M.; Driesen, N.R.; Skudlarski, P.; Gore, J.C.; Constable, R.T. Brain connectivity related to working memory performance. J. Neurosci. 2006, 26, 13338–13343. [Google Scholar] [CrossRef]
- Hahn, B.; Ross, T.J.; Stein, E.A. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb. Cortex 2007, 17, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Leech, R.; Kamourieh, S.; Beckmann, C.F.; Sharp, D.J. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011, 31, 3217–3224. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, A.; Gitelman, D.R.; Small, D.M.; Mesulam, M.M. The spatial attention network interacts with limbic and monoaminergic systems to modulate motivation-induced attention shifts. Cereb. Cortex 2008, 18, 2604–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafton, S.T.; Mazziotta, J.C.; Woods, R.P.; Phelps, M.E. Human functional anatomy of visually guided finger movements. Brain 1992, 115 Pt 2, 565–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanlierde, A.; De Volder, A.G.; Wanet-Defalque, M.C.; Veraart, C. Occipito-parietal cortex activation during visuo-spatial imagery in early blind humans. Neuroimage 2003, 19, 698–709. [Google Scholar] [CrossRef]
- Wolinski, N.; Cooper, N.R.; Sauseng, P.; Romei, V. The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS Biol. 2018, 16, e2005348. [Google Scholar] [CrossRef] [Green Version]
- Spyropoulos, G.; Bosman, C.A.; Fries, P. A theta rhythm in macaque visual cortex and its attentional modulation. Proc. Natl. Acad. Sci. USA 2018, 115, E5614–E5623. [Google Scholar] [CrossRef] [Green Version]
- Mudie, M.H.; Matyas, T.A. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil. Rehabil. 2000, 22, 23–37. [Google Scholar] [CrossRef]
- Han, Y.L.; Dai, Z.P.; Ridwan, M.C.; Lin, P.H.; Zhou, H.L.; Wang, H.F.; Yao, Z.J.; Lu, Q. Connectivity of the frontal cortical oscillatory dynamics underlying inhibitory control during a go/no-go task as a predictive biomarker in major depression. Front. Psychiatry 2020, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, S.M. Coherence a measure of the brain networks: Past and present. Neuropsychiatr. Electrophysiol. 2016, 2, 1–12. [Google Scholar] [CrossRef]



| Sex/Age (Years) | Lesion Location | Stroke Type | Stroke Duration (Months) | FMA-UE |
|---|---|---|---|---|
| M/38 | Right corona radiata | Ischemic | 2 | 60 |
| M/46 | Right corona radiata | Ischemic | 2 | 48 |
| M/46 | Right MCA | Ischemic | 5 | 60 |
| M/55 | Left caudate head | Ischemic | 7 | 56 |
| M/48 | Left internal capsule | Ischemic | 11 | 51 |
| M/62 | Right corona radiata | Ischemic | 6 | 51 |
| M/63 | Right corona radiata | Ischemic | 9 | 41 |
| M/47 | Left corona radiata | Ischemic | 4 | 58 |
| M/37 | Right MCA | Ischemic | 1 | 55 |
| M/55 | Right precentral gyrus | Ischemic | 1 | 60 |
| M/42 | Right putamen | Hemorrhagic | 4 | 57 |
| M/62 | Right basal ganglion | Hemorrhagic | 11 | 58 |
| M/38 | Left basal ganglion | Hemorrhagic | 4 | 37 |
| M/49 | Right basal ganglion | Hemorrhagic | 8 | 53 |
| M/56 | Right putamen | Hemorrhagic | 1 | 63 |
| ROIs | Center of SCS Coordinate | Vertice Number | Area (cm2) |
|---|---|---|---|
| Right M1 | (20, −47, 95) | 353 | 43.74 |
| Right PCC | (12, −1, 81) | 93 | 12.66 |
| Right V1 | (−64, −30, 58) | 367 | 45.57 |
| Right precuneus | (−23, −9, 84) | 325 | 37.95 |
| Right STG | (28, −57, 45) | 257 | 39.58 |
| Left M1 | (17, 53, 97) | 339 | 49.58 |
| Left PCC | (10, 1, 80) | 85 | 11.51 |
| Left V1 | (−66, 26, 57) | 371 | 45.71 |
| Left precuneus | (−26, 0, 84) | 314 | 36.76 |
| Left STG | (22, 57, 49) | 290 | 43.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, R.-Y.; Zhu, J.-D.; Chen, C.-C.; Hsieh, Y.-W.; Cheng, C.-H. Modulation of Functional Connectivity in Response to Mirror Visual Feedback in Stroke Survivors: An MEG Study. Brain Sci. 2021, 11, 1284. https://doi.org/10.3390/brainsci11101284
Tai R-Y, Zhu J-D, Chen C-C, Hsieh Y-W, Cheng C-H. Modulation of Functional Connectivity in Response to Mirror Visual Feedback in Stroke Survivors: An MEG Study. Brain Sciences. 2021; 11(10):1284. https://doi.org/10.3390/brainsci11101284
Chicago/Turabian StyleTai, Ruei-Yi, Jun-Ding Zhu, Chih-Chi Chen, Yu-Wei Hsieh, and Chia-Hsiung Cheng. 2021. "Modulation of Functional Connectivity in Response to Mirror Visual Feedback in Stroke Survivors: An MEG Study" Brain Sciences 11, no. 10: 1284. https://doi.org/10.3390/brainsci11101284
APA StyleTai, R.-Y., Zhu, J.-D., Chen, C.-C., Hsieh, Y.-W., & Cheng, C.-H. (2021). Modulation of Functional Connectivity in Response to Mirror Visual Feedback in Stroke Survivors: An MEG Study. Brain Sciences, 11(10), 1284. https://doi.org/10.3390/brainsci11101284






