Decreased Intracranial Pressure Elevation and Cerebrospinal Fluid Outflow Resistance: A Potential Mechanism of Hypothermia Cerebroprotection Following Experimental Stroke
Abstract
:1. Introduction
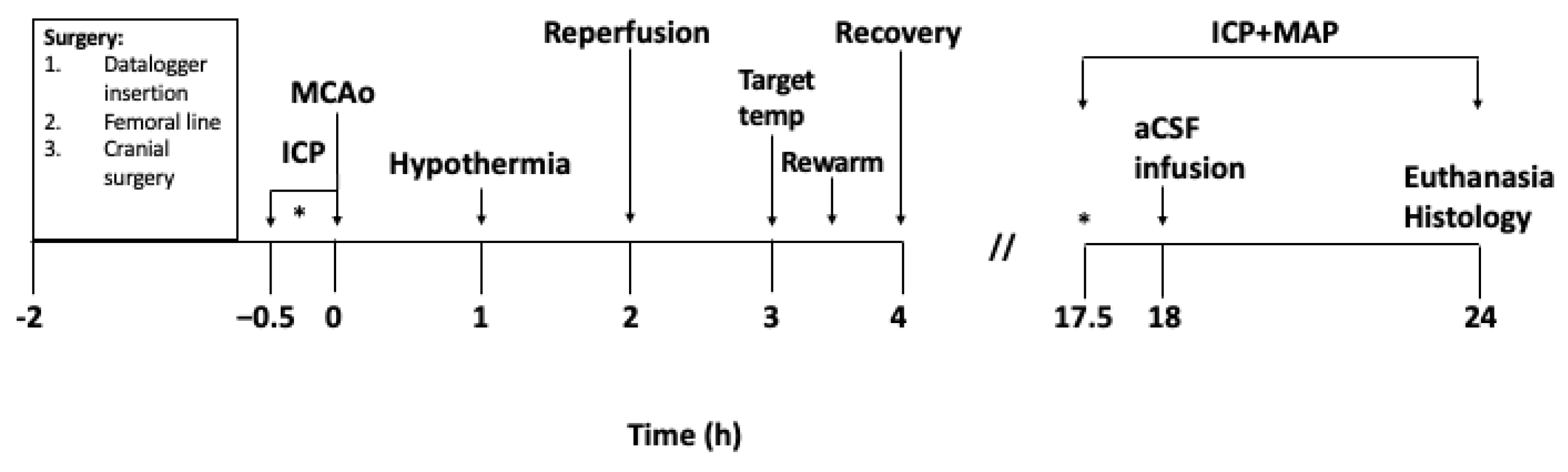
2. Materials and Methods
2.1. Animals
2.2. Anaesthesia and Physiological Monitoring
2.3. Implantation of Datalogger Device
2.4. Intracranial Pressure and Laser Doppler Measurement
2.5. Middle Cerebral Artery Occlusion
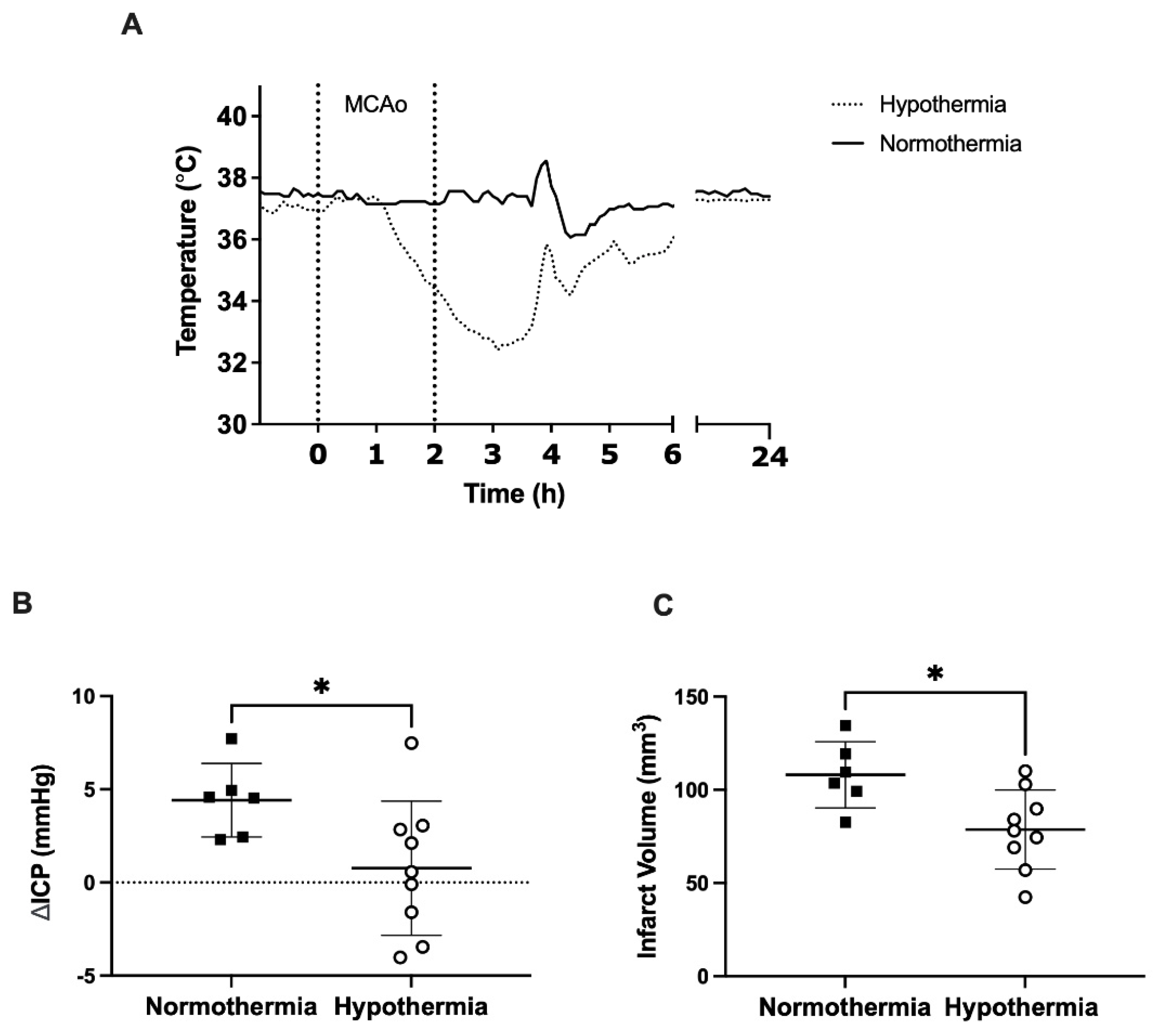
2.6. Hypothermia Treatment
2.7. Artificial Cerebrospinal Fluid (aCSF) Infusion
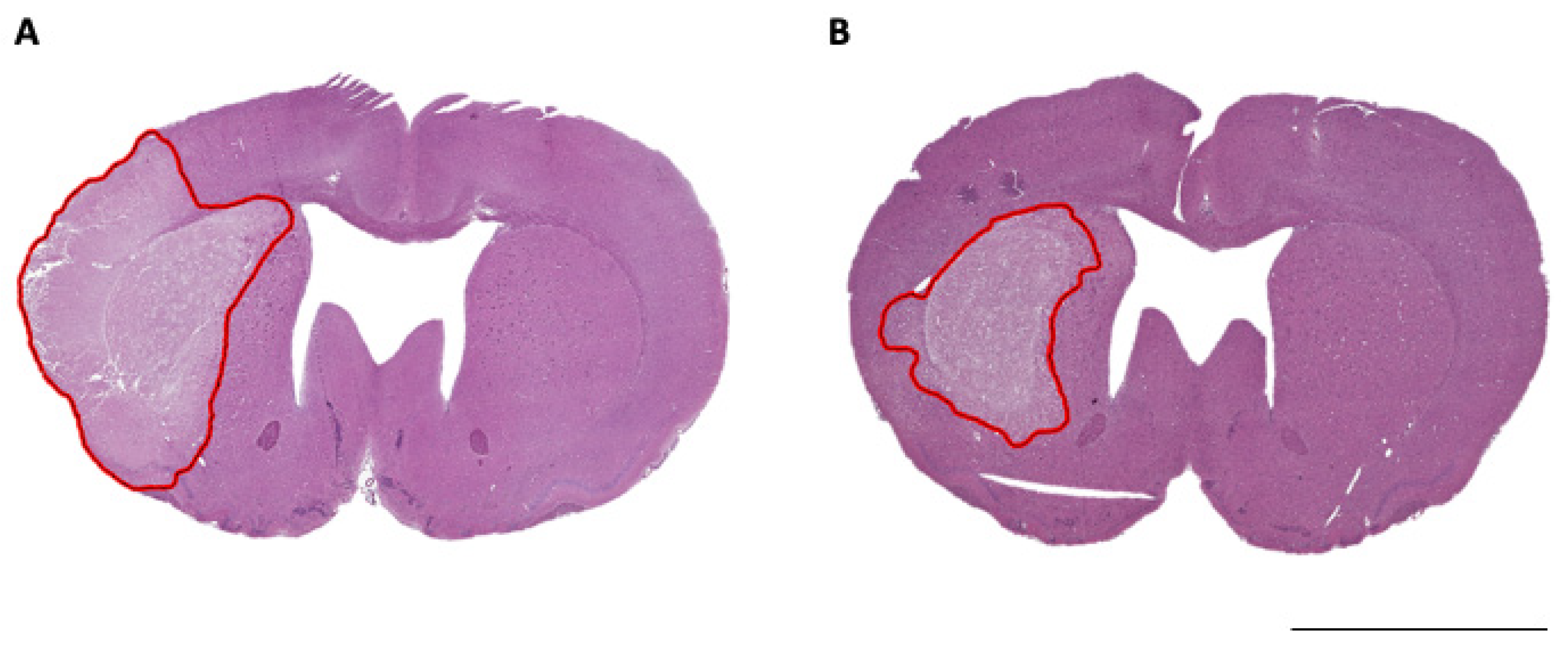
2.8. Histological Analysis
2.9. Exclusion Criteria and Statistical Analyses
3. Results
3.1. Normality Tests
3.2. ICP Elevation and Infarct Volume
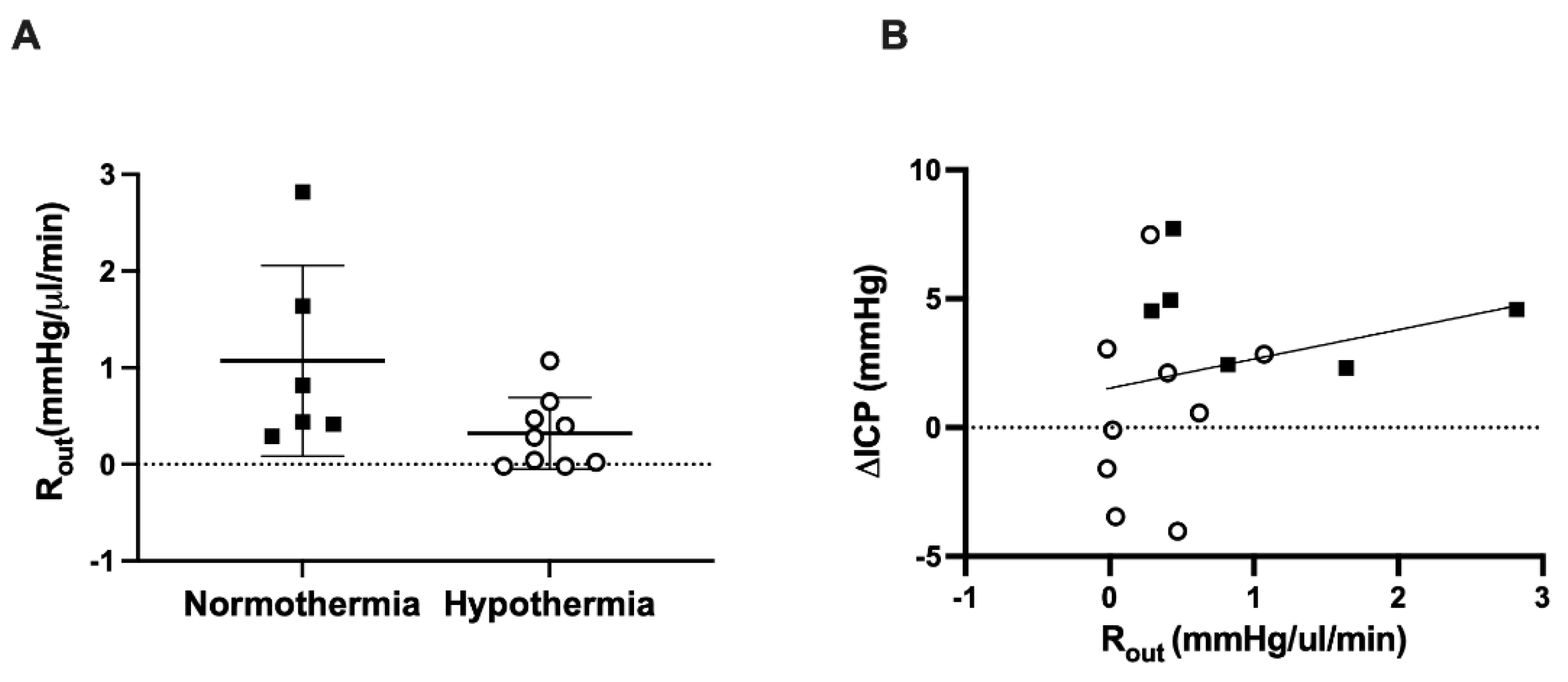
3.3. CSF Outflow Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Murtha, L.A.; McLeod, D.D.; McCann, S.K.; Pepperall, D.; Chung, S.; Levi, C.R.; Calford, M.B.; Spratt, N.J. Short-Duration Hypothermia after Ischemic Stroke Prevents Delayed Intracranial Pressure Rise. Int. J. Stroke 2014, 9, 553–559. [Google Scholar] [CrossRef]
- Murtha, L.A.; Mcleod, D.D.; Pepperall, D.; McCann, S.; Beard, D.J.; Tomkins, A.J.; Holmes, W.M.; McCabe, C.; Macrae, I.M.; Spratt, N.J. Intracranial pressure elevation after ischemic stroke in rats: Cerebral edema is not the only cause, and short-duration mild hypothermia is a highly effective preventive therapy. J. Cereb. Blood Flow Metab. 2015, 35, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Beard, D.J.; Mcleod, D.; Logan, C.L.; Murtha, L.; Imtiaz, M.S.; Van Helden, D.F.; Spratt, N.J. Intracranial pressure elevation reduces flow through collateral vessels and the penetrating arterioles they supply. A possible explanation for ‘collateral failure’ and infarct expansion after ischemic stroke. J. Cereb. Blood Flow Metab. 2015, 35, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Beard, D.J.; Bourke, J.T.; Pepperall, D.; McLeod, D.D.; Spratt, N.J. Intracranial Pressure Elevation 24 h after Ischemic Stroke in Aged Rats Is Prevented by Early, Short Hypothermia Treatment. Front. Aging Neurosci. 2016, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Beard, D.J.; Logan, C.L.; Mcleod, D.; Hood, R.; Pepperall, D.; Murtha, L.; Spratt, N.J. Ischemic penumbra as a trigger for intracranial pressure rise—A potential cause for collateral failure and infarct progression? J. Cereb. Blood Flow Metab. 2016, 36, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Patabendige, A.; MacKovski, N.; Pepperall, D.; Hood, R.; Spratt, N. Correction to: A26 Cerebrospinal fluid outflow resistance is increased following small-moderate ischaemic stroke. Fluids Barriers CNS 2019, 16, 22. [Google Scholar] [CrossRef]
- Urrutia, V.C.; Wityk, R.J. Blood pressure management in acute stroke. Neurol. Clin. 2008, 26, 565–583. [Google Scholar] [CrossRef] [PubMed]
- Charriaut-Marlangue, C.; Bonnin, P.; Gharib, A.; Leger, P.-L.; Villapol, S.; Pocard, M.; Gressens, P.; Renolleau, S.; Baud, O. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke 2012, 43, 3078–3084. [Google Scholar] [CrossRef] [Green Version]
- Terpolilli, N.A.; Kim, S.-W.; Thal, S.C.; Kataoka, H.; Zeisig, V.; Nitzsche, B.; Klaesner, B.; Zhu, C.; Schwarzmaier, S.; Meissner, L.; et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res. 2012, 110, 727–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beard, D.J.; Murtha, L.A.; McLeod, D.D.; Spratt, N.J. Intracranial Pressure and Collateral Blood Flow. Stroke 2016, 47, 1695–1700. [Google Scholar] [CrossRef] [Green Version]
- Monro, A. Observations on the Structure and Functions of the Nervous System. Lond. Med. J. 1783, 4, 113–115. [Google Scholar]
- Kellie, G. Reflections on the Pathology of the Brain: Part II. Trans. Med. Chir. Soc. Edinb. 1824, 1, 123–169. [Google Scholar]
- Alshuhri, M.S.; Gallagher, L.; McCabe, C.; Holmes, W.M. Change in CSF Dynamics Responsible for ICP Elevation after Ischemic Stroke in Rats: A New Mechanism for Unexplained end? Transl. Stroke Res. 2019, 11, 310–318. [Google Scholar] [CrossRef]
- Omileke, D.; Pepperall, D.; Bothwell, S.W.; Mackovski, N.; Azarpeykan, S.; Beard, D.J.; Coupland, K.; Patabendige, A.; Spratt, N.J. Ultra-Short Duration Hypothermia Prevents Intracranial Pressure Elevation Following Ischaemic Stroke in Rats. Front. Neurol. 2021, 12, 684353. [Google Scholar] [CrossRef]
- Omileke, D.; Bothwell, S.; Beard, D.J.; Mackovski, N.; Azarpeykan, S.; Coupland, K.; Patabendige, A.; Spratt, N. Short-Duration Hypothermia Induction in Rats using Models for Studies examining Clinical Relevance and Mechanisms. J. Vis. Exp. 2021, 169, e62325. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.; Mcleod, D.; Spratt, N. Epidural intracranial pressure measurement in rats using a fiber-optic pressure transducer. J. Vis. Exp. 2012, 62, e3689. [Google Scholar] [CrossRef]
- Spratt, N.J.; Fernandez, J.; Chen, M.; Rewell, S.; Cox, S.; van Raay, L.; Hogan, L.; Howells, D.W. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J. Neurosci. Methods 2006, 155, 285–290. [Google Scholar] [CrossRef]
- McLeod, D.D.; Beard, D.J.; Parsons, M.W.; Levi, C.R.; Calford, M.B.; Spratt, N.J. Inadvertent occlusion of the anterior choroidal artery explains infarct variability in the middle cerebral artery thread occlusion stroke model. PLoS ONE 2013, 8, e75779. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R.; Hussey, F. A simple constant-infusion manometric test for measurement of CSF absorption. I. Rationale and method. Neurology 1970, 20, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Davson, H.; Hollingsworth, G.; Segal, M.B. The mechanism of drainage of the cerebrospinal fluid. Brain 1970, 93, 665–678. [Google Scholar] [CrossRef]
- Bothwell, S.W.; Omileke, D.; Hood, R.J.; Pepperall, D.-G.; Azarpeykan, S.; Patabendige, A.; Spratt, N.J. Altered Cerebrospinal Fluid Clearance and Increased Intracranial Pressure in Rats 18 h After Experimental Cortical Ischaemia. Front. Mol. Neurosci. 2021, 14, 712779. [Google Scholar] [CrossRef]
- Snodgrass, S.R.; Lorenzo, A.V. Temperature and cerebrospinal fluid production rate. Am. J. Physiol. 1972, 222, 1524–1527. [Google Scholar] [CrossRef]
- Go, K.G.; Hochwald, G.M.; Koster-Otte, L.; van Zanten, A.K.; Gandhi, M. The effect of cold-induced brain edema on cerebrospinal fluid formation rate. J. Neurosurg. 1980, 53, 652–655. [Google Scholar] [CrossRef]
- Posnikoff, J.; Stratford, J.; Feindel, W. The effect of hypothermia and other factors on cerebrospinal fluid pressure. Can. Anaesth. Soc. J. 1960, 7, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Aldea, R.; Weller, R.O.; Wilcock, D.M.; Carare, R.O.; Richardson, G. Cerebrovascular Smooth Muscle Cells as the Drivers of Intramural Periarterial Drainage of the Brain. Front. Aging Neurosci. 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaberel, T.; Gakuba, C.; Goulay, R.; De Lizarrondo, S.M.; Hanouz, J.L.; Emery, E.; Touze, E.; Viven, D. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke 2014, 45, 3092–3096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalisvaart, A.C.J.; Wilkinson, C.M.; Gu, S.; Kung, T.F.C.; Yager, J.; Winship, I.R.; Van Landeghem, F.K.H.; Colbourne, F. An update to the Monro-Kellie doctrine to reflect tissue compliance after severe ischemic and hemorrhagic stroke. Sci. Rep. 2020, 10, 22013. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Verkman, A.S. CrossTalk opposing view: Going against the flow: Interstitial solute transport in brain is diffusive and aquaporin-4 independent. J. Physiol. 2019, 597, 4421–4424. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omileke, D.; Bothwell, S.W.; Pepperall, D.; Beard, D.J.; Coupland, K.; Patabendige, A.; Spratt, N.J. Decreased Intracranial Pressure Elevation and Cerebrospinal Fluid Outflow Resistance: A Potential Mechanism of Hypothermia Cerebroprotection Following Experimental Stroke. Brain Sci. 2021, 11, 1589. https://doi.org/10.3390/brainsci11121589
Omileke D, Bothwell SW, Pepperall D, Beard DJ, Coupland K, Patabendige A, Spratt NJ. Decreased Intracranial Pressure Elevation and Cerebrospinal Fluid Outflow Resistance: A Potential Mechanism of Hypothermia Cerebroprotection Following Experimental Stroke. Brain Sciences. 2021; 11(12):1589. https://doi.org/10.3390/brainsci11121589
Chicago/Turabian StyleOmileke, Daniel, Steven W. Bothwell, Debbie Pepperall, Daniel J. Beard, Kirsten Coupland, Adjanie Patabendige, and Neil J. Spratt. 2021. "Decreased Intracranial Pressure Elevation and Cerebrospinal Fluid Outflow Resistance: A Potential Mechanism of Hypothermia Cerebroprotection Following Experimental Stroke" Brain Sciences 11, no. 12: 1589. https://doi.org/10.3390/brainsci11121589
APA StyleOmileke, D., Bothwell, S. W., Pepperall, D., Beard, D. J., Coupland, K., Patabendige, A., & Spratt, N. J. (2021). Decreased Intracranial Pressure Elevation and Cerebrospinal Fluid Outflow Resistance: A Potential Mechanism of Hypothermia Cerebroprotection Following Experimental Stroke. Brain Sciences, 11(12), 1589. https://doi.org/10.3390/brainsci11121589





