Mechanisms of Nerve Damage in Neuropathies Associated with Hematological Diseases: Lesson from Nerve Biopsies
Abstract
:1. Introduction
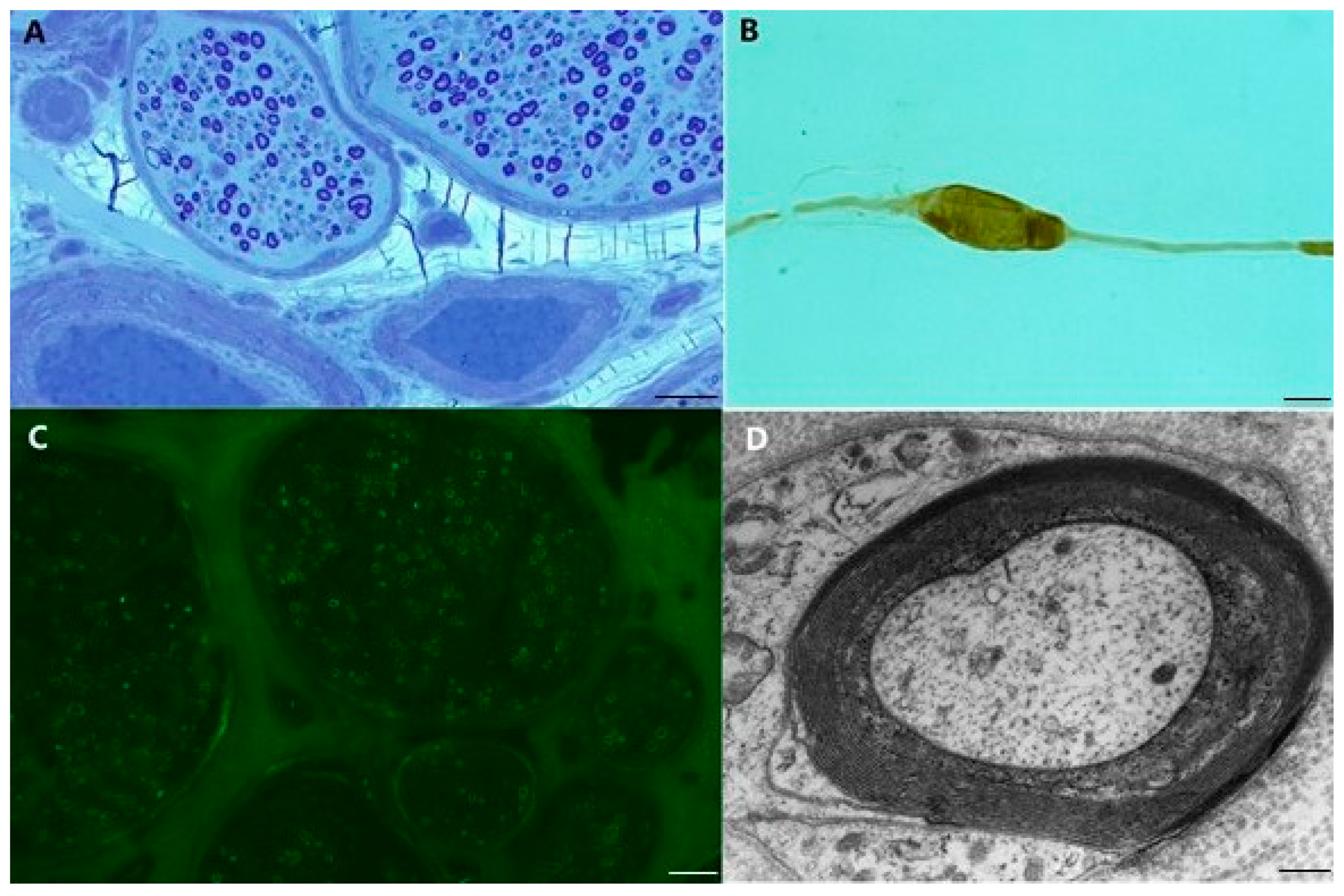
2. Polyneuropathy with Antibody to Myelin-Associated Glycoprotein (MAG)
- pathological studies of sural nerves show deposits of IgM and complement in myelin sheets, suggesting the need for complement activation in the demyelination process [19];
- feline nerves injected with the serum of patients with anti-MAG/SGPG IgM supplemented with additional complement, develop complement-mediated demyelination and conduction block within 2–9 days [21];
- systemic transfusion of chickens with anti-MAG IgM produces segmental demyelination with IgM deposits on external myelin sheets and consequent widening of myelin lamellae as observed in human pathology [22];
- cats immunized with purified SGPG develop an ataxic neuropathy with the involvement of dorsal root ganglia, similar to anti-MAG antibody neuropathy [23];
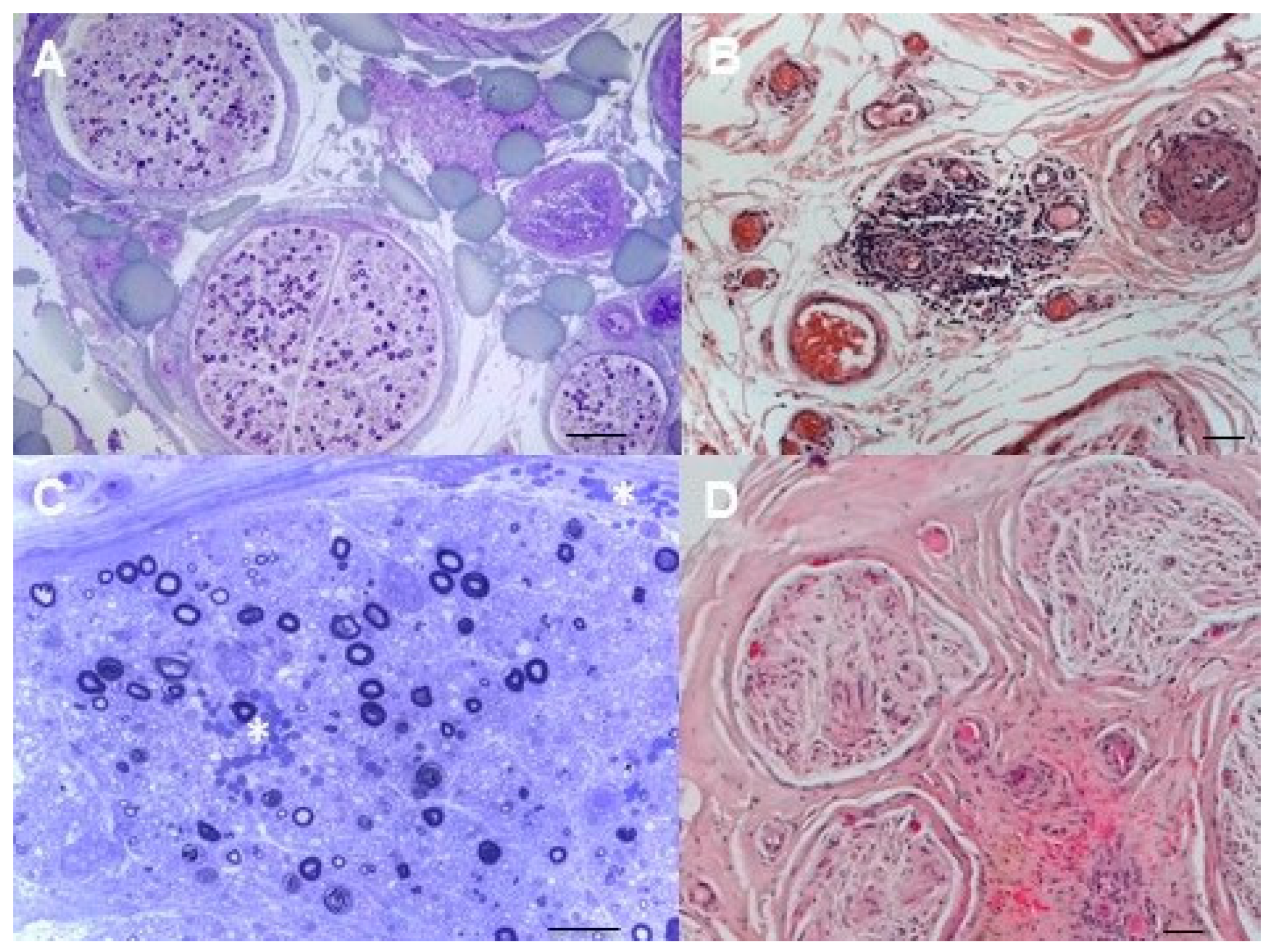
3. Cryoglobulinemic Neuropathies
4. Neurolymphomatosis
5. Amyloidosis
6. POEMS Syndrome
7. Chemotherapy-Induced Neurotoxicity (CIPN)
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- McLeod, J.G. Sural nerve biopsy. J. Neurol. Neurosurg. Psychiatry 2000, 69, 431. [Google Scholar] [CrossRef] [Green Version]
- Gasparotti, R.; Padua, L.; Briani, C.; Lauria, G. New technologies for the assessment of neuropathies. Nat. Rev. Neurol. 2017, 13, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotto, S.; Carta, S.; Bozzetti, S.; Zivelonghi, C.; Alberti, D.; Zanzoni, S.; Filosto, M.; Fusina, S.; Monaco, S.; Castellani, F.; et al. Sural nerve biopsy: Current role and comparison with serum neurofilament light chain levels. J. Neurol. 2020, 267, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, N.; Moretto, G.; Galiazzo Rizzuto, S. Clinical spectrum of the tomaculous neuropathies. Report of 60 cases and review of the literature. Ital. J. Neurol. Sci. 1993, 14, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Silberman, J.; Lonial, S. Review of peripheral neuropathy in plasma cell disorders. Hematol. Oncol. 2008, 26, 55–65. [Google Scholar] [CrossRef]
- Smith, I.S. The natural history of chronic demyelinating neuropathy associated with benign IgM paraproteinaemia. A clinical and neurophysiological study. Brain 1994, 117 Pt 5, 949–957. [Google Scholar] [CrossRef]
- Latov, N.; Sherman, W.H.; Nemni, R.; Galassi, G.; Shyong, J.S.; Penn, A.S.; Chess, L.; Olarte, M.R.; Rowland, L.P.; Osserman, E.F. Plasma-cell dyscrasia and peripheral neuropathy with a monoclonal antibody to peripheral-nerve myelin. N. Engl. J. Med. 1980, 303, 618–621. [Google Scholar] [CrossRef]
- Braun, P.E.; Frail, D.E.; Latov, N. Myelin-associated glycoprotein is the antigen for a monoclonal IgM in polyneuropathy. J. Neurochem. 1982, 39, 1261–1265. [Google Scholar] [CrossRef]
- Dalakas, M.C. Pathogenesis of immune-mediated neuropathies. Biochim. Biophys. Acta 2015, 1852, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Tropak, M.B.; Gerlai, R.; Clapoff, S.; Abramow-Newerly, W.; Trapp, B.; Peterson, A.; Roder, J. Myelination in the absence of myelin-associated glycoprotein. Nature 1994, 369, 747–750. [Google Scholar] [CrossRef]
- Montag, D.; Giese, K.P.; Bartsch, U.; Martini, R.; Lang, Y.; Bluthmann, H.; Karthigasan, J.; Kirschner, D.A.; Wintergerst, E.S.; Nave, K.A.; et al. Mice deficient for the myelin-associated glycoprotein show subtle abnormalities in myelin. Neuron 1994, 13, 229–246. [Google Scholar] [CrossRef]
- Ilyas, A.A.; Quarles, R.H.; MacIntosh, T.D.; Dobersen, M.J.; Trapp, B.D.; Dalakas, M.C.; Brady, R.O. IgM in a human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin-associated glycoprotein and to a ganglioside. Proc. Natl. Acad. Sci. USA 1984, 81, 1225–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendell, J.R.; Sahenk, Z.; Whitaker, J.N.; Trapp, B.D.; Yates, A.J.; Griggs, R.C.; Quarles, R.H. Polyneuropathy and IgM monoclonal gammopathy: Studies on the pathogenetic role of anti-myelin-associated glycoprotein antibody. Ann. Neurol. 1985, 17, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Quarles, R.H. Autoimmune ataxic neuropathies (sensory ganglionopathies): Are glycolipids the responsible autoantigens? Ann. Neurol. 1996, 39, 419–422. [Google Scholar] [CrossRef]
- Dalakas, M.C. Pathophysiology of autoimmune polyneuropathies. Presse Med. 2013, 42, e181–e192. [Google Scholar] [CrossRef]
- Dalakas, M.C. Pathogenesis and Treatment of Anti-MAG Neuropathy. Curr. Treat. Options Neurol. 2010, 12, 71–83. [Google Scholar] [CrossRef]
- Vital, A.; Vital, C.; Julien, J.; Baquey, A.; Steck, A.J. Polyneuropathy associated with IgM monoclonal gammopathy. Immunological and pathological study in 31 patients. Acta Neuropathol. 1989, 79, 160–167. [Google Scholar] [CrossRef]
- Vallat, J.M.; Magy, L.; Richard, L.; Piaser, M.; Sindou, P.; Calvo, J.; Ghorab, K.; Cros, D. Intranervous immunoglobulin deposits: An underestimated mechanism of neuropathy. Muscle Nerve 2008, 38, 904–911. [Google Scholar] [CrossRef]
- Monaco, S.; Bonetti, B.; Ferrari, S.; Moretto, G.; Nardelli, E.; Tedesco, F.; Mollnes, T.E.; Nobile-Orazio, E.; Manfredini, E.; Bonazzi, L.; et al. Complement-mediated demyelination in patients with IgM monoclonal gammopathy and polyneuropathy. N. Engl. J. Med. 1990, 322, 649–652. [Google Scholar] [CrossRef]
- Lombardi, R.; Erne, B.; Lauria, G.; Pareyson, D.; Borgna, M.; Morbin, M.; Arnold, A.; Czaplinski, A.; Fuhr, P.; Schaeren-Wiemers, N.; et al. IgM deposits on skin nerves in anti-myelin-associated glycoprotein neuropathy. Ann. Neurol. 2005, 57, 180–187. [Google Scholar] [CrossRef]
- Willison, H.J.; Trapp, B.D.; Bacher, J.D.; Dalakas, M.C.; Griffin, J.W.; Quarles, R.H. Demyelination induced by intraneural injection of human antimyelin-associated glycoprotein antibodies. Muscle Nerve 1988, 11, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Tatum, A.H. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti-myelin-associated glycoprotein. Ann. Neurol. 1993, 33, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.A.; Gu, Y.; Dalakas, M.C.; Quarles, R.H.; Bhatt, S. Induction of experimental ataxic sensory neuronopathy in cats by immunization with purified SGPG. J. Neuroimmunol. 2008, 193, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Lunn, M.P.; Nobile-Orazio, E. Immunotherapy for IgM anti-myelin-associated glycoprotein paraprotein-associated peripheral neuropathies. Cochrane Database Syst. Rev. 2016, 10, CD002827. [Google Scholar] [CrossRef] [Green Version]
- Castellani, F.; Visentin, A.; Campagnolo, M.; Salvalaggio, A.; Cacciavillani, M.; Candiotto, C.; Bertorelle, R.; Trentin, L.; Briani, C. The Bruton tyrosine kinase inhibitor ibrutinib improves anti-MAG antibody polyneuropathy. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briani, C.; Visentin, A.; Salvalaggio, A.; Cacciavillani, M.; Trentin, L. Obinutuzumab, a new anti-CD20 antibody, and chlorambucil are active and effective in anti-myelin-associated glycoprotein antibody polyneuropathy. Eur. J. Neurol. 2019, 26, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, L.; Briani, C.; Franciotta, D.; Carpo, M.; Padua, L.; Zara, G.; Zambello, R.; Sormani, M.P.; Mancardi, G.L.; Nobile-Orazio, E.; et al. Long-term effect of rituximab in anti-mag polyneuropathy. Neurology 2008, 71, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, L.; Briani, C.; Grandis, M.; Vigo, T.; Gobbi, M.; Ghiglione, E.; Carpo, M.; Cocito, D.; Caporale, C.M.; Sormani, M.P.; et al. Predictors of response to rituximab in patients with neuropathy and anti-myelin associated glycoprotein immunoglobulin M. J. Peripher. Nerv. Syst. 2007, 12, 102–107. [Google Scholar] [CrossRef]
- Briani, C.; Visentin, A.; Cerri, F.; Quattrini, A. From pathogenesis to personalized treatments of neuropathies in hematological malignancies. J. Peripher. Nerv. Syst. 2020, 25, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Visentin, A.; Campagnolo, M.; Salvalaggio, A.; Ferrari, S.; Cavallaro, T.; Manara, R.; Gasparotti, R.; Piazza, F. Peripheral nervous system involvement in lymphomas. J. Peripher. Nerv. Syst. 2019, 24, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Muchtar, E.; Magen, H.; Gertz, M.A. How I treat cryoglobulinemia. Blood 2017, 129, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbingi, A.; Innocenti, I.; Tomasso, A.; Pasquale, R.; Visentin, A.; Varettoni, M.; Flospergher, E.; Autore, F.; Morelli, F.; Trentin, L.; et al. Monoclonal gammopathy and serum immunoglobulin levels as prognostic factors in chronic lymphocytic leukaemia. Br. J. Haematol. 2020, 190, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Visentin, A.; Imbergamo, S.; Gurrieri, C.; Frezzato, F.; Trimarco, V.; Martini, V.; Severin, F.; Raggi, F.; Scomazzon, E.; Facco, M.; et al. Major infections, secondary cancers and autoimmune diseases occur in different clinical subsets of chronic lymphocytic leukaemia patients. Eur. J. Cancer 2017, 72, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Baehring, J.M.; Damek, D.; Martin, E.C.; Betensky, R.A.; Hochberg, F.H. Neurolymphomatosis. Neuro-Oncol. 2003, 5, 104–115. [Google Scholar] [CrossRef]
- Campagnolo, M.; Cacciavillani, M.; Cavallaro, T.; Ferrari, S.; Gasparotti, R.; Zambello, R.; Briani, C. Neurolymphomatosis, a rare manifestation of peripheral nerve involvement in lymphomas: Suggestive features and diagnostic challenges. J. Peripher. Nerv. Syst. 2020, 25, 312–315. [Google Scholar] [CrossRef]
- Grisariu, S.; Avni, B.; Batchelor, T.T.; van den Bent, M.J.; Bokstein, F.; Schiff, D.; Kuittinen, O.; Chamberlain, M.C.; Roth, P.; Nemets, A.; et al. Neurolymphomatosis: An International Primary CNS Lymphoma Collaborative Group report. Blood 2010, 115, 5005–5011. [Google Scholar] [CrossRef] [Green Version]
- Briani, C.; Visentin, A.; Cavallaro, T.; Cacciavillani, M.; Cabrini, I.; Ferrari, S.; Zambello, R.; Trentin, L. Primary neurolymphomatosis as clinical onset of chronic lymphocytic leukemia. Ann. Hematol. 2017, 96, 159–161. [Google Scholar] [CrossRef]
- Odabasi, Z.; Parrott, J.H.; Reddy, V.V.; Oh, S.J. Neurolymphomatosis associated with muscle and cerebral involvement caused by natural killer cell lymphoma: A case report and review of literature. J. Peripher. Nerv. Syst. 2001, 6, 197–203. [Google Scholar] [CrossRef]
- Briani, C.; Visentin, A.; Salvalaggio, A.; Imbergamo, S.; Piazza, F.; Cacciavillani, M.; Campagnolo, M.; Frezzato, F.; Semenzato, G.; Trentin, L. Peripheral neuropathies in chronic lymphocytic leukemia: A single center experience on 816 patients. Haematologica 2017, 102, e140–e143. [Google Scholar] [CrossRef] [Green Version]
- Visentin, A.; Bonaldi, L.; Rigolin, G.M.; Mauro, F.R.; Martines, A.; Frezzato, F.; Imbergamo, S.; Scomazzon, E.; Pravato, S.; Bardi, M.A.; et al. The combination of complex karyotype subtypes and IGHV mutational status identifies new prognostic and predictive groups in chronic lymphocytic leukaemia. Br. J. Cancer 2019, 121, 150–156. [Google Scholar] [CrossRef]
- Tomita, M.; Koike, H.; Kawagashira, Y.; Iijima, M.; Adachi, H.; Taguchi, J.; Abe, T.; Sako, K.; Tsuji, Y.; Nakagawa, M.; et al. Clinicopathological features of neuropathy associated with lymphoma. Brain 2013, 136, 2563–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Bergh, P.Y.; Hadden, R.D.; Bouche, P.; Cornblath, D.R.; Hahn, A.; Illa, I.; Koski, C.L.; Leger, J.M.; Nobile-Orazio, E.; Pollard, J.; et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First revision. Eur. J. Neurol. 2010, 17, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.H.; Younger, D.S. Neurolymphomatosis. Neurology 1996, 46, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.; de Bruin, H.G.; Beun, G.D.; Vecht, C.J. Neurolymphomatosis of the median nerve. Neurology 1995, 45, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Baehring, J.M.; Batchelor, T.T. Diagnosis and management of neurolymphomatosis. Cancer J. 2012, 18, 463–468. [Google Scholar] [CrossRef]
- Duchesne, M.; Roussellet, O.; Maisonobe, T.; Gachard, N.; Rizzo, D.; Armand, M.; Viala, K.; Richard, L.; Delage-Corre, M.; Jaccard, A.; et al. Pathology of Nerve Biopsy and Diagnostic Yield of PCR-Based Clonality Testing in Neurolymphomatosis. J. Neuropathol. Exp. Neurol. 2018, 77, 769–781. [Google Scholar] [CrossRef]
- Palladini, G.; Milani, P.; Merlini, G. Management of AL amyloidosis in 2020. Hematology Am. Soc. Hematol. Educ. Program 2020, 2020, 363–371. [Google Scholar] [CrossRef]
- Visentin, A.; Briani, C.; Imbergamo, S.; Frezzato, F.; Angelini, A.; Fedrigo, M.; Cacciavillani, M.; Altinier, S.; Piazza, F.; Semenzato, G.; et al. Idelalisib plus rituximab is effective in systemic AL amyloidosis secondary to chronic lymphocytic leukaemia. Hematol. Oncol. 2018, 36, 366–369. [Google Scholar] [CrossRef]
- Fernandes, A.; Coelho, T.; Rodrigues, A.; Felgueiras, H.; Oliveira, P.; Guimaraes, A.; Melo-Pires, M.; Taipa, R. Clinicopathological correlations of sural nerve biopsies in TTR Val30Met familial amyloid polyneuropathy. Brain Commun. 2019, 1, fcz032. [Google Scholar] [CrossRef] [Green Version]
- Araki, S.; Yi, S. Pathology of familial amyloidotic polyneuropathy with TTR met 30 in Kumamoto, Japan. Neuropathology 2000, 20, S47–S51. [Google Scholar] [CrossRef]
- Luigetti, M.; Romozzi, M.; Bisogni, G.; Cardellini, D.; Cavallaro, T.; Di Paolantonio, A.; Fabrizi, G.M.; Fenu, S.; Gentile, L.; Grandis, M.; et al. hATTR Pathology: Nerve Biopsy Results from Italian Referral Centers. Brain Sci. 2020, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Suhr, O.B.; Lundgren, E.; Westermark, P. One mutation, two distinct disease variants: Unravelling the impact of transthyretin amyloid fibril composition. J. Intern. Med. 2017, 281, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, C.E.; Saraiva, M.J.; Sousa, M.M. Transthyretin enhances nerve regeneration. J. Neurochem. 2007, 103, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A. POEMS Syndrome: 2019 Update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019, 94, 812–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, O.; Maruyama, I.; Arimura, K.; Kitajima, I.; Arimura, H.; Hanatani, M.; Matsuo, K.; Arisato, T.; Osame, M. Overproduction of vascular endothelial growth factor/vascular permeability factor is causative in Crow-Fukase (POEMS) syndrome. Muscle Nerve 1998, 21, 1390–1397. [Google Scholar] [CrossRef]
- Scarlato, M.; Previtali, S.C.; Carpo, M.; Pareyson, D.; Briani, C.; Del Bo, R.; Nobile-Orazio, E.; Quattrini, A.; Comi, G.P. Polyneuropathy in POEMS syndrome: Role of angiogenic factors in the pathogenesis. Brain 2005, 128, 1911–1920. [Google Scholar] [CrossRef] [Green Version]
- Briani, C.; Fabrizi, G.M.; Ruggero, S.; Torre, C.D.; Ferrarini, M.; Campagnolo, M.; Cavallaro, T.; Ferrari, S.; Scarlato, M.; Taioli, F.; et al. Vascular endothelial growth factor helps differentiate neuropathies in rare plasma cell dyscrasias. Muscle Nerve 2011, 43, 164–167. [Google Scholar] [CrossRef]
- Mauermann, M.L.; Sorenson, E.J.; Dispenzieri, A.; Mandrekar, J.; Suarez, G.A.; Dyck, P.J.; Dyck, P.J. Uniform demyelination and more severe axonal loss distinguish POEMS syndrome from CIDP. J. Neurol. Neurosurg. Psychiatry 2012, 83, 480–486. [Google Scholar] [CrossRef]
- Karam, C.; Klein, C.J.; Dispenzieri, A.; Dyck, P.J.; Mandrekar, J.; D’Souza, A.; Mauermann, M.L. Polyneuropathy improvement following autologous stem cell transplantation for POEMS syndrome. Neurology 2015, 84, 1981–1987. [Google Scholar] [CrossRef] [Green Version]
- Koike, H.; Iijima, M.; Mori, K.; Yamamoto, M.; Hattori, N.; Watanabe, H.; Tanaka, F.; Doyu, M.; Sobue, G. Neuropathic pain correlates with myelinated fibre loss and cytokine profile in POEMS syndrome. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1171–1179. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Kourelis, T.; Buadi, F. POEMS Syndrome: Diagnosis and Investigative Work-up. Hematol. Oncol. Clin. N. Am. 2018, 32, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Nasu, S.; Misawa, S.; Sekiguchi, Y.; Shibuya, K.; Kanai, K.; Fujimaki, Y.; Ohmori, S.; Mitsuma, S.; Koga, S.; Kuwabara, S. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J. Neurol. Neurosurg. Psychiatry 2012, 83, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Vital, C.; Vital, A.; Ferrer, X.; Viallard, J.F.; Pellegrin, J.L.; Bouillot, S.; Larrieu, J.M.; Lequen, L.; Larrieu, J.L.; Brechenmacher, C.; et al. Crow-Fukase (POEMS) syndrome: A study of peripheral nerve biopsy in five new cases. J. Peripher. Nerv. Syst. 2003, 8, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Fedrigo, M.; Manara, R.; Castellani, C.; Zambello, R.; Citton, V.; Campagnolo, M.; Dalla Torre, C.; Lucchetta, M.; Orvieto, E.; et al. Pachymeningeal involvement in POEMS syndrome: MRI and histopathological study. J. Neurol. Neurosurg. Psychiatry 2012, 83, 33–37. [Google Scholar] [CrossRef]
- Briani, C.; Manara, R.; Lessi, F.; Citton, V.; Zambello, R.; Adami, F. Pachymeningeal involvement in POEMS syndrome: Dramatic cerebral MRI improvement after lenalidomide therapy. Am. J. Hematol. 2012, 87, 539–541. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Park, S.B.; Islam, B.; Tamburin, S.; Velasco, R.; Alberti, P.; Bruna, J.; Psimaras, D.; Cavaletti, G.; Cornblath, D.R.; et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1361–1369. [Google Scholar] [CrossRef] [Green Version]
- Brahmer, J.R.; Lacchetti, C.; Thompson, J.A. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2018, 14, 247–249. [Google Scholar] [CrossRef]
- Dalakas, M.C. Neurological complications of immune checkpoint inhibitors: What happens when you ‘take the brakes off’ the immune system. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418799864. [Google Scholar] [CrossRef] [Green Version]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef]
- Magge, R.S.; DeAngelis, L.M. The double-edged sword: Neurotoxicity of chemotherapy. Blood Rev. 2015, 29, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity--focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sul, J.K.; Deangelis, L.M. Neurologic complications of cancer chemotherapy. Semin. Oncol. 2006, 33, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, B.; Lustberg, M.; Staff, N.P.; Kolb, N.; Alberti, P.; Argyriou, A.A. Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. 2), S63–S73. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, P.G.; Dyck, P.J.; Kiely, J.M. Vinca alkaloid neuropathy: Nerve biopsy studies in rats and in man. Neurology 1968, 18, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Topp, K.S.; Tanner, K.D.; Levine, J.D. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J. Comp. Neurol. 2000, 424, 563–576. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Woolf, C.J.; Gibson, S.J.; Mallaburn, P.S. Alterations in the structure, function, and chemistry of C fibers following local application of vinblastine to the sciatic nerve of the rat. J. Neurosci. 1984, 4, 430–441. [Google Scholar] [CrossRef]
- Gagelmann, N.; Kroger, N. The role of novel agents for consolidation after autologous transplantation in newly diagnosed multiple myeloma: A systematic review. Ann. Hematol. 2020. [Google Scholar] [CrossRef]
- Mohty, B.; El-Cheikh, J.; Yakoub-Agha, I.; Moreau, P.; Harousseau, J.L.; Mohty, M. Peripheral neuropathy and new treatments for multiple myeloma: Background and practical recommendations. Haematologica 2010, 95, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Briani, C.; Zara, G.; Rondinone, R.; Della Libera, S.; Ermani, M.; Ruggero, S.; Ghirardello, A.; Zampieri, S.; Doria, A. Thalidomide neurotoxicity: Prospective study in patients with lupus erythematosus. Neurology 2004, 62, 2288–2290. [Google Scholar] [CrossRef]
- Chaudhry, V.; Cornblath, D.R.; Corse, A.; Freimer, M.; Simmons-O’Brien, E.; Vogelsang, G. Thalidomide-induced neuropathy. Neurology 2002, 59, 1872–1875. [Google Scholar] [CrossRef]
- De Iongh, R.U. A quantitative ultrastructural study of motor and sensory lumbosacral nerve roots in the thalidomide-treated rabbit fetus. J. Neuropathol. Exp. Neurol. 1990, 49, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Dalla Torre, C.; Zambello, R.; Cacciavillani, M.; Campagnolo, M.; Berno, T.; Salvalaggio, A.; De March, E.; Barila, G.; Lico, A.; Lucchetta, M.; et al. Lenalidomide long-term neurotoxicity: Clinical and neurophysiologic prospective study. Neurology 2016, 87, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Nozza, A.; Terenghi, F.; Gallia, F.; Adami, F.; Briani, C.; Merlini, G.; Giordano, L.; Santoro, A.; Nobile-Orazio, E. Lenalidomide and dexamethasone in patients with POEMS syndrome: Results of a prospective, open-label trial. Br. J. Haematol. 2017, 179, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riva, M.; Lessi, F.; Berno, T.; Visentin, A.; Campagnolo, M.; Semenzato, G.; Adami, F.; Briani, C. Bortezomib-based regimens in patients with POEMS syndrome: A case series in newly diagnosed and relapsed patients. Leuk. Lymphoma 2019, 60, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Marmiroli, P.; Scuteri, A.; Cornblath, D.R.; Cavaletti, G. Pain in chemotherapy-induced peripheral neurotoxicity. J. Peripher. Nerv. Syst. 2017, 22, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Thawani, S.P.; Tanji, K.; De Sousa, E.A.; Weimer, L.H.; Brannagan, T.H., 3rd. Bortezomib-associated demyelinating neuropathy--clinical and pathologic features. J. Clin. Neuromuscul. Dis. 2015, 16, 202–209. [Google Scholar] [CrossRef]
- Geisler, S. Vincristine- and bortezomib-induced neuropathies—From bedside to bench and back. Exp. Neurol. 2020, 336, 113519. [Google Scholar] [CrossRef]
- Landowski, T.H.; Megli, C.J.; Nullmeyer, K.D.; Lynch, R.M.; Dorr, R.T. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005, 65, 3828–3836. [Google Scholar] [CrossRef] [Green Version]
- Argyriou, A.A.; Cavaletti, G.; Bruna, J.; Kyritsis, A.P.; Kalofonos, H.P. Bortezomib-induced peripheral neurotoxicity: An update. Arch. Toxicol. 2014, 88, 1669–1679. [Google Scholar] [CrossRef]
- Mariotto, S.; Ferrari, S.; Monaco, S. Brentuximab vedotin-induced peripheral neuropathy: Looking at microtubules. J. Neurooncol. 2018, 137, 665–666. [Google Scholar] [CrossRef]
- Pastorelli, F.; Derenzini, E.; Plasmati, R.; Pellegrini, C.; Broccoli, A.; Casadei, B.; Argnani, L.; Salvi, F.; Pileri, S.; Zinzani, P.L. Severe peripheral motor neuropathy in a patient with Hodgkin lymphoma treated with brentuximab vedotin. Leuk. Lymphoma 2013, 54, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Fargeot, G.; Dupel-Pottier, C.; Stephant, M.; Lazarovici, J.; Thomas, L.; Mouthon-Reignier, C.; Riad, B.; Carde, P.; Berzero, G.; Tafani, C.; et al. Brentuximab vedotin treatment associated with acute and chronic inflammatory demyelinating polyradiculoneuropathies. J. Neurol. Neurosurg. Psychiatry 2020, 91, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.C.; Haggiagi, A. Neurologic Immune-Related Adverse Events Associated with Immune Checkpoint Inhibition. Curr. Oncol. Rep. 2019, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; David, W.S.; Amato, A.A.; Reynolds, K.L.; Clement, N.F.; Chute, D.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; et al. Varied phenotypes and management of immune checkpoint inhibitor-associated neuropathies. Neurology 2019, 93, e1093–e1103. [Google Scholar] [CrossRef] [PubMed]
- Psimaras, D.; Velasco, R.; Birzu, C.; Tamburin, S.; Lustberg, M.; Bruna, J.; Argyriou, A.A. Immune checkpoint inhibitors-induced neuromuscular toxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. 2), S74–S85. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef]
- Johansen, A.; Christensen, S.J.; Scheie, D.; Hojgaard, J.L.S.; Kondziella, D. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: Systematic review. Neurology 2019, 92, 663–674. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briani, C.; Ferrari, S.; Campagnolo, M.; Tagliapietra, M.; Castellani, F.; Salvalaggio, A.; Mariotto, S.; Visentin, A.; Cavallaro, T. Mechanisms of Nerve Damage in Neuropathies Associated with Hematological Diseases: Lesson from Nerve Biopsies. Brain Sci. 2021, 11, 132. https://doi.org/10.3390/brainsci11020132
Briani C, Ferrari S, Campagnolo M, Tagliapietra M, Castellani F, Salvalaggio A, Mariotto S, Visentin A, Cavallaro T. Mechanisms of Nerve Damage in Neuropathies Associated with Hematological Diseases: Lesson from Nerve Biopsies. Brain Sciences. 2021; 11(2):132. https://doi.org/10.3390/brainsci11020132
Chicago/Turabian StyleBriani, Chiara, Sergio Ferrari, Marta Campagnolo, Matteo Tagliapietra, Francesca Castellani, Alessandro Salvalaggio, Sara Mariotto, Andrea Visentin, and Tiziana Cavallaro. 2021. "Mechanisms of Nerve Damage in Neuropathies Associated with Hematological Diseases: Lesson from Nerve Biopsies" Brain Sciences 11, no. 2: 132. https://doi.org/10.3390/brainsci11020132
APA StyleBriani, C., Ferrari, S., Campagnolo, M., Tagliapietra, M., Castellani, F., Salvalaggio, A., Mariotto, S., Visentin, A., & Cavallaro, T. (2021). Mechanisms of Nerve Damage in Neuropathies Associated with Hematological Diseases: Lesson from Nerve Biopsies. Brain Sciences, 11(2), 132. https://doi.org/10.3390/brainsci11020132





