Anatomical Location of the Vestibulocerebellar Tract in the Healthy Human Brain: A Diffusion Tensor Imaging Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Subjects
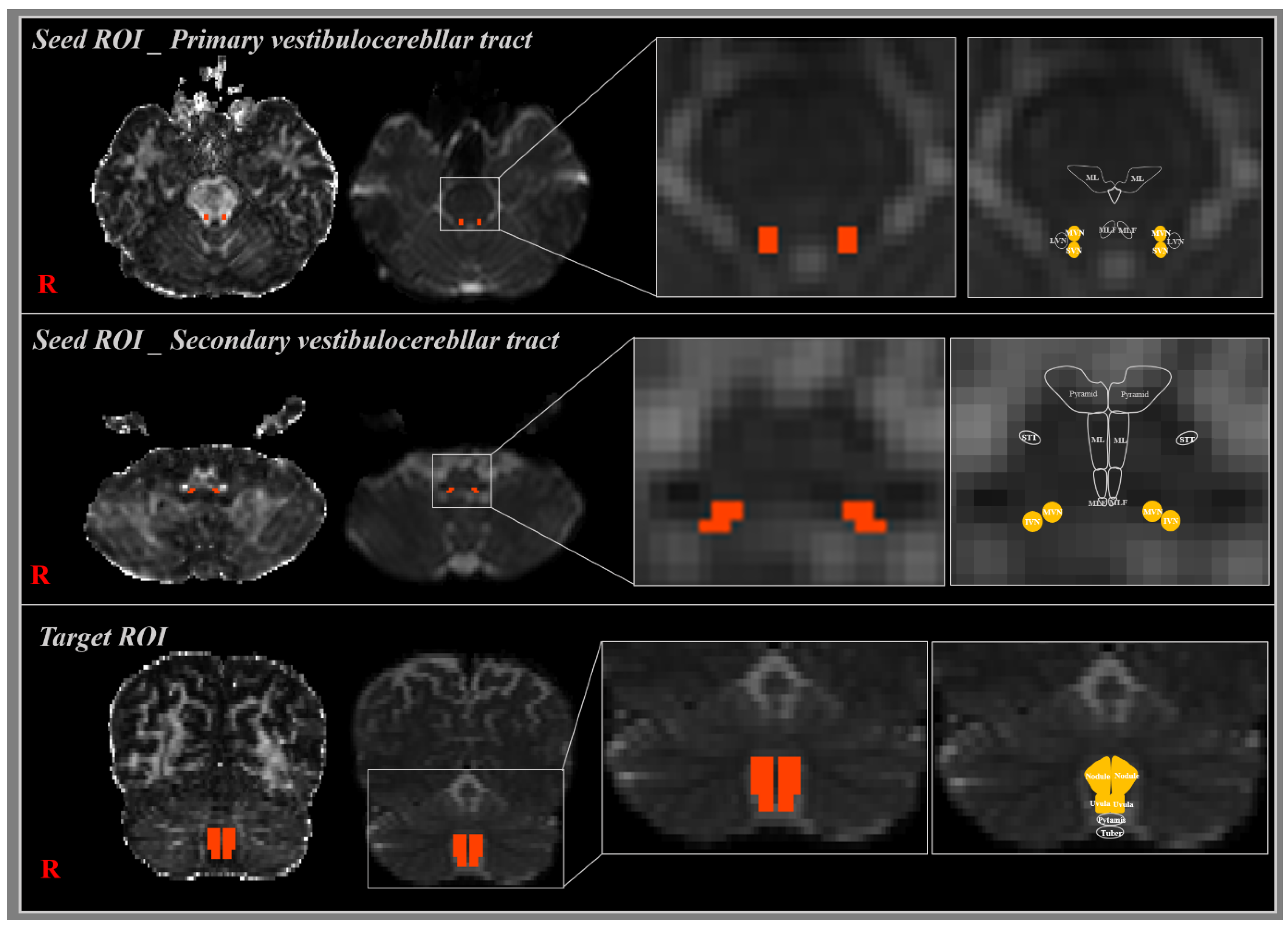
2.2. Diffusion Tensor Image Tractography
2.3. Probabilistic Fiber Tracking
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.; Arshad, Q.; Seemungal, B.M. Vestibular Deficits in Neurodegenerative Disorders: Balance, Dizziness, and Spatial Disorientation. Front. Neurol. 2017, 8, 538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winser, S.J.; Schubert, M.C.; Chan, A.Y.Y.; Kannan, P.; Whitney, S.L. Can pre-screening vestibulocerebellar involvement followed by targeted training improve the outcomes of balance in cerebellar ataxia? Med. Hypotheses 2018, 117, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, M.B. Core Text of Neuroanatomy. Williams & Wilkins: Philadelphia, PA, USA, 1991. [Google Scholar]
- Li, P.; Gu, H.; Xu, J.; Zhang, Z.; Li, F.; Feng, M.; Tian, Q.; Shang, C.; Zhuang, J. Purkinje cells of vestibulocerebellum play an important role in acute vestibular migraine. J. Integr. Neurosci. 2019, 18, 409–414. [Google Scholar] [PubMed]
- Herdman, S.J.; Clendaniel, R. Vestibular Rehabilitation, 4th ed.; F. A. Davis Company: Philadelphia, PA, USA, 2014. [Google Scholar]
- Glasauer, S.; Dieterich, M.; Brandt, T. Computational neurology of gravity perception involving semicircular canal dysfunction in unilateral vestibular lesions. Prog. Brain Res. 2019, 248, 303–317. [Google Scholar] [PubMed]
- Barmack, N.H. Central vestibular system: Vestibular nuclei and posterior cerebellum. Brain Res. Bull. 2003, 60, 511–541. [Google Scholar] [CrossRef]
- Mendoza, J.; Foundas, A. Clinical Neuroanatomy: A Neurobehavioral Approach; Springer: New York, NY, USA, 2007. [Google Scholar]
- Jang, S.H.; Kim, J.H.; Kim, D.H.; Kwon, H.G. The Vestibulocerebellar Tract in the Human Brain: A Diffusion Tensor Tractography Study. Curr. Med. Imaging Rev. 2017, 14, 617–620. [Google Scholar] [CrossRef]
- Brodal, A.; Brodal, P. Observations on the secondary vestibulocerebellar projections in the macaque monkey. Exp. Brain Res. 1985, 58, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H.; Yakhnitsa, V. Vestibulocerebellar Connections. In Handbook of the Cerebellum and Cerebellar Disorders; Manto, M., Schmahmann, J.D., Rossi, F., Gruol, D.L., Koibuchi, N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 357–375. [Google Scholar]
- Wang, Q.; Yap, P.-T.; Wu, G.; Shen, D. Diffusion tensor image registration using hybrid connectivity and tensor features. Hum. Brain Mapp. 2014, 35, 3529–3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. MN 2008, 34, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Liu, D.; Li, G.; Guo, Z.; Fan, Y.; Niu, Y. Evaluations of diffusion tensor image registration based on fiber tractography. Biomed. Eng. Online 2017, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galons, J.P. Diffusion weighted and diffusion tensor imaging: A clinical guide. J. Magn. Reson. Imaging 2017, 46. [Google Scholar] [CrossRef] [PubMed]
- Takeshige, H.; Ueno, Y.; Kamagata, K.; Sasaki, F.; Yamashiro, K.; Tanaka, R.; Aoki, S.; Hattori, N. Pathways Linked to Internuclear Ophthalmoplegia on Diffusion-Tensor Imaging in a Case with Midbrain Infarction. J. Stroke Cerebrovasc. Dis. 2016, 25, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Korte, G.E.; Mugnaini, E. The cerebellar projection of the vestibular nerve in the cat. J. Comp. Neurol. 1979, 184, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Carleton, S.C.; Carpenter, M.B. Distribution of primary vestibular fibers in the brainstem and cerebellum of the monkey. Brain Res. 1984, 294, 281–298. [Google Scholar] [CrossRef]
- Schwarz, I.E.; Schwarz, D.W. The primary vestibular projection to the cerebellar cortex in the pigeon (Columba livia). J. Comp. Neurol. 1983, 216, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, N.M.; Epema, A.H.; van Linge, A.; Dalm, E. The primary vestibulocerebellar projection in the rabbit: Absence of primary afferents in the flocculus. Neurosci. Lett. 1989, 105, 27–33. [Google Scholar] [CrossRef]
- Barmack, N.H.; Baughman, R.W.; Errico, P.; Shojaku, H. Vestibular primary afferent projection to the cerebellum of the rabbit. J. Comp. Neurol. 1993, 327, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Rubertone, J.A.; Haines, D.E. Secondary vestibulocerebellar projections to flocculonodular lobe in a prosimian primate, Galago senegalensis. J. Comp. Neurol. 1981, 200, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Arslan, O.E. Neuroanatomical Basis of Clinical Neurology, Second Edition, 2nd ed.; Taylor & Francis: Abingdon, UK, 2014. [Google Scholar]
- Barahona, M.L.; Encinas, J.P.M.; Pascual, R.Q.; Prado, J.A.-L.; Presmanes, Y.G.; Gil, M.Á.F. Structural and Functional Anatomy of Cerebellum. More than a Motor Conception; European Society of Radiology Electronic Presentation Online System: Vienna, Austria, 2011. [Google Scholar]

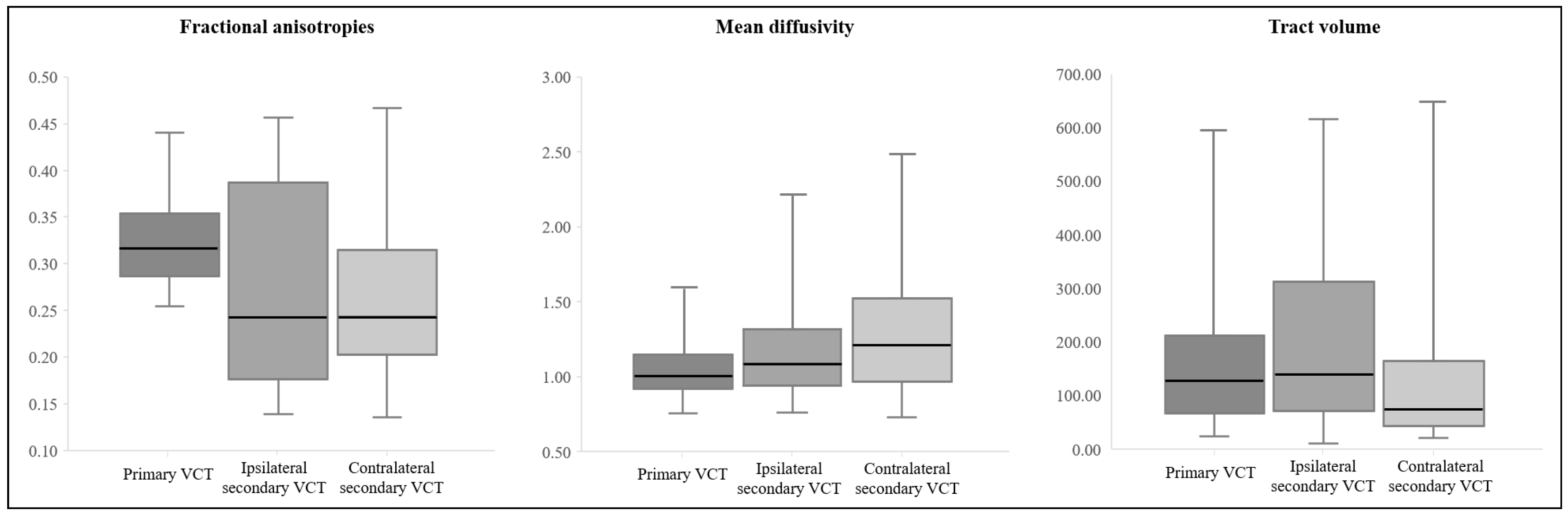
| FA | MD | Tract Volume | ||
|---|---|---|---|---|
| Primary VCT | 0.28 | 1.05 | 176.53 | |
| (0.06) | (0.19) | (142.50) | ||
| Secondary VCT | Ipsilateral | 0.22 | 1.18 | 204.92 |
| (0.07) | (0.32) | (166.10) | ||
| Contralateral | 0.22 | 1.29 | 125.87 | |
| (0.08) | (0.40) | (121.62) | ||
| A vs. B | 0.000 * | 0.024 * | 0.276 | |
| A vs. C | 0.000 * | 0.000 * | 0.053 | |
| B vs. C | 0.902 | 0.043 * | 0.003 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Yeo, S.S.; Jang, S.H.; Cho, I.H. Anatomical Location of the Vestibulocerebellar Tract in the Healthy Human Brain: A Diffusion Tensor Imaging Study. Brain Sci. 2021, 11, 199. https://doi.org/10.3390/brainsci11020199
Park SY, Yeo SS, Jang SH, Cho IH. Anatomical Location of the Vestibulocerebellar Tract in the Healthy Human Brain: A Diffusion Tensor Imaging Study. Brain Sciences. 2021; 11(2):199. https://doi.org/10.3390/brainsci11020199
Chicago/Turabian StylePark, Seo Yoon, Sang Seok Yeo, Sung Ho Jang, and In Hee Cho. 2021. "Anatomical Location of the Vestibulocerebellar Tract in the Healthy Human Brain: A Diffusion Tensor Imaging Study" Brain Sciences 11, no. 2: 199. https://doi.org/10.3390/brainsci11020199






