Bliss in and Out of the Body: The (Extra)Corporeal Space Is Impervious to Social Pleasant Touch
Abstract
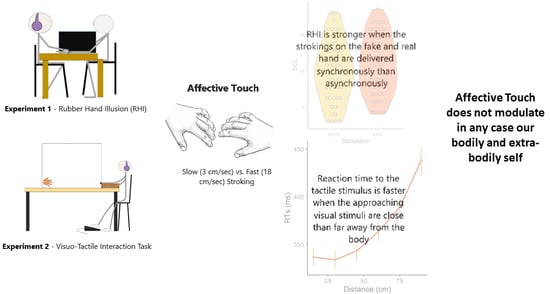
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
3. Results
4. Discussion
5. Material and Methods
5.1. Participants
5.2. Procedure
6. Results
7. Discussion
8. General Discussion
Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slatman, J. The sense of life: Husserl and Merleau-Ponty touching and being touched. Chiasmi Int. 2005, 7, 305–324. [Google Scholar] [CrossRef]
- McGlone, F.; Reilly, D. The cutaneous sensory system. Neurosci. Biobehav. Rev. 2010, 34, 148–159. [Google Scholar] [CrossRef]
- McGlone, F.; Wessberg, J.; Olausson, H. Discriminative and affective touch: Sensing and feeling. Neuron 2014, 82, 737–755. [Google Scholar] [CrossRef] [Green Version]
- Löken, L.S.; Wessberg, J.; McGlone, F.; Olausson, H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Löken, L.S.; Evert, M.; Wessberg, J. Pleasantness of touch in human glabrous and hairy skin: Order effects on affective ratings. Brain Res. 2011, 1417, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Voos, A.C.; Bennett, R.H.; Bolling, D.Z.; Pelphrey, K.A.; Kaiser, M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapping 2013, 34, 914–922. [Google Scholar] [CrossRef]
- Croy, I.; Geide, H.; Paulus, M.; Weidner, K.; Olausson, H. Affective touch awareness in mental health and disease relates to autistic traits–An explorative neurophysiological investigation. Psychiatry Res. 2016, 245, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Sailer, U.; Hausmann, M.; Croy, I. Pleasantness only? How sensory and affective attributes describe touch targeting C-tactile fibers. Exp. Psychol. 2020, 67, 224. [Google Scholar] [CrossRef]
- Ackerley, R.; Wasling, H.B.; Liljencrantz, J.; Olausson, H.; Johnson, R.D.; Wessberg, J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J. Neurosci. 2014, 34, 2879–2883. [Google Scholar] [CrossRef]
- Morrison, I.; Löken, L.S.; Olausson, H. The skin as a social organ. Exp. Brain Res. 2010, 204, 305–314. [Google Scholar] [CrossRef]
- Masson, H.L.; de Beeck, H.O.; Boets, B. Reduced task-dependent modulation of functional network architecture for positive versus negative affective touch processing in autism spectrum disorders. NeuroImage 2020, 219, 117009. [Google Scholar] [CrossRef]
- Van Stralen, H.E.; van Zandvoort, M.J.; Hoppenbrouwers, S.S.; Vissers, L.M.; Kappelle, L.J.; Dijkerman, H.C. Affective touch modulates the rubber hand illusion. Cognition 2014, 131, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Krahé, C.; Drabek, M.M.; Paloyelis, Y.; Fotopoulou, A. Affective touch and attachment style modulate pain: A laser-evoked potentials study. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Mohr, M.; Kirsch, L.P.; Fotopoulou, A. The soothing function of touch: Affective touch reduces feelings of social exclusion. Sci. Rep. 2017, 7, 1–9. [Google Scholar]
- Bershad, A.K.; Mayo, L.M.; Van Hedger, K.; McGlone, F.; Walker, S.C.; de Wit, H. Effects of MDMA on attention to positive social cues and pleasantness of affective touch. Neuropsychopharmacology 2019, 44, 1698–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawling, R.; Trotter, P.D.; McGlone, F.P.; Walker, S.C. A positive touch: C-tactile afferent targeted skin stimulation carries an appetitive motivational value. Biol. Psychol. 2017, 129, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Von Mohr, M.; Crowley, M.J.; Walthall, J.; Mayes, L.C.; Pelphrey, K.A.; Rutherford, H.J. EEG captures affective touch: CT-optimal touch and neural oscillations. Cogn. Affect. Behav. Neurosci. 2018, 18, 155–166. [Google Scholar] [CrossRef]
- Haggarty, C.J.; Malinowski, P.; McGlone, F.P.; Walker, S.C. Autistic traits modulate cortical responses to affective but not discriminative touch. Eur. J. Neurosci. 2020, 51, 1844–1855. [Google Scholar] [CrossRef]
- Ree, A.; Mayo, L.M.; Leknes, S.; Sailer, U. Touch targeting C-tactile afferent fibers has a unique physiological pattern: A combined electrodermal and facial electromyography study. Biol. Psychol. 2019, 140, 55–63. [Google Scholar] [CrossRef]
- McGlone, F.; Vallbo, A.B.; Olausson, H.; Loken, L.; Wessberg, J. Discriminative touch and emotional touch. Can. J. Exp. Psychol. Rev. Can. Psychol. Expérimentale 2007, 61, 173. [Google Scholar] [CrossRef] [Green Version]
- Rolls, E.T. Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn. 2009, 110, 4–19. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Bechara, A. The hidden island of addiction: The insula. Trends Neurosci. 2016, 32, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, L.P.; Besharati, S.; Papadaki, C.; Crucianelli, L.; Bertagnoli, S.; Ward, N.; Fotopoulou, A. Damage to the right insula disrupts the perception of affective touch. Elife 2020, 9, e47895. [Google Scholar] [CrossRef]
- Botvinick, M. Probing the neural basis of body ownership. Science 2004, 305, 782–783. [Google Scholar] [CrossRef] [PubMed]
- Crucianelli, L.; Metcalf, N.K.; Fotopoulou, A.K.; Jenkinson, P.M. Bodily pleasure matters: Velocity of touch modulates body ownership during the rubber hand illusion. Front. Psychol. 2013, 4, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crucianelli, L.; Krahé, C.; Jenkinson, P.M.; Fotopoulou, A.K. Interoceptive ingredients of body ownership: Affective touch and cardiac awareness in the rubber hand illusion. Cortex 2018, 104, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef]
- Rohde, M.; Di Luca, M.; Ernst, M.O. The rubber hand illusion: Feeling of ownership and proprioceptive drift do not go hand in hand. PLoS ONE 2011, 6, e21659. [Google Scholar] [CrossRef] [Green Version]
- Holle, H.; McLatchie, N.; Maurer, S.; Ward, J. Proprioceptive drift without illusions of ownership for rotated hands in the “rubber hand illusion” paradigm. Cogn. Neurosci. 2011, 2, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, Z.; Ehrsson, H.H. No causal link between changes in hand position sense and feeling of limb ownership in the rubber hand illusion. Atten. Percept. Psychophys. 2016, 78, 707–720. [Google Scholar] [CrossRef] [Green Version]
- De Jong, J.R.; Keizer, A.; Engel, M.M.; Dijkerman, H.C. Does affective touch influence the virtual reality full body illusion? Exp. Brain Res. 2017, 235, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawling, R.; Cannon, P.R.; McGlone, F.P.; Walker, S.C. C-tactile afferent stimulating touch carries a positive affective value. PLoS ONE 2017, 12, e0173457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etzi, R.; Carta, C.; Gallace, A. Stroking and tapping the skin: Behavioral and electrodermal effects. Exp. Brain Res. 2018, 236, 453–461. [Google Scholar] [CrossRef]
- Van Hooijdonk, R.; Mathot, S.; Schat, E.; Spencer, H.; van der Stigchel, S.; Dijkerman, H.C. Touch-induced pupil size reflects stimulus intensity, not subjective pleasantness. Exp. Brain Res. 2019, 237, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Cardinali, L.; Brozzoli, C.; Farne, A. Peripersonal space and body schema: Two labels for the same concept? Brain Topogr. 2009, 21, 252–260. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Scandolara, C.; Matelli, M.; Gentilucci, M. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav. Brain Res. 1981, 2, 147–163. [Google Scholar] [CrossRef]
- Serino, A.; Noel, J.P.; Galli, G.; Canzoneri, E.; Marmaroli, P.; Lissek, H.; Blanke, O. Body part-centered and full body-centered peripersonal space representations. Sci. Rep. 2015, 5, 18603. [Google Scholar] [CrossRef]
- Fogassi, L.; Gallese, V.; Fadiga, L.; Luppino, G.; Matelli, M.; Rizzolatti, G. Coding of peripersonal space in inferior premotor cortex (area F4). J. Neurophysiol. 1996, 76, 141–157. [Google Scholar] [CrossRef]
- Spaccasassi, C.; Romano, D.; Maravita, A. Everything is worth when it is close to my body: How spatial proximity and stimulus valence affect visuo-tactile integration. Acta Psychol. 2019, 192, 42–51. [Google Scholar] [CrossRef]
- Di Pino, G.; Romano, D.; Spaccasassi, C.; Mioli, A.; D’Alonzo, M.; Sacchetti, R.; Maravita, A. Sensory–and action-oriented embodiment of neurally-interfaced robotic hand prostheses. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- De Haan, A.M.; Smit, M.; Van der Stigchel, S.; Dijkerman, H.C. Approaching threat modulates visuotactile interactions in peripersonal space. Exp. Brain Res. 2016, 234, 1875–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellena, G.; Starita, F.; Haggard, P.; Làdavas, E. The spatial logic of fear. Cognition 2020, 203, 104336. [Google Scholar] [CrossRef] [PubMed]
- MacKay, W.A.; Crammond, D.J. Neuronal correlates in posterior parietal lobe of the expectation of events. Behav. Brain Res. 1987, 24, 167–179. [Google Scholar] [CrossRef]
- Ferri, F.; Tajadura-Jiménez, A.; Väljamäe, A.; Vastano, R.; Costantini, M. Emotion-inducing approaching sounds shape the boundaries of multisensory peripersonal space. Neuropsychologia 2015, 70, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaccasassi, C.; Maravita, A. Peripersonal space is diversely sensitive to a temporary vs permanent state of anxiety. Cognition 2020, 195, 104133. [Google Scholar] [CrossRef]
- Figner, B.; Murphy, R.O. Using skin conductance in judgment and decision making research. In A Handbook of Process Tracing Methods for Decision Research; Psychology Press: East Sussex, UK, 2011; pp. 163–184. [Google Scholar]
- Storm, H.; Fremming, A.; Ødegaard, S.; Martinsen, Ø.G.; Moerkrid, L. The development of a software program for analyzing spontaneous and externally elicited skin conductance changes in infants and adults. Clin. Neurophysiol. 2000, 111, 1889–1898. [Google Scholar] [CrossRef]
- Günther, A.C.; Bottai, M.; Schandl, A.R.; Storm, H.; Rossi, P.; Sackey, P.V. Palmar skin conductance variability and the relation to stimulation, pain and the motor activity assessment scale in intensive care unit patients. Crit. Care 2013, 17, R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiris, M.; Haggard, P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 80. [Google Scholar] [CrossRef] [Green Version]
- Riemer, M.; Trojan, J.; Beauchamp, M.; Fuchs, X. The rubber hand universe: On the impact of methodological differences in the rubber hand illusion. Neurosci. Biobehav. Rev. 2019, 104, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Jiménez, A.T.; Costantini, M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. B Biol. Sci. 2011, 278, 2470–2476. [Google Scholar] [CrossRef] [Green Version]
- Olausson, H.; Cole, J.; Rylander, K.; McGlone, F.; Lamarre, Y.; Wallin, B.G. Functional role of unmyelinated tactile afferents in human hairy skin: Sympathetic response and perceptual localization. Exp. Brain Res. 2008, 184, 135–140. [Google Scholar] [CrossRef]
- Olausson, H.; Wessberg, J.; McGlone, F.; Vallbo, Å. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010, 34, 185–191. [Google Scholar] [CrossRef]
- Longo, M.R.; Schüür, F.; Kammers, M.P.; Tsakiris, M.; Haggard, P. What is embodiment? A psychometric approach. Cognition 2008, 107, 978–998. [Google Scholar] [CrossRef] [Green Version]
- Elbrecht, C.; Antcliff, L.R. Being touched through touch. Trauma treatment through haptic perception at the Clay Field: A sensorimotor art therapy. Int. J. Art Ther. 2014, 19, 19–30. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Tuominen, L.; Dunbar, R.; Hirvonen, J.; Manninen, S.; Arponen, E.; Sams, M. Social touch modulates endogenous μ-opioid system activity in humans. NeuroImage 2016, 138, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Ainley, V.; Apps, M.A.; Fotopoulou, A.; Tsakiris, M. ‘Bodily precision’: A predictive coding account of individual differences in interoceptive accuracy. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160003. [Google Scholar] [CrossRef]
- Harjunen, V.J.; Spapé, M.; Ahmed, I.; Jacucci, G.; Ravaja, N. Individual differences in affective touch: Behavioral inhibition and gender define how an interpersonal touch is perceived. Personal. Individ. Differ. 2017, 107, 88–95. [Google Scholar] [CrossRef]
- Croy, I.; Bierling, A.; Sailer, U.; Ackerley, R. Individual variability of pleasantness ratings to stroking touch over different velocities. Neuroscience 2020. [Google Scholar] [CrossRef]
- Voos, A.C.; Pelphrey, K.A.; Kaiser, M.D. Autistic traits are associated with diminished neural response to affective touch. Soc. Cogn. Affect. Neurosci. 2013, 8, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, C.; McGlone, F.; Folger, S.; Tannan, V.; Baranek, G.; Pelphrey, K.A.; Essick, G. Tactile perception in adults with autism: A multidimensional psychophysical study. J. Autism Dev. Disord. 2008, 38, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, C.J.; Moana-Filho, E.J.; Guest, S.; Nebel, M.B.; Weisner, J.; Baranek, G.T.; Essick, G.K. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res. 2012, 5, 231–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellingsen, D.M.; Leknes, S.; Løseth, G.; Wessberg, J.; Olausson, H. The neurobiology shaping affective touch: Expectation, motivation, and meaning in the multisensory context. Front. Psychol. 2016, 6, 1986. [Google Scholar] [CrossRef]
- Krahé, C.; von Mohr, M.; Gentsch, A.; Guy, L.; Vari, C.; Nolte, T.; Fotopoulou, A. Sensitivity to CT-optimal, affective touch depends on adult attachment style. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Spitoni, G.F.; Zingaretti, P.; Giovanardi, G.; Antonucci, G.; Galati, G.; Lingiardi, V.; Boccia, M. Disorganized attachment pattern affects the perception of affective touch. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Russo, V.; Ottaviani, C.; Spitoni, G.F. Affective touch: A meta-analysis on sex differences. Neurosci. Biobehav. Rev. 2020, 108, 445–452. [Google Scholar] [CrossRef]
- Fiorio, M.; Recchia, S.; Corra, F.; Simonetto, S.; Garcia-Larrea, L.; Tinazzi, M. Enhancing non-noxious perception: Behavioural and neurophysiological correlates of a placebo-like manipulation. Neuroscience 2012, 217, 96–104. [Google Scholar] [CrossRef]
- Beaver, J.D.; Lawrence, A.D.; Van Ditzhuijzen, J.; Davis, M.H.; Woods, A.; Calder, A.J. Individual differences in reward drive predict neural responses to images of food. J. Neurosci. 2006, 26, 5160–5166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.E.; Yager, L.M.; Cogan, E.S.; Saunders, B.T. On the motivational properties of reward cues: Individual differences. Neuropharmacology 2014, 76, 450–459. [Google Scholar] [CrossRef] [Green Version]
- Marotta, A.; Tinazzi, M.; Cavedini, C.; Zampini, M.; Fiorio, M. Individual differences in the rubber hand illusion are related to sensory suggestibility. PLoS ONE 2016, 11, e0168489. [Google Scholar] [CrossRef] [PubMed]
- Germine, L.; Benson, T.L.; Cohen, F.; Hooker, C.I.L. Psychosis-proneness and the rubber hand illusion of body ownership. Psychiatry Res. 2013, 207, 45–52. [Google Scholar] [CrossRef]
- Asai, T.; Mao, Z.; Sugimori, E.; Tanno, Y. Rubber hand illusion, empathy, and schizotypal experiences in terms of self-other representations. Conscious. Cogn. 2011, 20, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Bufacchi, R.J.; Iannetti, G.D. An action field theory of peripersonal space. Trends Cogn. Sci. 2018, 22, 1076–1090. [Google Scholar] [CrossRef] [Green Version]
- Mayo, L.M.; Lindé, J.; Olausson, H.; Heilig, M. Putting a good face on touch: Facial expression reflects the affective valence of caress-like touch across modalities. Biol. Psychol. 2018, 137, 83–90. [Google Scholar] [CrossRef]
- Nguyen, V.H.H.; Palmer, S.B.; Aday, J.S.; Davoli, C.C.; Bloesch, E.K. Meditation alters representations of peripersonal space: Evidence from auditory evoked potentials. Conscious. Cogn. 2020, 83, 102978. [Google Scholar] [CrossRef]
- Ardizzi, M.; Ferri, F. Interoceptive influences on peripersonal space boundary. Cognition 2018, 177, 79–86. [Google Scholar] [CrossRef]
- Tajadura-Jiménez, A.; Pantelidou, G.; Rebacz, P.; Västfjäll, D.; Tsakiris, M. I-space: The effects of emotional valence and source of music on interpersonal distance. PLoS ONE 2011, 6, e26083. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, G.; Frassinetti, F.; Coello, Y.; Rapuano, M.; Di Cola, A.S.; Iachini, T. The effect of facial expressions on peripersonal and interpersonal spaces. Psychol. Res. 2017, 81, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Monroe, C.M. The effects of therapeutic touch on pain. J. Holist. Nurs. 2009, 27, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, A.; Ng, T.; Ebstein, R.P. Vicarious social touch biases gazing at faces and facial emotions. Emotion 2018, 18, 1097. [Google Scholar] [CrossRef]
- Kress, I.U.; Minati, L.; Ferraro, S.; Critchley, H.D. Direct skin-to-skin vs. indirect touch modulates neural responses to stroking vs. tapping. Neuroreport 2011, 22, 646. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.D.; Rytting, M.; Heslin, R. Hands touching hands: Affective and evaluative effects of an interpersonal touch. Sociometry 1976, 416–421. [Google Scholar] [CrossRef] [Green Version]
- Guest, S.; Essick, G.; Dessirier, J.M.; Blot, K.; Lopetcharat, K.; McGlone, F. Sensory and affective judgments of skin during inter-and intrapersonal touch. Acta Psychol. 2009, 130, 115–126. [Google Scholar] [CrossRef]
- Gentsch, A.; Panagiotopoulou, E.; Fotopoulou, A. Active interpersonal touch gives rise to the social softness illusion. Curr. Biol. 2015, 25, 2392–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvilehto, J.T.; Glerean, E.; Dunbar, R.I.; Hari, R.; Nummenmaa, L. Topography of social touching depends on emotional bonds between humans. Proc. Natl. Acad. Sci. USA 2015, 112, 13811–13816. [Google Scholar] [CrossRef] [Green Version]
- Scheele, D.; Kendrick, K.M.; Khouri, C.; Kretzer, E.; Schläpfer, T.E.; Stoffel-Wagner, B.; Hurlemann, R. An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 2014, 39, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.D.; Yang, D.Y.J.; Voos, A.C.; Bennett, R.H.; Gordon, I.; Pretzsch, C.; Pelphrey, K.A. Brain mechanisms for processing affective (and nonaffective) touch are atypical in autism. Cereb. Cortex 2016, 26, 2705–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, H.L.; Pillet, I.; Amelynck, S.; Van De Plas, S.; Hendriks, M.; de Beeck, H.O.; Boets, B. Intact neural representations of affective meaning of touch but lack of embodied resonance in autism: A multi-voxel pattern analysis study. Mol. Autism 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Crucianelli, L.; Cardi, V.; Treasure, J.; Jenkinson, P.M.; Fotopoulou, A. The perception of affective touch in anorexia nervosa. Psychiatry Res. 2016, 239, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, E.; Filippetti, M.L.; Gentsch, A.; Fotopoulou, A. Dissociable sources of erogeneity in social touch: Imagining and perceiving C-Tactile optimal touch in erogenous zones. PLoS ONE 2018, 13, e0203039. [Google Scholar] [CrossRef]
- Routasalo, P.; Isola, A. The right to touch and be touched. Nurs. Ethics 1996, 3, 165–176. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaccasassi, C.; Frigione, I.; Maravita, A. Bliss in and Out of the Body: The (Extra)Corporeal Space Is Impervious to Social Pleasant Touch. Brain Sci. 2021, 11, 225. https://doi.org/10.3390/brainsci11020225
Spaccasassi C, Frigione I, Maravita A. Bliss in and Out of the Body: The (Extra)Corporeal Space Is Impervious to Social Pleasant Touch. Brain Sciences. 2021; 11(2):225. https://doi.org/10.3390/brainsci11020225
Chicago/Turabian StyleSpaccasassi, Chiara, Ivana Frigione, and Angelo Maravita. 2021. "Bliss in and Out of the Body: The (Extra)Corporeal Space Is Impervious to Social Pleasant Touch" Brain Sciences 11, no. 2: 225. https://doi.org/10.3390/brainsci11020225
APA StyleSpaccasassi, C., Frigione, I., & Maravita, A. (2021). Bliss in and Out of the Body: The (Extra)Corporeal Space Is Impervious to Social Pleasant Touch. Brain Sciences, 11(2), 225. https://doi.org/10.3390/brainsci11020225