Dynamical EEG Indices of Progressive Motor Inhibition and Error-Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
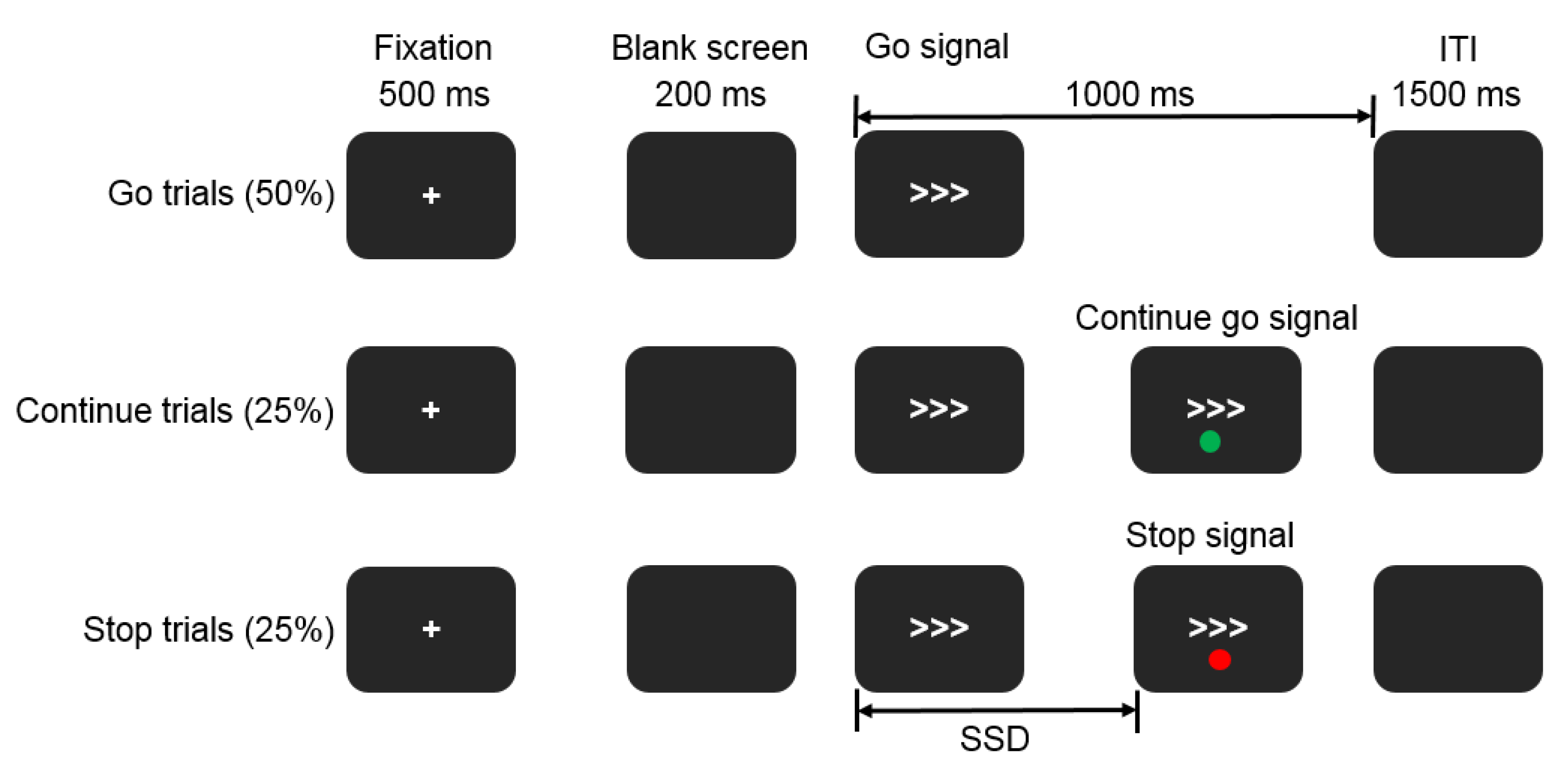
2.2. Selective Stop Signal Task
2.3. Electroencephalography Recording
2.4. Data Analysis
2.4.1. Behavioral Analysis
2.4.2. EEG Data
Hilbert–Huang Transform (HHT)
3. Results
3.1. Behavior Results
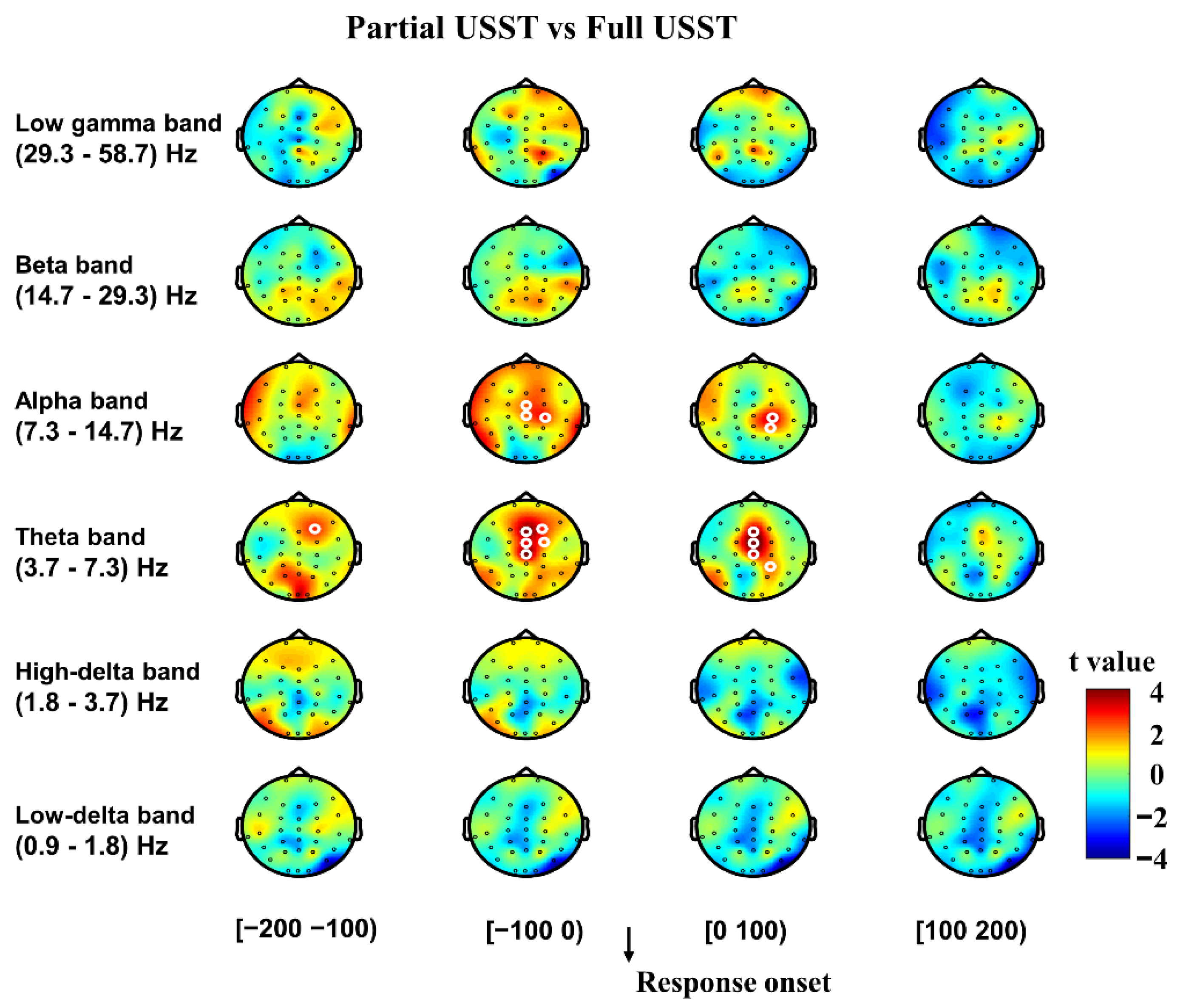
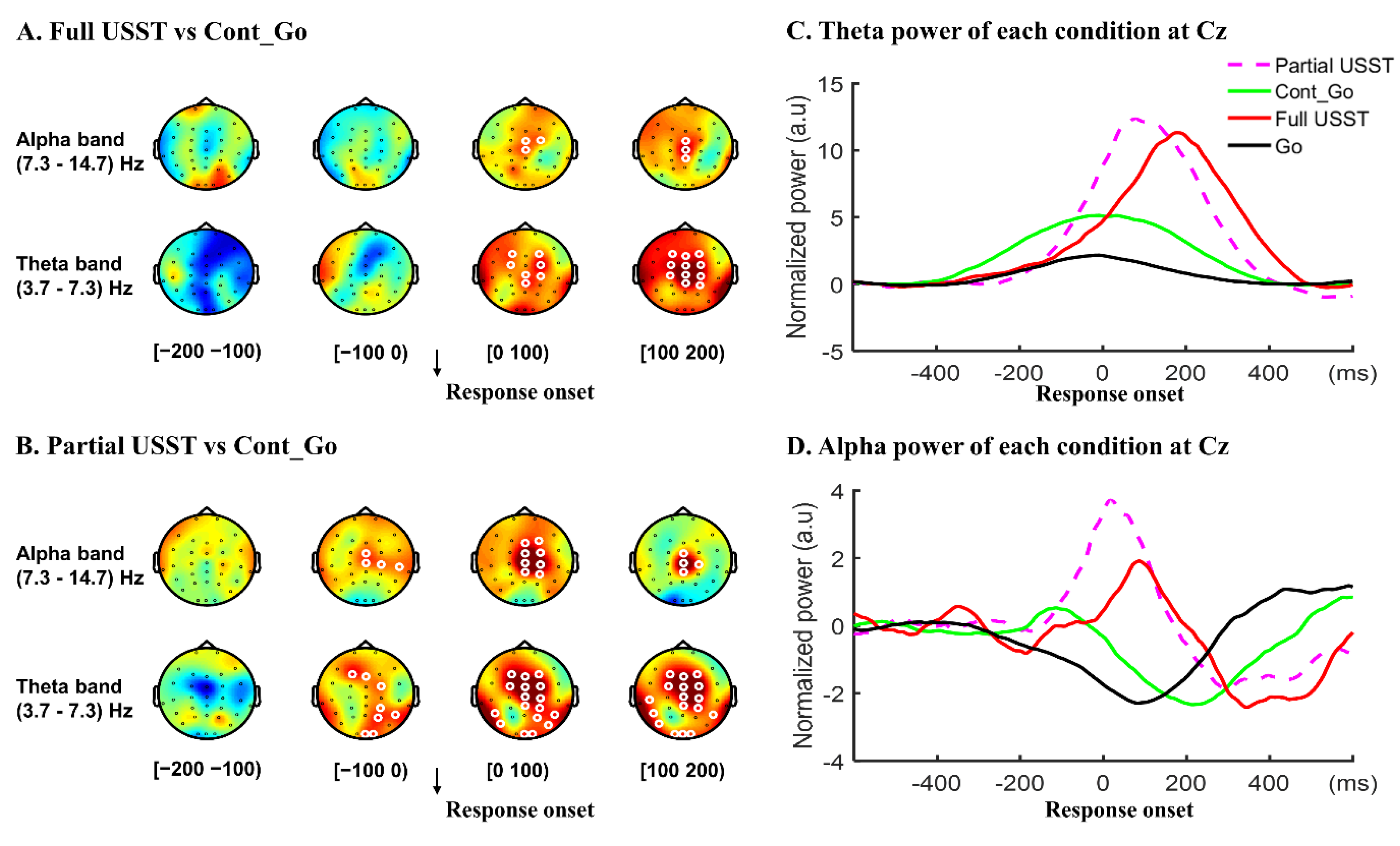
3.2. HHT Results
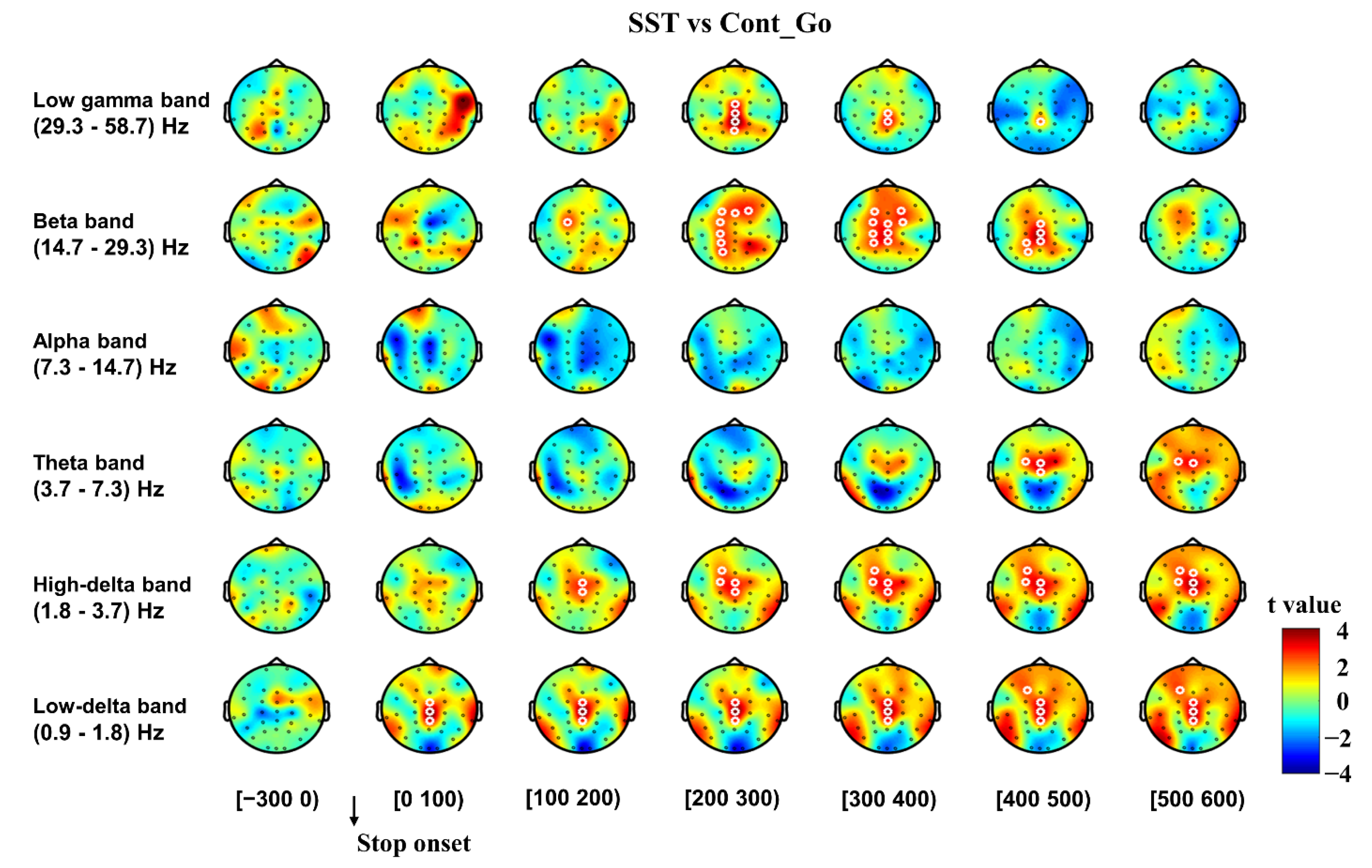
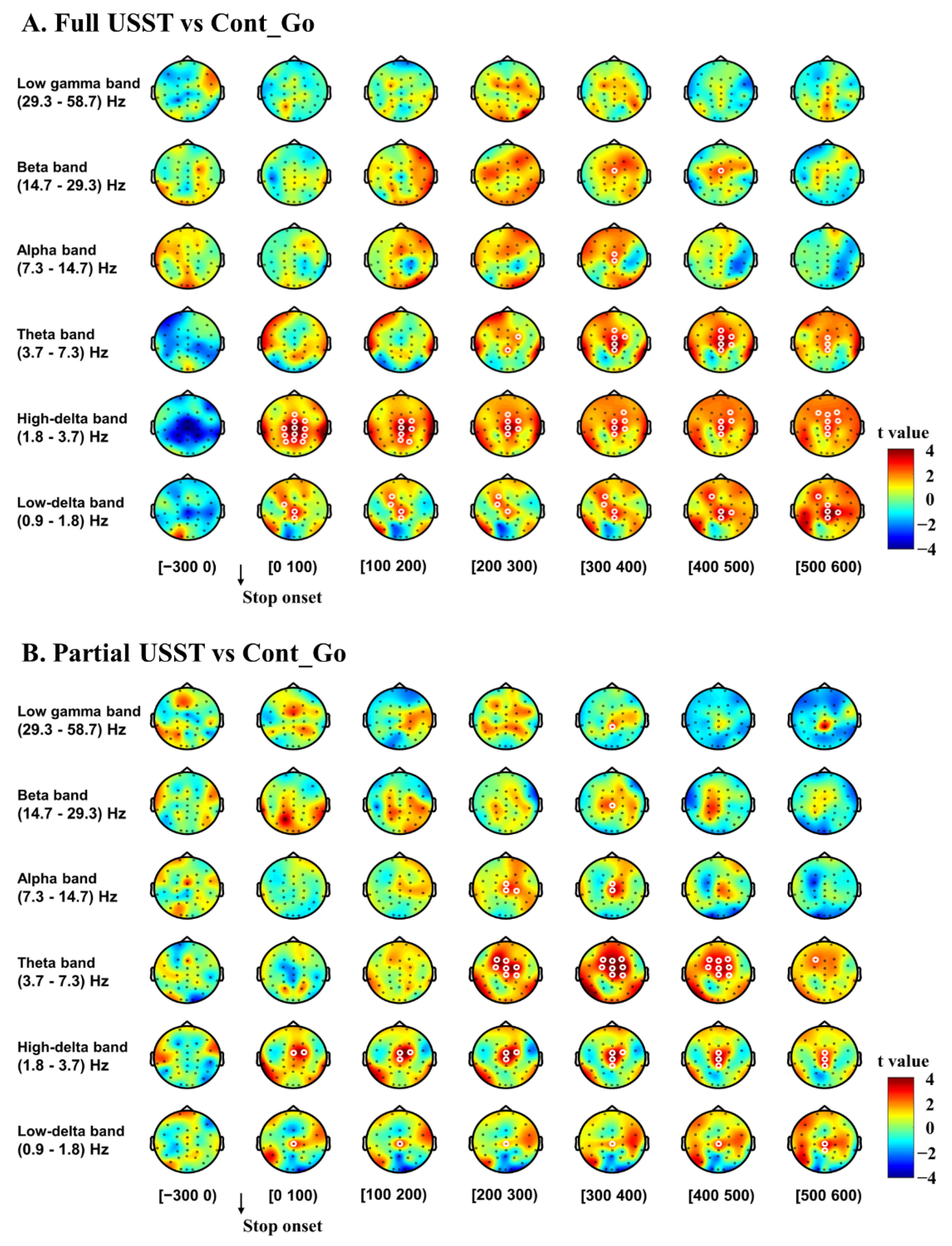
3.2.1. Inhibitory Control
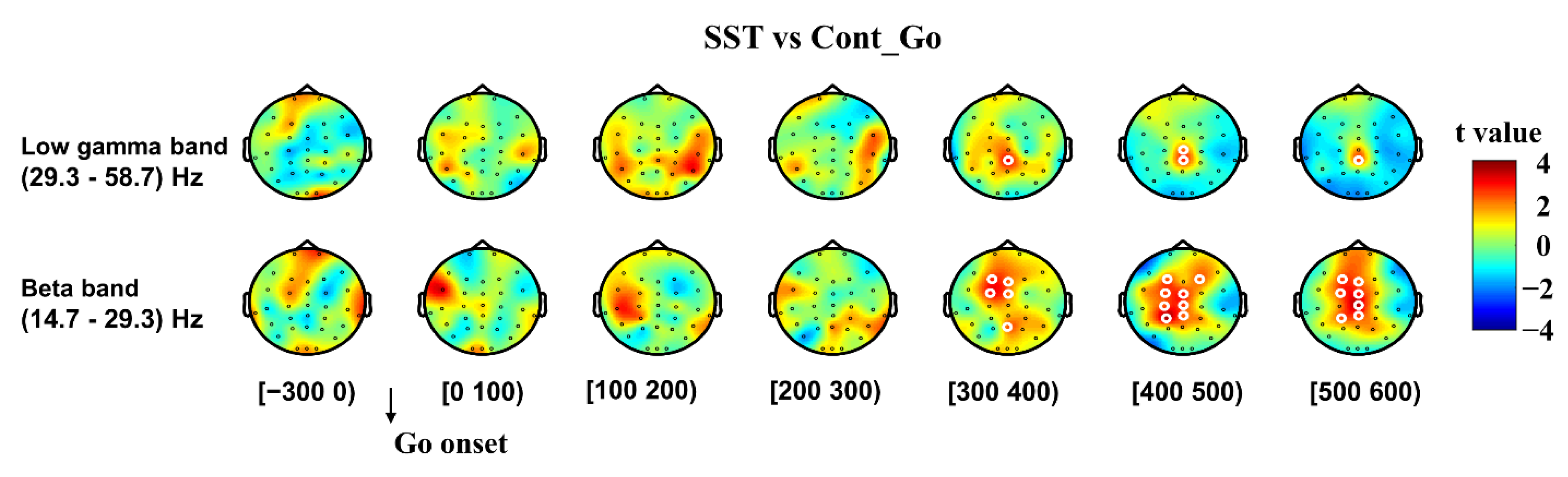
3.2.2. Error Detection and Error Correction
4. Discussion
4.1. Neural Mechanisms of Motor Inhibitory Control
4.2. Neural Mechanisms of Error Detection and Correction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, I.T.; Munoz, D.P. Inhibitory control of eye movements during oculomotor countermanding in adults with attention-deficit hyperactivity disorder. Exp. Brain Res. 2003, 152, 444–452. [Google Scholar] [CrossRef]
- Ma, C.M.; Cardona, F.; Baglioni, V.; Panunzi, S.; Pantano, P.; Suppa, A.; Mirabella, G. Inhibition is impaired in children with obsessive-compulsive symptoms but not in those with tics. Mov. Disord. 2018, 33, 950–959. [Google Scholar] [CrossRef]
- Wylie, S.A.; Claassen, D.O.; Kanoff, K.E.; Ridderinkhof, K.R.; Wildenberg, W.P.V.D. Impaired inhibition of prepotent motor actions in patients with Tourette syndrome. J. Psychiatry Neurosci. 2013, 38, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeso, I.; Wilkinson, L.; Casabona, E.; Speekenbrink, M.; Bringas, M.L.; Álvarez, M.; Álvarez, L.; Pavón, N.; Rodríguez-Oroz, M.C.; Macías, R.; et al. The subthalamic nucleus and inhibitory control: Impact of subthalamotomy in Parkinson’s disease. Brain 2014, 137, 1470–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahanshahi, M.; Ardouin, C.M.A.; Brown, R.G.; Rothwell, J.C.; Obeso, J.; Albanese, A.; Rodriguez-Oroz, M.C.; Moro, E.; Benabid, A.L.; Pollak, P.; et al. The impact of deep brain stimulation on executive function in Parkinson’s disease. Brain 2000, 123, 1142–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabella, G.; Fragola, M.; Giannini, G.; Modugno, N.; Lakens, D.D. Inhibitory control is not lateralized in Parkinson’s patients. Neuropsychologia 2017, 102, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Manza, P.; Amandola, M.; Tatineni, V.; Li, C.-S.R.; Leung, H.-C. Response inhibition in Parkinson’s disease: A meta-analysis of dopaminergic medication and disease duration effects. npj Parkinsons Dis. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 295–327. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009, 33, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Logan, G.D.; Van Zandt, T.; Verbruggen, F.; Wagenmakers, E.-J. On the ability to inhibit thought and action: General and special theories of an act of control. Psychol. Rev. 2014, 121, 66–95. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, F.; Aron, A.R.; Band, G.P.; Beste, C.; Bissett, P.G.; Brockett, A.T.; Brown, J.W.; Chamberlain, S.R.; Chambers, C.D.; Colonius, H.; et al. A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. eLife 2019, 8. [Google Scholar] [CrossRef]
- Boucher, L.; Palmeri, T.J.; Logan, G.D.; Schall, J.D. Inhibitory control in mind and brain: An interactive race model of countermanding saccades. Psychol. Rev. 2007, 114, 376–397. [Google Scholar] [CrossRef]
- Schall, J.D.; Palmeri, T.J.; Logan, G.D. Models of inhibitory control. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160193. [Google Scholar] [CrossRef] [PubMed]
- Eichele, H.; Juvodden, H.T.; Ullsperger, M.; Eichele, T. Eichele Mal-adaptation of event-related EEG responses preceding performance errors. Front. Hum. Neurosci. 2010, 4, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabbitt, P.M. Errors and error correction in choice-response tasks. J. Exp. Psychol. 1966, 71, 264–272. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D.; Liefooghe, B.; Vandierendonck, A. Short-term aftereffects of response inhibition: Repetition priming or between-trial control adjustments? J. Exp. Psychol. Hum. Percept. Perform. 2008, 34, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Schachar, R.J.; Chen, S.; Logan, G.D.; Ornstein, T.J.; Crosbie, J.; Ickowicz, A.; Pakulak, A. Evidence for an Error Monitoring Deficit in Attention Deficit Hyperactivity Disorder. J. Abnorm. Child Psychol. 2004, 32, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Cohen, M.X.; Allen, J.J.B. Prelude to and Resolution of an Error: EEG Phase Synchrony Reveals Cognitive Control Dynamics during Action Monitoring. J. Neurosci. 2009, 29, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.X. Comparison of different spatial transformations applied to EEG data: A case study of error processing. Int. J. Psychophysiol. 2015, 97, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Tucker, D.M.; Makeig, S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clin. Neurophysiol. 2004, 115, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci. 2014, 37, 480–490. [Google Scholar] [CrossRef]
- Cohen, M.X.; Van Gaal, S. Dynamic Interactions between Large-Scale Brain Networks Predict Behavioral Adaptation after Perceptual Errors. Cereb. Cortex 2012, 23, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Cavanagh, J.F.; Shackman, A.J. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J. Physiol. 2015, 109, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, R.; Coles, M.G.H.; Logan, G.D.; Gratton, G. In search of the point of no return: The control of response processes. J. Exp. Psychol. Hum. Percept. Perform. 1990, 16, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-T.; Alsford, T.; Miller, J. Inhibitory effects on response force in the stop-signal paradigm. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Van Nguyen, T.; Hsu, C.-Y.; Jaiswal, S.; Muggleton, N.G.; Liang, W.-K.; Juan, C.-H. To Go or Not to Go: Degrees of Dynamic Inhibitory Control Revealed by the Function of Grip Force and Early Electrophysiological Indices. Front. Hum. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Carbonnell, L.; Falkenstein, M. Does the error negativity reflect the degree of response conflict? Brain Res. 2006, 1095, 124–130. [Google Scholar] [CrossRef]
- Vidal, F.; Burle, B.; Hasbroucq, T. Errors and Action Monitoring: Errare Humanum Est Sed Corrigere Possibile. Front. Hum. Neurosci. 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Goel, P.; Kopell, N.; Pohja, M.; Hari, R.; Ermentrout, B. On the human sensorimotor-cortex beta rhythm: Sources and modeling. NeuroImage 2005, 26, 347–355. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Liang, W.-K.; Lee, H.-W.; Wang, C.-H.; Tzeng, O.J.L.; Hung, D.L.; Cheng, S.-K.; Juan, C.-H. The Neural Development of Response Inhibition in 5- and 6-Year-Old Preschoolers: An ERP and EEG Study. Dev. Neuropsychol. 2013, 38, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.; Tandon, N.; Canolty, R.; Ellmore, T.M.; McEvoy, L.K.; Dreyer, S.; Disano, M.; Aron, A.R. Intracranial EEG Reveals a Time- and Frequency-Specific Role for the Right Inferior Frontal Gyrus and Primary Motor Cortex in Stopping Initiated Responses. J. Neurosci. 2009, 29, 12675–12685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, N.C.; Cai, W.; Conner, C.R.; Pieters, T.A.; Claffey, M.P.; George, J.S.; Aron, A.R.; Tandon, N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. NeuroImage 2012, 59, 2860–2870. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Y.; Bressler, S.; Ding, M. Response preparation and inhibition: The role of the cortical sensorimotor beta rhythm. Neuroscience 2008, 156, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubia, K.; Smith, A.B.; Brammer, M.J.; Taylor, E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage 2003, 20, 351–358. [Google Scholar] [CrossRef]
- Lee, H.W.; Lu, M.-S.; Chen, C.-Y.; Muggleton, N.G.; Hsu, T.-Y.; Juan, C.-H. Roles of the pre-SMA and rIFG in conditional stopping revealed by transcranial magnetic stimulation. Behav. Brain Res. 2016, 296, 459–467. [Google Scholar] [CrossRef]
- Sharp, D.J.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.F.; James, S.G.; Patel, M.C.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef] [Green Version]
- Huster, R.J.; Enriquez-Geppert, S.; Lavallee, C.F.; Falkenstein, M.; Herrmann, C.S. Electroencephalography of response inhibition tasks: Functional networks and cognitive contributions. Int. J. Psychophysiol. 2013, 87, 217–233. [Google Scholar] [CrossRef]
- Spronk, M.; Jonkman, L.M.; Kemner, C. Response inhibition and attention processing in 5- to 7-year-old children with and without symptoms of ADHD: An ERP study. Clin. Neurophysiol. 2008, 119, 2738–2752. [Google Scholar] [CrossRef]
- Huang, N.E.; Long, S.R.; Shen, Z. The Mechanism for Frequency Downshift in Nonlinear Wave Evolution. In Advances in Applied Mechanics; Elsevier: Amsterdam, The Netherlands, 1996; Volume 32, pp. 59–117. [Google Scholar]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.-C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. A Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Huang, N.E.; Wu, Z.; Long, S.R.; Arnold, K.C.; Chen, X.; Blank, K. On Instantaneous Frequency. Adv. Adapt. Data Anal. 2009, 1, 177–229. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.-K.; Lo, M.-T.; Yang, A.C.; Peng, C.-K.; Cheng, S.-K.; Tseng, P.; Juan, C.-H. Revealing the brain’s adaptability and the transcranial direct current stimulation facilitating effect in inhibitory control by multiscale entropy. NeuroImage 2014, 90, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Tsai, S.-Y.; Juan, C.-H.; Muggleton, N.G.; Liang, W.-K. Low delta and high alpha power are associated with better conflict control and working memory in high mindfulness, low anxiety individuals. Soc. Cogn. Affect. Neurosci. 2019, 14, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, J.; Ridderinkhof, K.R.; Cohen, M.X. Not All Errors Are Alike: Theta and Alpha EEG Dynamics Relate to Differences in Error-Processing Dynamics. J. Neurosci. 2012, 32, 16795–16806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, A.K.; Fries, P. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, T.; Lalo, E.; Doyle, L.; Di Lazzaro, V.; Cioni, B.; Brown, P. Existing Motor State Is Favored at the Expense of New Movement during 13-35 Hz Oscillatory Synchrony in the Human Corticospinal System. J. Neurosci. 2005, 25, 7771–7779. [Google Scholar] [CrossRef] [Green Version]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting Cortical Activity at Beta-Band Frequencies Slows Movement in Humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Joundi, R.A.; Jenkinson, N.; Brittain, J.-S.; Aziz, T.Z.; Brown, P. Driving Oscillatory Activity in the Human Cortex Enhances Motor Performance. Curr. Biol. 2012, 22, 403–407. [Google Scholar] [CrossRef] [Green Version]
- Van Belle, J.; Vink, M.; Durston, S.; Zandbelt, B.B. Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. NeuroImage 2014, 103, 65–74. [Google Scholar] [CrossRef]
- Zandbelt, B.B.; Vink, M. On the Role of the Striatum in Response Inhibition. PLoS ONE 2010, 5, e13848. [Google Scholar] [CrossRef]
- Duann, J.-R.; Ide, J.S.; Luo, X.; Li, C.-S.R. Functional Connectivity Delineates Distinct Roles of the Inferior Frontal Cortex and Presupplementary Motor Area in Stop Signal Inhibition. J. Neurosci. 2009, 29, 10171–10179. [Google Scholar] [CrossRef] [Green Version]
- Rae, C.L.; Hughes, L.E.; Anderson, M.C.; Rowe, J.B. The Prefrontal Cortex Achieves Inhibitory Control by Facilitating Subcortical Motor Pathway Connectivity. J. Neurosci. 2015, 35, 786–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachev, P.; Wydell, H.; O’Neill, K.; Husain, M.; Kennard, C. The role of the pre-supplementary motor area in the control of action. NeuroImage 2007, 36 (Suppl. 2), T155–T163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef]
- Aron, A.R.; Fletcher, P.C.; Bullmore, E.T.; Sahakian, B.J.; Robbins, T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003, 6, 115–116. [Google Scholar] [CrossRef]
- Hampshire, A.; Sharp, D. Inferior PFC Subregions Have Broad Cognitive Roles. Trends Cogn. Sci. 2015, 19, 712–713. [Google Scholar] [CrossRef]
- Benis, D.; David, O.; Lachaux, J.-P.; Seigneuret, E.; Krack, P.; Fraix, V.; Chabardès, S.; Bastin, J. Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson’s disease. NeuroImage 2014, 91, 273–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavala, B.; Damera, S.; Dong, J.W.; Lungu, C.; Brown, P.; Zaghloul, K.A. Human Subthalamic Nucleus Theta and Beta Oscillations Entrain Neuronal Firing During Sensorimotor Conflict. Cereb. Cortex 2015, 27, 496–508. [Google Scholar] [CrossRef] [Green Version]
- Wildenberg, W.P.M.V.D.; Van Boxtel, G.J.M.; Van Der Molen, M.W.; Bosch, D.A.; Speelman, J.D.; Brunia, C.H.M. Stimulation of the Subthalamic Region Facilitates the Selection and Inhibition of Motor Responses in Parkinson’s Disease. J. Cogn. Neurosci. 2006, 18, 626–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabella, G.; Iaconelli, S.; Romanelli, P.; Modugno, N.; Lena, F.; Manfredi, M.; Cantore, G. Deep Brain Stimulation of Subthalamic Nuclei Affects Arm Response Inhibition in Parkinson’s Patients. Cereb. Cortex 2011, 22, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Swann, N.; Poizner, H.; Houser, M.; Gould, S.; Greenhouse, I.; Cai, W.; Strunk, J.; George, J.; Aron, A.R. Deep Brain Stimulation of the Subthalamic Nucleus Alters the Cortical Profile of Response Inhibition in the Beta Frequency Band: A Scalp EEG Study in Parkinson’s Disease. J. Neurosci. 2011, 31, 5721–5729. [Google Scholar] [CrossRef] [Green Version]
- Mancini, C.; Modugno, N.; Santilli, M.; Pavone, L.; Grillea, G.; Morace, R.; Mirabella, G. Unilateral Stimulation of Subthalamic Nucleus Does Not Affect Inhibitory Control. Front. Neurol. 2019, 9, 1149. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Pani, P.; Ferraina, S. Neural correlates of cognitive control of reaching movements in the dorsal premotor cortex of rhesus monkeys. J. Neurophysiol. 2011, 106, 1454–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattia, M.; Pani, P.; Mirabella, G.; Costa, S.; Del Giudice, P.; Ferraina, S. Heterogeneous Attractor Cell Assemblies for Motor Planning in Premotor Cortex. J. Neurosci. 2013, 33, 11155–11168. [Google Scholar] [CrossRef] [PubMed]
- Coxon, J.P.; Stinear, C.M.; Byblow, W.D. Intracortical Inhibition During Volitional Inhibition of Prepared Action. J. Neurophysiol. 2006, 95, 3371–3383. [Google Scholar] [CrossRef] [PubMed]
- Mattia, M.; Spadacenta, S.; Pavone, L.; Quarato, P.; Esposito, V.; Sparano, A.; Sebastiano, F.; Di Gennaro, G.; Morace, R.; Cantore, G.; et al. Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements. Front. Neuroeng. 2012, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, T.; Shimoyama, I.; Ito, T.; Abla, D.; Iwasa, H.; Koseki, K.; Yamanouchi, N.; Sato, T.; Nakajima, Y. Event-related dynamics of the gamma-band oscillation in the human brain: Information processing during a GO/NOGO hand movement task. Neurosci. Res. 1999, 33, 215–222. [Google Scholar] [CrossRef]
- Kaiser, J.; Simon, N.A.; Sauseng, P.; Schütz-Bosbach, S. Midfrontal neural dynamics distinguish between general control and inhibition-specific processes in the stopping of motor actions. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.S.; Darriba, Á.; Karayanidis, F.; Barceló, F. Contextually sensitive power changes across multiple frequency bands underpin cognitive control. NeuroImage 2016, 132, 499–511. [Google Scholar] [CrossRef]
- Xu, K.Z.; Anderson, B.A.; Emeric, E.E.; Sali, A.W.; Stuphorn, V.; Yantis, S.; Courtney, S.M. Neural Basis of Cognitive Control over Movement Inhibition: Human fMRI and Primate Electrophysiology Evidence. Neuron 2017, 96, 1447–1458. [Google Scholar] [CrossRef] [Green Version]
- Hampshire, A.; Highfield, R.R.; Parkin, B.L.; Owen, A.M. Fractionating Human Intelligence. Neuron 2012, 76, 1225–1237. [Google Scholar] [CrossRef] [Green Version]
- Kok, A.; Ramautar, J.R.; De Ruiter, M.B.; Band, G.P.H.; Ridderinkhof, K.R. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Zambrano-Vazquez, L.; Allen, J.J.B. Theta lingua franca: A common mid-frontal substrate for action monitoring processes. Psychophysiology 2011, 49, 220–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, M.X. Error-related medial frontal theta activity predicts cingulate-related structural connectivity. NeuroImage 2011, 55, 1373–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, X.; Wang, Y.; Sun, J.; Li, C.; Tong, S. Segregating Top-Down Selective Attention from Response Inhibition in a Spatial Cueing Go/NoGo Task: An ERP and Source Localization Study. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Benedek, M.; Bergner, S.; Könen, T.; Fink, A.; Neubauer, A.C. EEG alpha synchronization is related to top-down processing in convergent and divergent thinking. Neuropsychologia 2011, 49, 3505–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavone, E.F.; Tieri, G.; Rizza, G.; Tidoni, E.; Grisoni, L.; Aglioti, S.M. Embodying Others in Immersive Virtual Reality: Electro-Cortical Signatures of Monitoring the Errors in the Actions of an Avatar Seen from a First-Person Perspective. J. Neurosci. 2016, 36, 268–279. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.V.; Balachandran, P.; Muggleton, N.G.; Liang, W.-K.; Juan, C.-H. Dynamical EEG Indices of Progressive Motor Inhibition and Error-Monitoring. Brain Sci. 2021, 11, 478. https://doi.org/10.3390/brainsci11040478
Nguyen TV, Balachandran P, Muggleton NG, Liang W-K, Juan C-H. Dynamical EEG Indices of Progressive Motor Inhibition and Error-Monitoring. Brain Sciences. 2021; 11(4):478. https://doi.org/10.3390/brainsci11040478
Chicago/Turabian StyleNguyen, Trung Van, Prasad Balachandran, Neil G. Muggleton, Wei-Kuang Liang, and Chi-Hung Juan. 2021. "Dynamical EEG Indices of Progressive Motor Inhibition and Error-Monitoring" Brain Sciences 11, no. 4: 478. https://doi.org/10.3390/brainsci11040478





