Chewing Behavior Attenuates the Tumor Progression-Enhancing Effects of Psychological Stress in a Breast Cancer Model Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal and Xenograft Breast Cancer Model
2.3. Tumor Samples Collection and Serum Corticosterone Assay
2.4. Immunohistochemistry
2.5. Western Blot Analyses
2.6. Statistical Analysis
3. Results
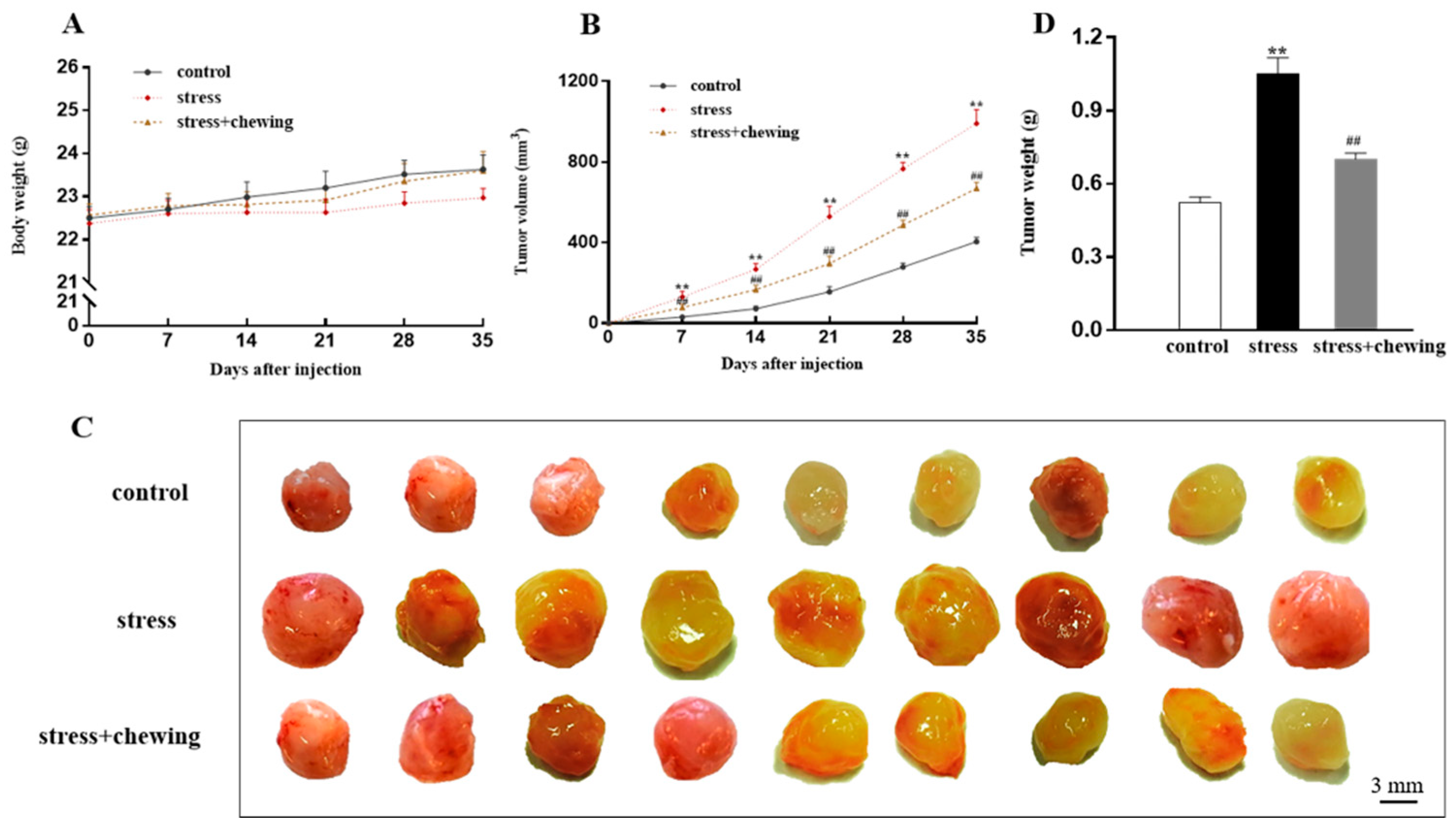
3.1. Chewing Behavior, Psychological Stress, and Tumor Growth
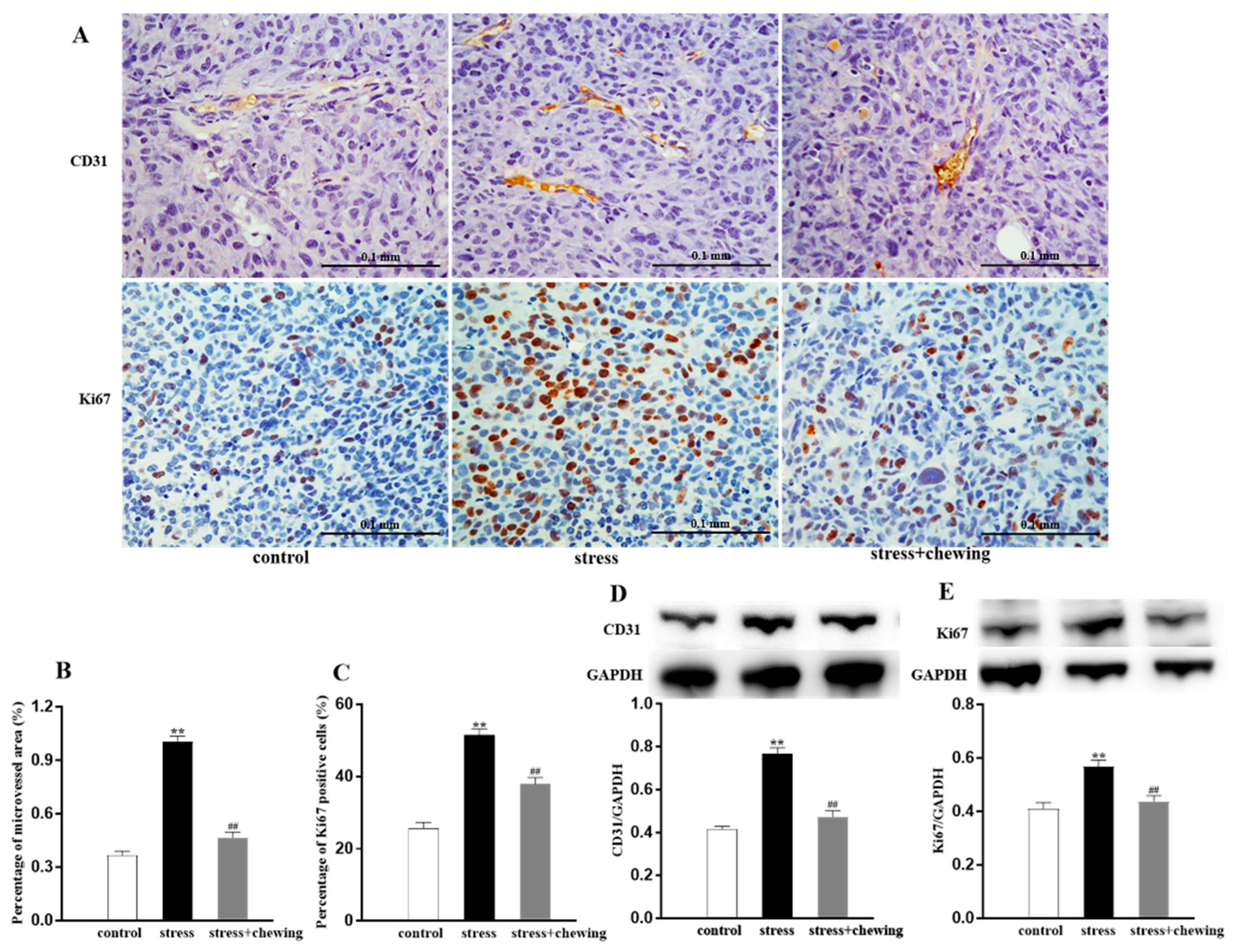
3.2. Chewing Behavior, Psychological Stress, and Tumor Angiogenesis and Proliferation
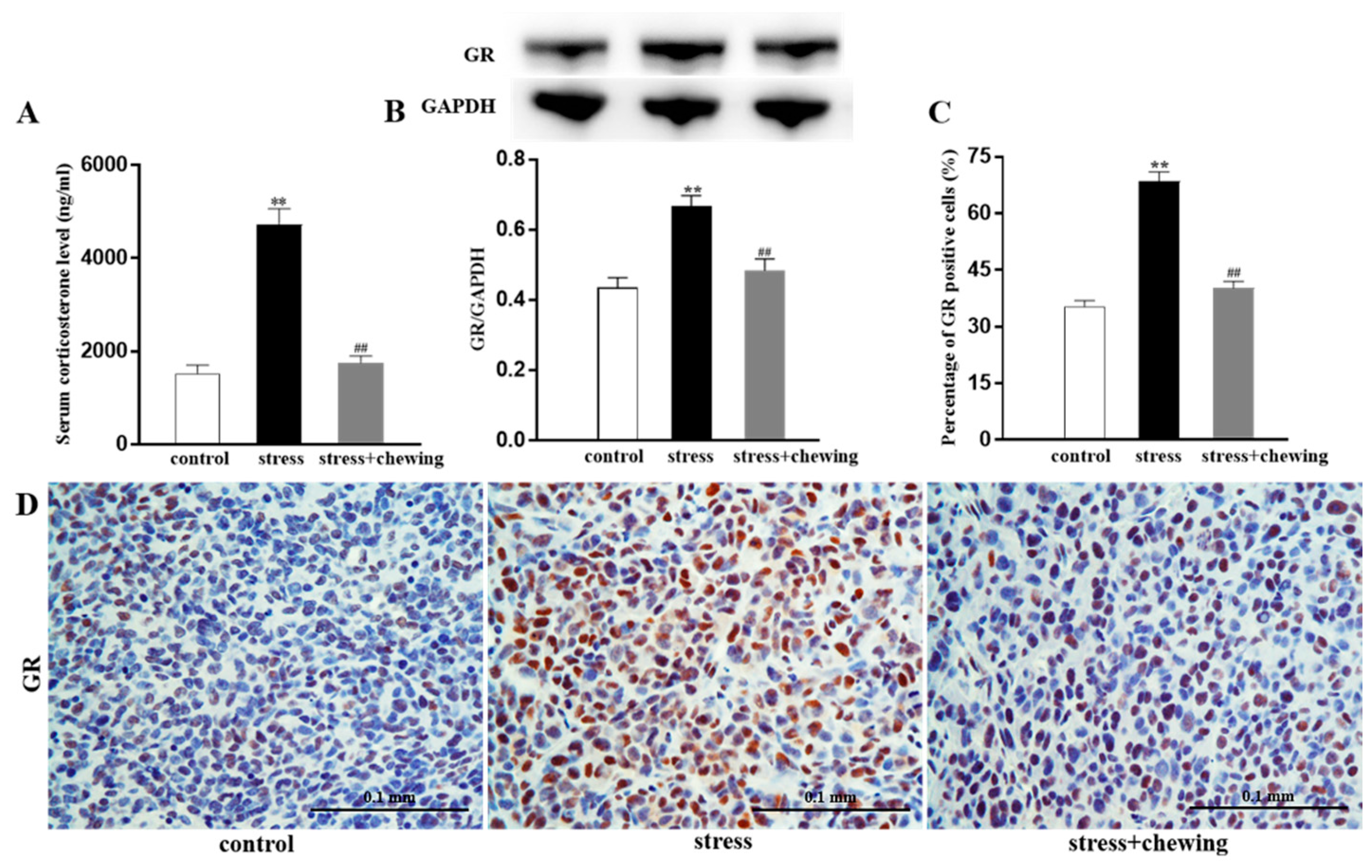
3.3. Chewing Behavior, Psychological Stress, Serum Corticosterone Levels, and Glucocorticoid Receptor Expression in the Tumor
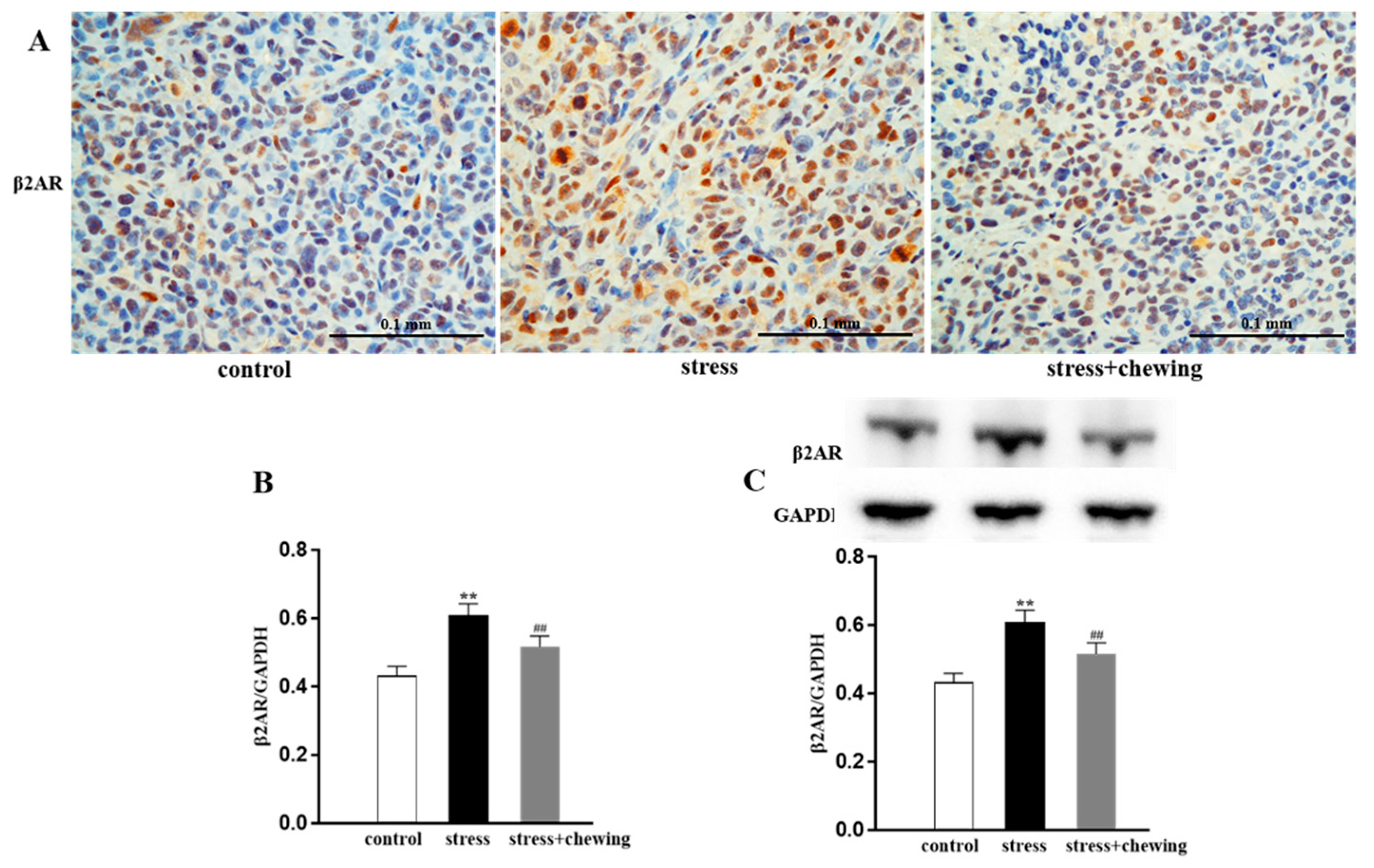
3.4. Chewing Behavior, Psychological Stress, and β2-Adrenergic Receptor Expression in the Tumor
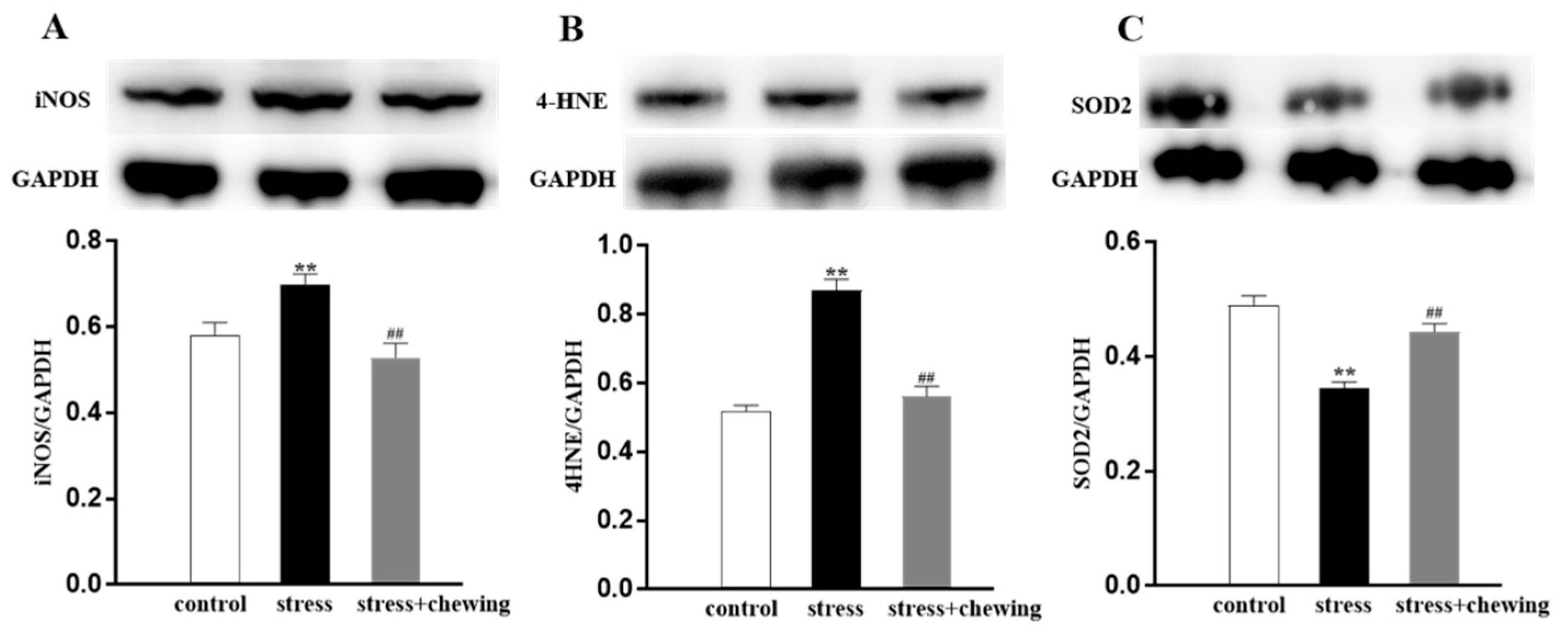
3.5. Chewing Behavior, Psychological Stress, and Oxidative Stress in the Tumor
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Narod, S.A.; Foulkes, W.D. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 2004, 4, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Luo, Y.; Tian, P.; Peng, F.; Lu, J.; Yang, Y.; Su, Q.; Liu, B.; Yu, J.; Luo, X.; et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Investig. 2019, 129, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef]
- Azuma, K.; Adachi, Y.; Hayashi, H.; Kubo, K.Y. Chronic Psychological Stress as a Risk Factor of Osteoporosis. J. UOEH 2015, 37, 245–253. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.; Tremblay, M.E. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Chiarelli, F. The effects of acute and chronic stress on diabetes control. Sci. Signal 2012, 5, pt10. [Google Scholar] [CrossRef] [PubMed]
- Proietti, R.; Mapelli, D.; Volpe, B.; Bartoletti, S.; Sagone, A.; Dal Bianco, L.; Daliento, L. Mental stress and ischemic heart disease: Evolving awareness of a complex association. Future Cardiol. 2011, 7, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006, 12, 939–944. [Google Scholar] [CrossRef]
- Xie, H.; Li, C.; He, Y.; Griffin, R.; Ye, Q.; Li, L. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 2015, 51, 991–997. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, J.; Liang, F.; Li, A.; Zhang, Y.; Sun, J. Effect of Chronic Psychological Stress on Liver Metastasis of Colon Cancer in Mice. PLoS ONE 2015, 10, e0139978. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Le, C.P.; Walker, A.K.; Creed, S.J.; Pon, C.K.; Albold, S.; Carroll, D.; Halls, M.L.; Lane, J.R.; Riedel, B.; et al. beta2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav. Immun. 2016, 57, 106–115. [Google Scholar] [CrossRef]
- Furuzawa, M.; Chen, H.; Fujiwara, S.; Yamada, K.; Kubo, K.Y. Chewing ameliorates chronic mild stress-induced bone loss in senescence-accelerated mouse (SAMP8), a murine model of senile osteoporosis. Exp. Gerontol. 2014, 55, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Obradović, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.M.; Munst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids promote breast cancer metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.L.; Intabli, H.; Falcinelli, M.; Bucca, G.; Hesketh, A.; Patel, B.A.; Allen, M.C.; Smith, C.P.; Flint, M.S. Stress hormone-mediated acceleration of breast cancer metastasis is halted by inhibition of nitric oxide synthase. Cancer Lett. 2019, 459, 59–71. [Google Scholar] [CrossRef]
- Flaherty, R.L.; Owen, M.; Fagan-Murphy, A.; Intabli, H.; Healy, D.; Patel, A.; Allen, M.C.; Patel, B.A.; Flint, M.S. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017, 19, 35. [Google Scholar] [CrossRef]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef]
- Li, Y.P.; Tian, F.G.; Shi P., C.; Guo, L.Y.; Wu, H.M.; Chen, R.Q.; Xue, J.M. 4-Hydroxynonenal promotes growth and angiogenesis of breast cancer cells through HIF-1α stabilization. Asian Pac. J. Cancer Prev. 2014, 15, 10151–10156. [Google Scholar] [CrossRef]
- Breitzig, M.; Bhimineni, C.; Lockey, R.; Kolliputi, N. 4-Hydroxy-2-nonenal: A critical target in oxidative stress? Am. J. Physiol. Cell Physiol. 2016, 311, C537–C543. [Google Scholar] [CrossRef]
- Kim, Y.S.; Vallur, P.G.; Phaëton, R.; Mythreye, K.; Hempe, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef]
- Chang, C.F.; Diers, A.R.; Hogg, N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic. Biol. Med. 2015, 79, 324–336. [Google Scholar] [CrossRef]
- Azuma, K.; Zhou, Q.; Niwa, M.; Kubo, K.Y. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1687. [Google Scholar] [CrossRef]
- Kubo, K.Y.; Iinuma, M.; Chen, H. Mastication as a Stress-Coping Behavior. Biomed. Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef]
- Smith, A. Effects of chewing gum on stress and health: A replication and investigation of dose-response. Stress Health 2013, 29, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Haskell, C.; Robertson, B.; Kennedy, D.; Milne, A.; Wetherell, M. Chewing gum alleviates negative mood and reduces cortisol during acute laboratory psychological stress. Physiol. Behav. 2009, 97, 304–312. [Google Scholar] [CrossRef]
- Azuma, K.; Furuzawa, M.; Fujiwara, S.; Yamada, K.; Kubo, K.Y. Effects of Active Mastication on Chronic Stress-Induced Bone Loss in Mice. Int. J. Med. Sci. 2015, 12, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Minamisawa, S.; Sasaguri, K.; Onozuka, M.; Sato, S.; Ono, Y. Chewing reduces sympathetic nervous response to stress and prevents poststress arrhythmias in rats. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1551–H1558. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, A.; Kikuchi, M.; Nakanishi, K.; Ueda, T.; Yamashita, S.; Sakurai, K. Psychological stress-relieving effects of chewing—Relationship between masticatory function-related factors and stress-relieving effects. J. Prosthodont. Res. 2018, 62, 50–55. [Google Scholar] [CrossRef]
- Gannon, A.L.; O’Hara, L.; Mason, J.I.; Rebourcet, D.; Smith, S.; Traveres, A.; Alcaide-Corral, C.J.; Frederiksen, H.; Jorgensen, A.; Milne, L.; et al. Ablation of glucocorticoid receptor in the hindbrain of the mouse provides a novel model to investigate stress disorders. Sci. Rep. 2019, 9, 3250. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Marrero, L.; Rodriguez, P.; Del Valle, L.; Ochoa, A.; Cui, Y. Trp53 inactivation in the tumor microenvironment promotes tumor progression by expanding the immunosuppressive lymphoid-like stromal network. Cancer Res. 2013, 73, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xiao, J.; Zhou, Z.; Wu, J.; Ge, F.; Li, Z.; Zhang, H.; Sun, J.; Li, F.; Liu, R.; et al. Hypoxia induces miR-153 through the IRE1α-XBP1 pathway to fine tune the HIF1α/VEGFA axis in breast cancer angiogenesis. Oncogene 2018, 37, 1961–1975. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Mena, J.; Hernandez-Unzueta, I.; Benedicto, A.; Sanz, E.; Arteta, B.; Olaso, E. Ocoxin® oral solution slows down tumor growth in an experimental model of colorectal cancer metastasis to the liver in Balb/c mice. Oncol. Rep. 2016, 35, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Liu, J.; Deng, G.H.; Zhang, J.; Wang, Y.; Xia, X.Y.; Luo, X.M.; Deng, Y.T.; He, S.S.; Mao, Y.Y.; Peng, X.C.; et al. The effect of chronic stress on anti-angiogenesis of sunitinib in colorectal cancer models. Psychoneuroendocrinology 2015, 52, 130–142. [Google Scholar] [CrossRef]
- Zhou, Q.; Suzuki, A.; Iinuma, M.; Wang, K.Y.; Kubo, K.Y.; Azuma, K. Effects of Maternal Chewing on Prenatal Stress-Induced Cognitive Impairments in the Offspring via Multiple Molecular Pathways. Int. J. Mol. Sci. 2020, 21, 5627. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- He, L.; Yuan, L.; Sun, Y.; Wang, P.; Zhang, H.; Feng, X.; Wang, Z.; Zhang, W.; Yang, C.; Zeng, Y.A.; et al. Glucocorticoid Receptor Signaling Activates TEAD4 to Promote Breast Cancer Progression. Cancer Res. 2019, 79, 4399–4411. [Google Scholar] [CrossRef]
- Puhr, M.; Hoefer, J.; Eigentler, A.; Ploner, C.; Handle, F.; Schaefer, G.; Kroon, J.; Leo, A.; Heidegger, I.; Eder, I.; et al. The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clin. Cancer Res. 2018, 24, 927–938. [Google Scholar] [CrossRef]
- Ogara, M.F.; Rodriguez-Segui, S.A.; Marini, M.; Nacht, A.S.; Stortz, M.; Levi, V.; Presman, D.M.; Vicent, G.P.; Pecci, A. The glucocorticoid receptor interferes with progesterone receptor-dependent genomic regulation in breast cancer cells. Nucleic Acids Res. 2019, 47, 10645–10661. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, G.; Ruggeri, N.; Zannini, A.; Ingallina, E.; Bertolio, R.; Marotta, C.; Neri, C.; Cappuzzello, E.; Forcato, M.; Rosato, A.; et al. Glucocorticoid receptor signalling activates YAP in breast cancer. Nat. Commun. 2017, 8, 14073. [Google Scholar] [CrossRef]
- Fukushima-Nakayama, Y.; Ono, T.; Hayashi, M.; Inoue, M.; Wake, H.; Ono, T.; Nakashima, T. Reduced Mastication Impairs Memory Function. J. Dent. Res. 2017, 96, 1058–1066. [Google Scholar] [CrossRef]
- Miyake, S.; Yoshikawa, G.; Yamada, K.; Sasaguri, K.; Yamamoto, T.; Onozuka, M.; Sato, S. Chewing ameliorates stress-induced suppression of spatial memory by increasing glucocorticoid receptor expression in the hippocampus. Brain Res. 2012, 1446, 34–39. [Google Scholar] [CrossRef]
- Reiche, E.M.; Nunes, S.O.; Morimoto, H.K. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004, 5, 617–625. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Qin, J.F.; Jin, F.J.; Li, N.; Guan, H.T.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Creed, S.J.; Le, C.P.; Hassan, M.; Pon, C.K.; Albold, S.; Chan, K.T.; Berginski, M.E.; Huang, Z.; Bear, J.E.; Lane, J.R.; et al. β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015, 17, 145. [Google Scholar] [CrossRef]
- Zhi, X.; Li, B.; Li, Z.; Zhang, J.; Yu, J.; Zhang, L.; Xu, Z. Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int. J. Oncol. 2019, 54, 1625–1638. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Zhang, L.; Hu, X.; Wang, Z.; Ni, H.; Wang, Y.; Qin, J. Activation of β2-Adrenergic Receptor Promotes Growth and Angiogenesis in Breast Cancer by Down-regulating PPARγ. Cancer Res. Treat. 2020, 52, 830–847. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Guo, L.; Cheng, X.; Guo, N.; Shi, M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J. Pathol. 2018, 244, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Lee, S.B.; Won, J.; Choi, H.Y.; Kim, K.; Yang, G.M.; Dayem, A.A.; Cho, S.G. Correlation between Oxidative Stress, Nutrition, and Cancer Initiation. Int. J. Mol. Sci. 2017, 18, 1544. [Google Scholar] [CrossRef]
- Canli, O.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Lower, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883 e5. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef]
- Thomas, S.R.; Chen, K.; Keaney, J.F., Jr. Oxidative stress and endothelial nitric oxide bioactivity. Antioxid. Redox Signal 2003, 5, 181–194. [Google Scholar] [CrossRef]
- Salimian Rizi, B.; Achreja, A.; Nagrath, D. Nitric Oxide: The Forgotten Child of Tumor Metabolism. Trends Cancer 2017, 3, 659–672. [Google Scholar] [CrossRef]
- Singh, A.K.; Awasthi, D.; Dubey, M.; Nagarkoti, S.; Kumar, A.; Chandra, T.; Barthwal, M.K.; Tripathi, A.K.; Dikshit, M. High oxidative stress adversely affects NFκB mediated induction of inducible nitric oxide synthase in human neutrophils: Implications in chronic myeloid leukemia. Nitric Oxide 2016, 58, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Kenne Sarenmalm, E.; Mårtensson, L.B.; Andersson, B.A.; Karlsson, P.; Bergh, I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017, 6, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, H.; Zheng, Y. Effectiveness of mindfulness-based stress reduction (MBSR) on symptom variables and health-related quality of life in breast cancer patients-a systematic review and meta-analysis. Support Care Cancer 2019, 27, 771–781. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Manufacturer | Product Number | Dilution (Western Bolt (WB)) | Dilution (Immunohistochemistry (IH)) |
|---|---|---|---|---|
| GAPDH | Cell Signaling Technology | 2118 | 1:1000 | − |
| CD31 | Abcam | ab28364 | 1:500 | 1:300 |
| Ki67 | Abcam | ab16667 | 1:1000 | 1:200 |
| GR | Cell Signaling Technology | 12041 | 1:1000 | 1:400 |
| β2AR | Abcam | ab135641 | 1:100 | 1:50 |
| iNOS | Thermo Scientific | PA3-030A | 1:2000 | − |
| SOD2 | Cell Signaling Technology | 13141 | 1:1000 | − |
| 4HNE | Japan Institute for the Control of Aging | MHN-100P | 100 µg/mL | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Katano, M.; Zhang, J.-H.; Liu, X.; Wang, K.-Y.; Iinuma, M.; Kubo, K.-y.; Azuma, K. Chewing Behavior Attenuates the Tumor Progression-Enhancing Effects of Psychological Stress in a Breast Cancer Model Mouse. Brain Sci. 2021, 11, 479. https://doi.org/10.3390/brainsci11040479
Zhou Q, Katano M, Zhang J-H, Liu X, Wang K-Y, Iinuma M, Kubo K-y, Azuma K. Chewing Behavior Attenuates the Tumor Progression-Enhancing Effects of Psychological Stress in a Breast Cancer Model Mouse. Brain Sciences. 2021; 11(4):479. https://doi.org/10.3390/brainsci11040479
Chicago/Turabian StyleZhou, Qian, Masahisa Katano, Jia-He Zhang, Xiao Liu, Ke-Yong Wang, Mitsuo Iinuma, Kin-ya Kubo, and Kagaku Azuma. 2021. "Chewing Behavior Attenuates the Tumor Progression-Enhancing Effects of Psychological Stress in a Breast Cancer Model Mouse" Brain Sciences 11, no. 4: 479. https://doi.org/10.3390/brainsci11040479
APA StyleZhou, Q., Katano, M., Zhang, J.-H., Liu, X., Wang, K.-Y., Iinuma, M., Kubo, K.-y., & Azuma, K. (2021). Chewing Behavior Attenuates the Tumor Progression-Enhancing Effects of Psychological Stress in a Breast Cancer Model Mouse. Brain Sciences, 11(4), 479. https://doi.org/10.3390/brainsci11040479






