Are the Acute Effects of THC Different in Aging Adults?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.1.1. Baseline Session
2.1.2. Follow-Up Session
2.2. Measures
2.2.1. Demographics and Substance Use
2.2.2. Assessment of Blood Cannabinoids Levels
2.2.3. Cognitive Performance
2.2.4. Subjective Intoxication
2.2.5. Subjective Craving
2.2.6. Subjective Anxiety
2.2.7. Subjective Dizziness
2.3. Statistical Analysis
3. Results
3.1. Sample Characteristics
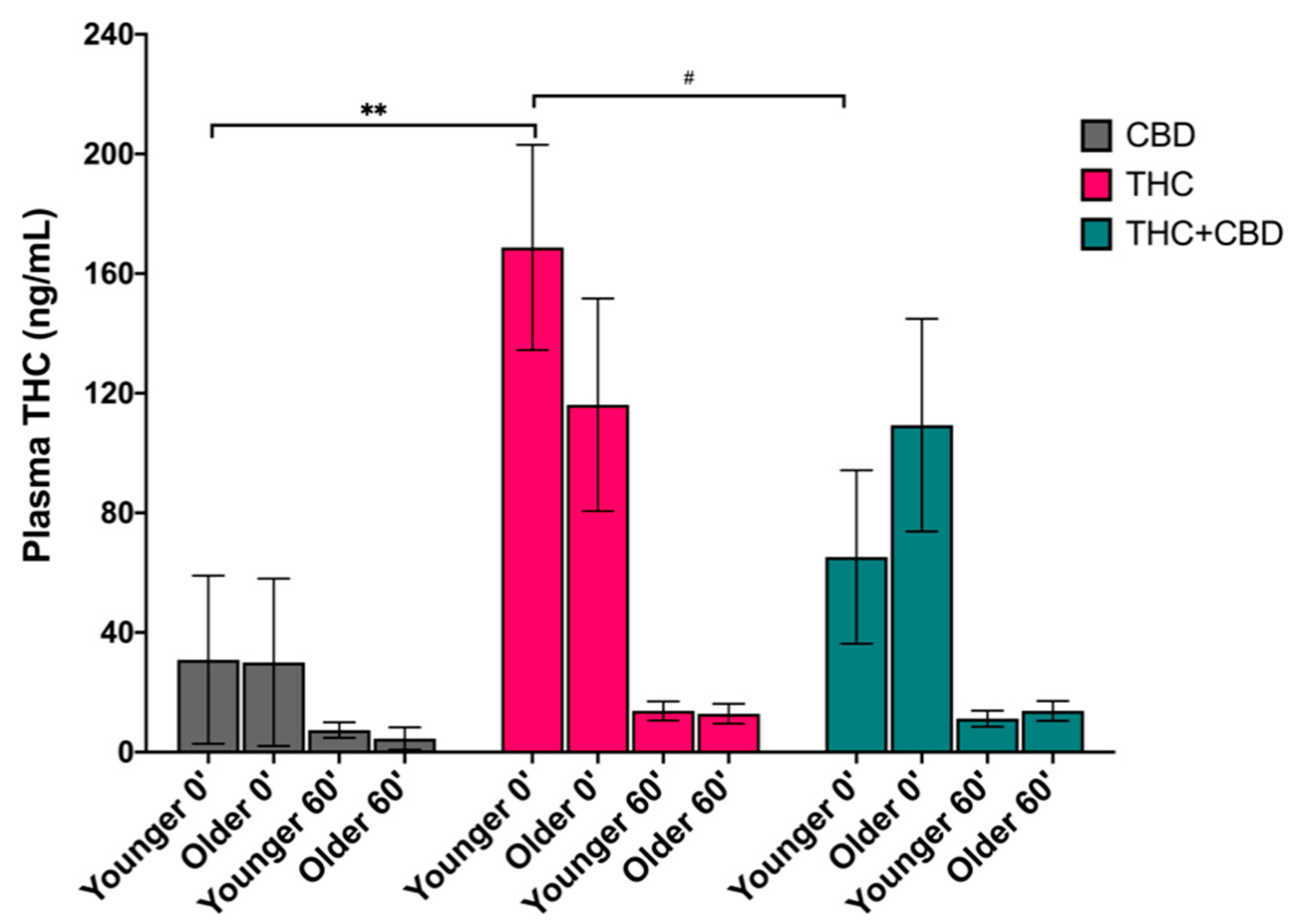
3.2. Effects of Age on Blood Plasma THC Levels
3.3. Effects of Age and Chemovar on Cognitive Performance
3.4. Effects of Age and Chemovar on Subjective Responses
3.4.1. Intoxication
3.4.2. Craving
3.4.3. Anxiety
3.4.4. Dizziness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bobitt, J.; Qualls, S.H.; Schuchman, M.; Wickersham, R.; Lum, H.D.; Arora, K.; Milavetz, G.; Kaskie, B. Qualitative Analysis of Cannabis Use Among Older Adults in Colorado. Drugs Aging 2019, 36, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.A.; Mitzner, T.L. Envisioning the future for older adults: Autonomy, health, well-being, and social connectedness with technology support. Futures 2017, 87, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Branches, P.; Donaghue, T.; Gravity, D.; Gravity, D.; Ridge, O. Effects of marihuana on the solution of anagrams, memory and appetite. Nature 1971, 231, 260–261. [Google Scholar]
- Heishman, S.J.; Stitzer, M.L.; Yingling, J.E. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol. Biochem. Behav. 1989, 34, 173–179. [Google Scholar] [CrossRef]
- Lundqvist, T. Cognitive consequences of cannabis use: Comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol. Biochem. Behav. 2005, 81, 319–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganathan, M.; D’Souza, D.C. The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology 2006, 188, 425–444. [Google Scholar] [CrossRef]
- Chait, L.D.; Evans, S.M.; Grant, K.A.; Kamien, J.B.; Johanson, C.E.; Schuster, C.R. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology 1988, 94, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Chait, L.D.; Zacny, J.P. Reinforcing and subjective effects of oral Δ9-THC and smoked marijuana in humans. Psychopharmacology 1992, 107, 255–262. [Google Scholar] [CrossRef]
- Heishman, S.J.; Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Acute and residual effects of marijuana: Profiles of plasma THC levels, physiological, subjective, and performance measures. Pharmacol. Biochem. Behav. 1990, 37, 561–565. [Google Scholar] [CrossRef]
- Schacht, J.P.; Selling, R.E.; Hutchison, K.E. Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology 2008, 203, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.C.; Slomiak, S.T.; Jones, J.D.; Rosen, A.F.G.; Moore, T.M.; Gur, R.C. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry 2018, 75, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Sarne, Y. THC for age-related cognitive decline? Aging 2018, 10, 3628–3629. [Google Scholar] [CrossRef] [PubMed]
- Sarne, Y.; Toledano, R.; Rachmany, L.; Sasson, E.; Doron, R. Reversal of age-related cognitive impairments in mice by an extremely low dose of tetrahydrocannabinol. Neurobiol. Aging 2018, 61, 177–186. [Google Scholar] [CrossRef] [PubMed]
- YorkWilliams, S.L.; Gibson, L.P.; Gust, C.J.; Giordano, G.; Hutchison, K.E.; Bryan, A.D. Exercise Intervention Outcomes with Cannabis Users and Nonusers Aged 60 and Older. Am. J. Health Behav. 2020, 44, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Fiske, A.; Wetherell, J.L.; Gatz, M. Depression in Older Adults. Annu. Rev. Clin. Psychol. 2009, 5, 363–389. [Google Scholar] [CrossRef] [PubMed]
- Cravello, L.; Di Santo, S.; Varrassi, G.; Benincasa, D.; Marchettini, P.; De Tommaso, M.; Shofany, J.; Assogna, F.; Perotta, D.; Palmer, K.; et al. Chronic Pain in the Elderly with Cognitive Decline: A Narrative Review. Pain Ther. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vadasz, Z.; Haj, T.; Kessel, A.; Toubi, E. Age-related autoimmunity. BMC Med. 2013, 11, 94. [Google Scholar] [CrossRef]
- Simone, M.J.; Tan, Z.S. The Role of Inflammation in the Pathogenesis of Delirium and Dementia in Older Adults: A Review. CNS Neurosci. Ther. 2010, 17, 506–513. [Google Scholar] [CrossRef]
- Lupica, C.R.; Riegel, A.C.; Hoffman, A.F. Marijuana and cannabinoid regulation of brain reward circuits. Br. J. Pharmacol. 2004, 143, 227–234. [Google Scholar] [CrossRef]
- Ramaekers, J.; Moeller, M.; Van Ruitenbeek, P.; Theunissen, E.; Schneider, E.; Kauert, G. Cognition and motor control as a function of Δ9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol. Depend. 2006, 85, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, J.G.; Kauert, G.; Theunissen, E.L.; Toennes, S.W.; Moeller, M.R. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J. Psychopharmacol. 2008, 23, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Sherman, S.; Mauro, P.M.; Martins, S.S.; Rotenberg, J.; Palamar, J.J. Demographic trends among older cannabis users in the United States, 2006–2013. Addiction 2017, 112, 516–525. [Google Scholar] [CrossRef]
- Lloyd, S.L.; Striley, C.W. Marijuana Use Among Adults 50 Years or Older in the 21st Century. Gerontol. Geriatr. Med. 2018, 4, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, B.H.; Palamar, J.J. Trends in Cannabis Use Among Older Adults in the United States, 2015-2018. JAMA Intern. Med. 2020, 180, 609–611. [Google Scholar] [CrossRef]
- Stephens, R.S.; Roffman, R.A.; Curtin, L. Comparison of extended versus brief treatments for marijuana use. J. Consult. Clin. Psychol. 2000, 68, 898–908. [Google Scholar] [CrossRef]
- Sobell, L.C.; Sobell, M.B. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In Measuring Alcohol Consumption: Psychosocial and Biochemical Methods; Humana Press: Totowa, NJ, USA, 1992; pp. 41–72. [Google Scholar]
- Klawitter, J.; Sempio, C.; Mörlein, S.; Bloois, E.D.; Klepacki, J.; Henthorn, T.; Leehey, M.A.; Hoffenberg, E.J.; Knupp, K.; Wang, G.S.; et al. An Atmospheric Pressure Chemical Ionization MS/MS Assay Using Online Extraction for the Analysis of 11 Cannabinoids and Metabolites in Human Plasma and Urine. Ther. Drug Monit. 2017, 39, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition assessment using the NIH Toolbox. Neurology 2013, 80, S54–S64. [Google Scholar] [CrossRef] [Green Version]
- Shacham, S. A Shortened Version of the Profile of Mood States. J. Pers. Assess. 1983, 47, 305–306. [Google Scholar] [CrossRef]
- Matheson, J.; Mann, R.E.; Sproule, B.; Huestis, M.A.; Wickens, C.M.; Stoduto, G.; George, T.P.; Rehm, J.; Le Foll, B.; Brands, B. Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol. Biochem. Behav. 2020, 194, 172937. [Google Scholar] [CrossRef]
- Wolfe, D.; Corace, K.; Rice, D.; Smith, A.; Kanji, S.; Conn, D.; Willows, M.; Garber, G.E.; Puxty, J.; Moghadam, E.; et al. Effects of medical and non-medical cannabis use in older adults: Protocol for a scoping review. BMJ Open 2020, 10, e034301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minerbi, A.; Häuser, W.; Fitzcharles, M.-A. Medical Cannabis for Older Patients. Drugs Aging 2019, 36, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Gorey, C.; Kuhns, L.; Smaragdi, E.; Kroon, E.; Cousijn, J. Age-related differences in the impact of cannabis use on the brain and cognition: A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 37–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leishman, E.; Murphy, M.; Mackie, K.; Bradshaw, H.B. Δ9-Tetrahydrocannabinol changes the brain lipidome and transcriptome differentially in the adolescent and the adult. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 479–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mato, S.; Pazos, A. Influence of age, postmortem delay and freezing storage period on cannabinoid receptor density and functionality in human brain. Neuropharmacology 2004, 46, 716–726. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.G.; Ramos, M.L.S.; Amaro, A.J.; Dias, R.A.; Vieira, S.I. Gi/o-Protein Coupled Receptors in the Aging Brain. Front. Aging Neurosci. 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [Green Version]



| Characteristic [Mean (SD)] | Overall (n = 86) | CBD (n = 26) | THC (n = 30) | THC + CBD (n = 30) | ||||
|---|---|---|---|---|---|---|---|---|
| Younger (n = 54) | Older (n = 32) | Younger (n = 17) | Older (n = 9) | Younger (n = 18) | Older (n = 12) | Younger (n = 19) | Older (n = 11) | |
| Demographics | ||||||||
| Age | 22.45 (1.37) | 63.13 (4.66) *** | 22.94 (1.39) | 65 (4.66) | 22.11 (1.13) | 61.33 (5.03) | 22.42 (1.5) | 63.55 (3.85) |
| Gender (% male) | 66.7% | 59.4% | 58.8% | 66.7% | 72.8% | 58.3% | 68.4% | 54.5% |
| Race (% white) | 79.6% | 93.8% | 82.4% | 100% | 77.8% | 91.7% | 78.9% | 90.9% |
| Substance Use | ||||||||
| Age of onset | 17.41 (2.25) | 27.22 (16.25) ** | 17.65 (2.06) | 24.33 (14.83) | 16.33 (1.94) | 27 (16.64) | 18.21 (2.39) | 29.82 (17.96) |
| CUD Score | 3.59 (2.62) | 1.53 (2.38) *** | 4.18 (2.69) | 1 (1.11) | 3.89 (2.52) | 1.25 (0.62) | 2.79 (2.57) | 2.27 (3.9) |
| Cannabis use days a | 23.39 (6.97) | 22.28 (8.14) | 24.65 (6.16) | 21.67 (10.52) | 23.06 (7.84) | 21.17 (8.93) | 22.58 (7) | 24 (4.89) |
| Flower use days a | 18.44 (9.94) | 18.09 (11.13) | 18.06 (10.46) | 20.89 (10.16) | 18.61 (9.57) | 12.58 (12.62) | 18.63 (10.33) | 21.82 (8.21) |
| Edible use days a | 0.74 (1.5) | 5.91 (10.47) ** | 0.71 (0.92) | 4.22 (9.65) | 1 (2.09) | 7.25 (10.75) | 0.53 (1.31) | 5.82 (11.53) |
| Concentrate use days a | 7.56 (9.81) | 4.50 (9.02) | 10.29 (10.65) | 1.11 (2.61) | 7.28 (9.69) | 7.25 (11.27) | 5.37 (9.01) | 4.55 (9.35) |
| Follow-up Pre-Use | ||||||||
| PSM b | 109.04 (20.67) | 112.96 (16.45) | 110.31 (19.37) | 104.67 (19.09) | 108.19 (17.39) | 119.4 (13.67) | 108.68 (24.88) | 111.22 (16.35) |
| PCPS b | 125.67 (17.44) | 115.2 (13.59) | 130.5 (13.67) | 119.5 (9.64) | 125.19 (15.96) | 116.9 (15.11) | 122 (21.02) | 110.44 (14.01) |
| DCCS b | 108.33 (10.68) | 107.68 (12.95) | 108 (10.63) | 105.33 (13.95) | 105.75 (9.61) | 117.3 (9) | 110.79 (11.54) | 98.56 (8.77) |
| FICA b | 98.78 (15.96) | 99.76 (10.79) | 102.69 (18.96) | 106.17 (8.63) | 96.88 (17.86) | 96.6 (11.77) | 97.11 (11.09) | 99 (10.13) |
| Craving c | 2.34 (2.43) | 1.4 (2.02) | 3.28 (2.77) | 0.69 (1.08) | 1.93 (2.02) | 2.1 (1.89) | 1.92 (2.35) | 1.34 (2.6) |
| Anxiety d | 0.37 (0.6) | 0.31 (0.3) | 0.64 (0.93) | 0.42 (0.43) | 0.31 (0.32) | 0.35 (0.23) | 0.17 (0.2) | 0.18 (0.24) |
| THC (ng/mL) | 6.14 (9.65) | 4.58 (8.79) | 5.42 (6) | 2.68 (5.49) | 7.57 (14.31) | 5.89 (12) | 5.46 (2.93) | 4.69 (7.17) |
| Grams used | 0.31 (0.33) | 0.23 (0.27) | 0.39 (0.38) | 0.11 (0.06) | 0.23 (0.29) | 0.32 (0.33) | 0.31 (0.28) | 0.23 (0.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, R.L.; Ellingson, J.M.; Bidwell, L.C.; Bryan, A.D.; Hutchison, K.E. Are the Acute Effects of THC Different in Aging Adults? Brain Sci. 2021, 11, 590. https://doi.org/10.3390/brainsci11050590
Mueller RL, Ellingson JM, Bidwell LC, Bryan AD, Hutchison KE. Are the Acute Effects of THC Different in Aging Adults? Brain Sciences. 2021; 11(5):590. https://doi.org/10.3390/brainsci11050590
Chicago/Turabian StyleMueller, Raeghan L., Jarrod M. Ellingson, L. Cinnamon Bidwell, Angela D. Bryan, and Kent E. Hutchison. 2021. "Are the Acute Effects of THC Different in Aging Adults?" Brain Sciences 11, no. 5: 590. https://doi.org/10.3390/brainsci11050590
APA StyleMueller, R. L., Ellingson, J. M., Bidwell, L. C., Bryan, A. D., & Hutchison, K. E. (2021). Are the Acute Effects of THC Different in Aging Adults? Brain Sciences, 11(5), 590. https://doi.org/10.3390/brainsci11050590






