Effects of Different Anxiety Levels on the Behavioral Patternings Investigated through T-pattern Analysis in Wistar Rats Tested in the Hole-Board Apparatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Animals and Drugs
2.3. Procedure
2.4. Data Analysis
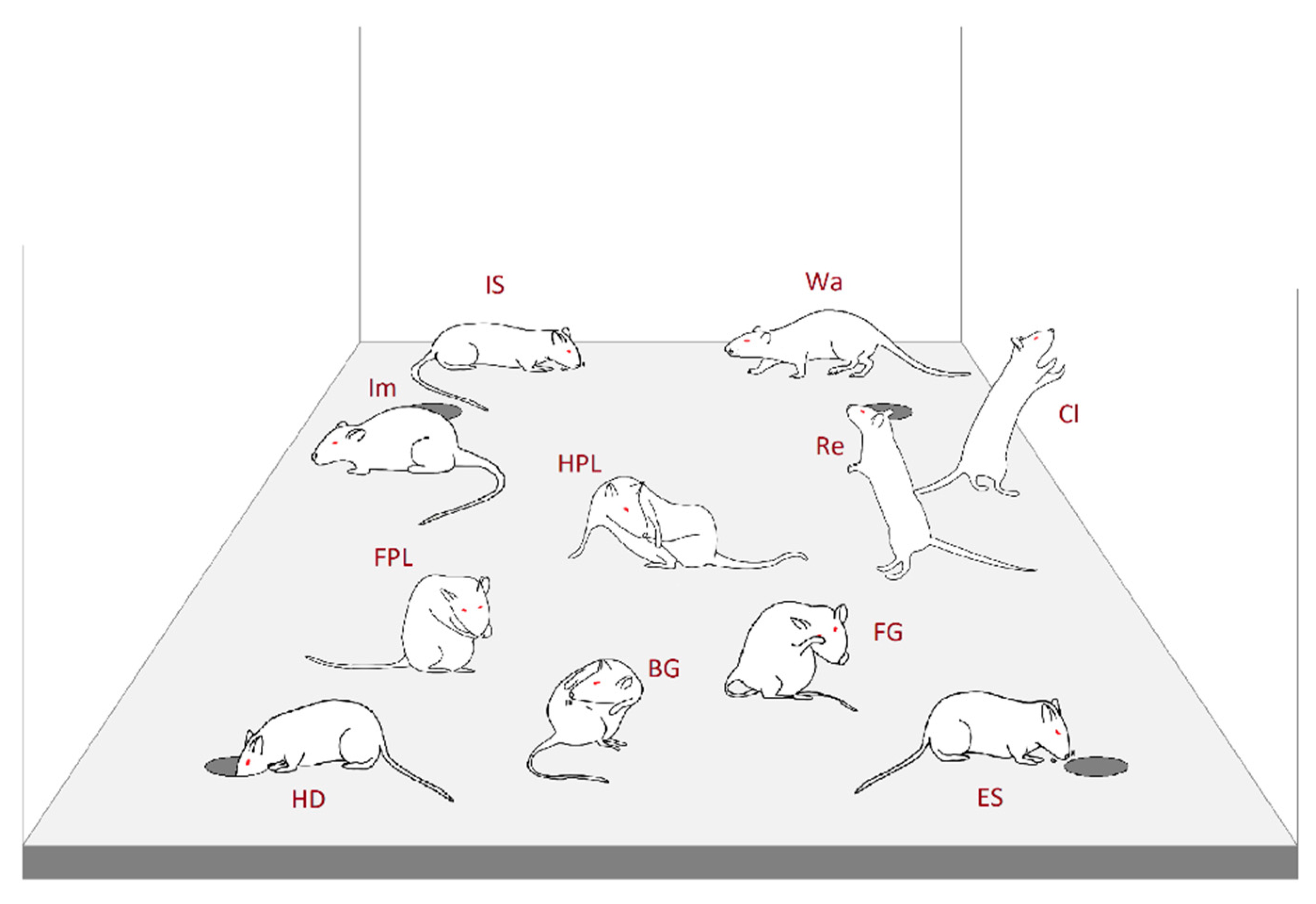
2.4.1. Ethogram
2.4.2. Temporal Structure of Behavior
2.4.3. Statistics
3. Results
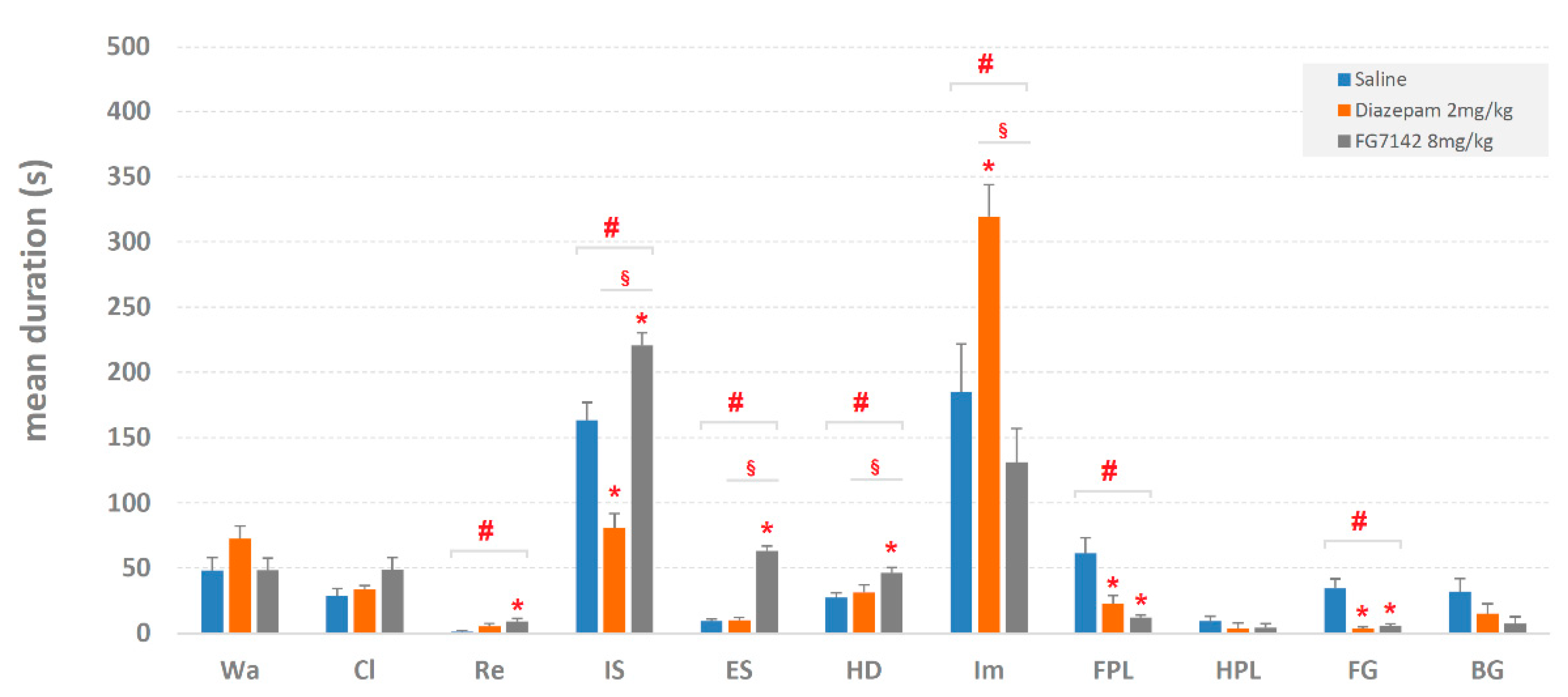
3.1. Quantitative Results
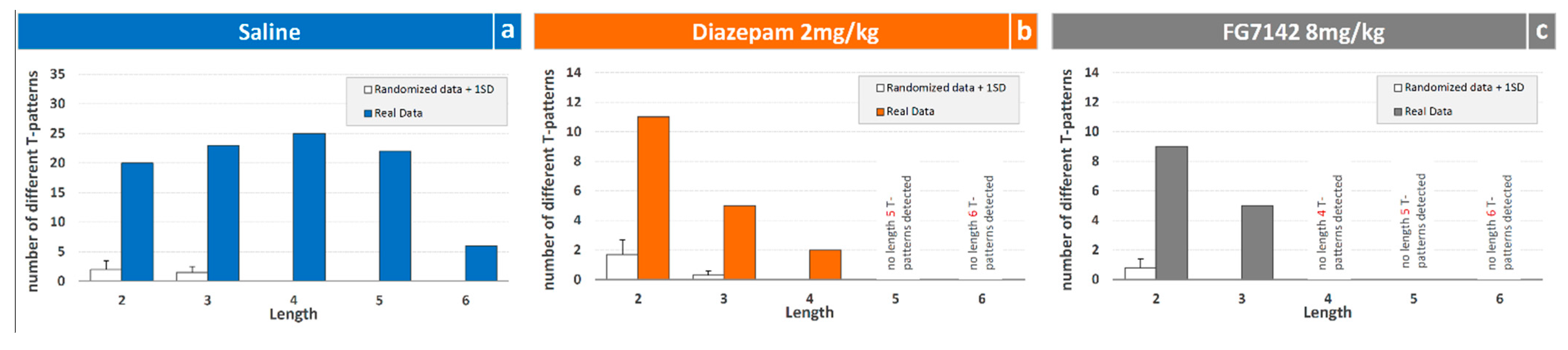
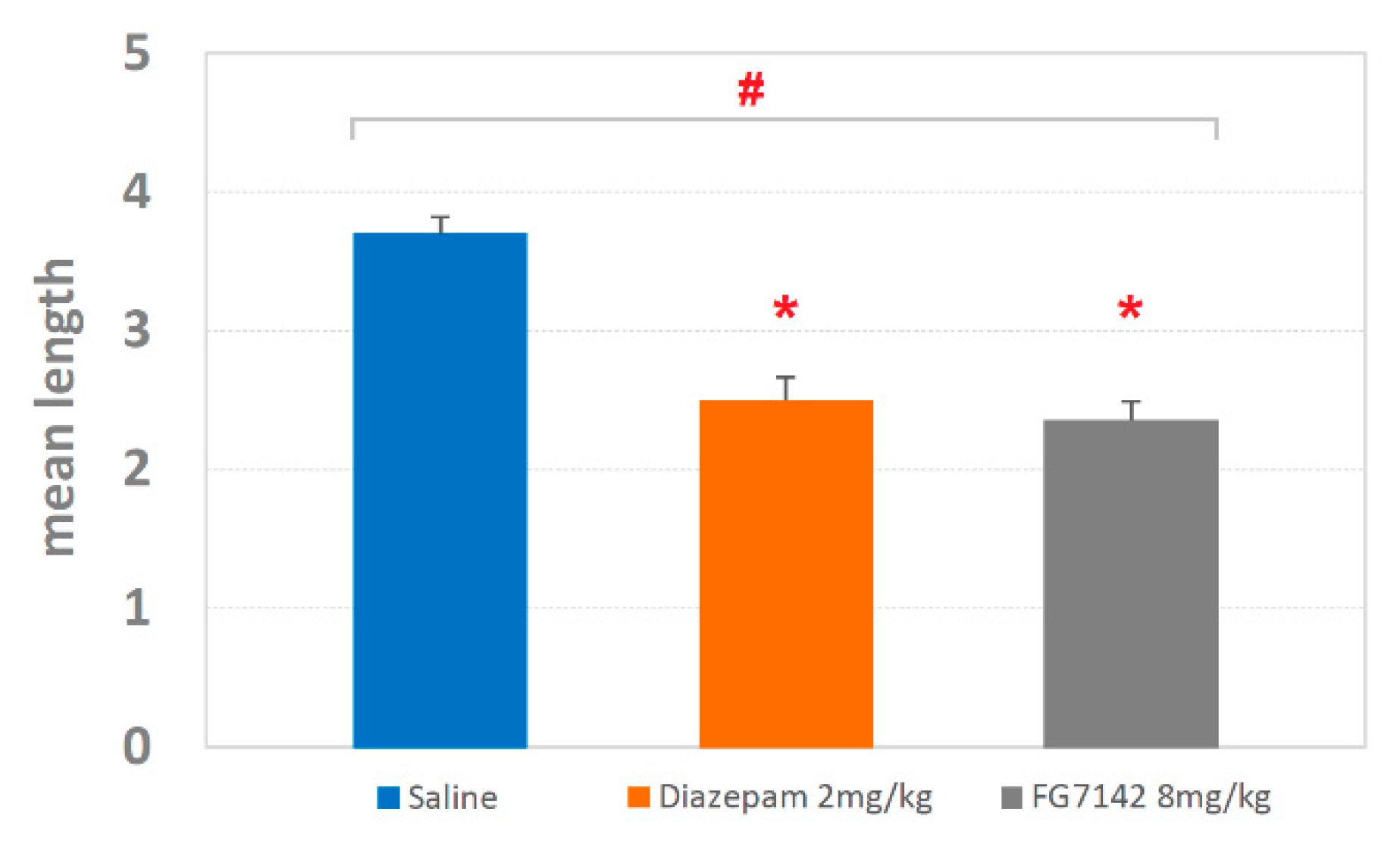
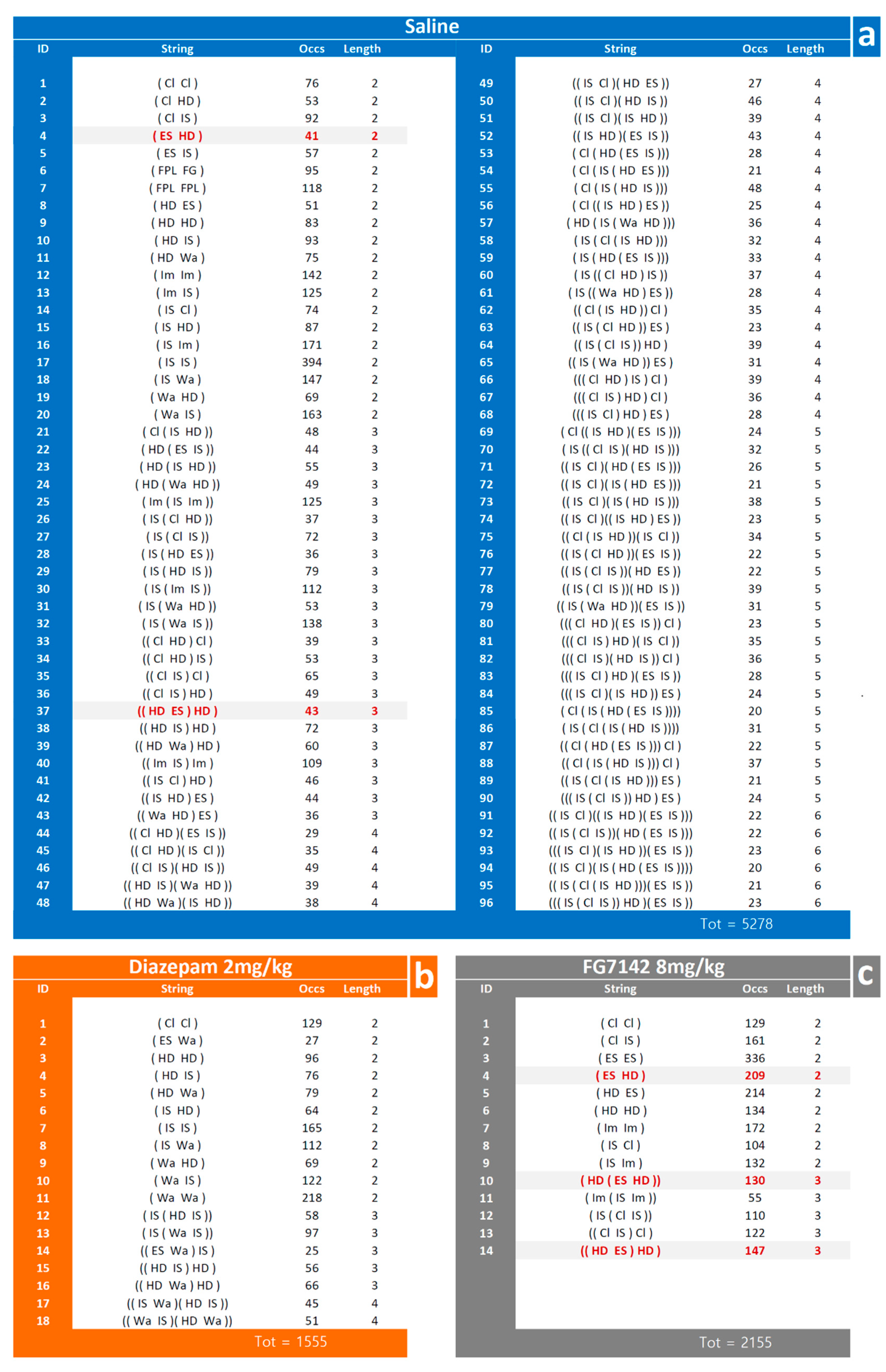
3.2. Detection and Analysis of T-Patterns
4. Discussion
4.1. Quantitative Analyses
4.2. The Head-Dip/Edge-Sniff Ratio
4.3. T-Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Greenberg, P.E.; Sisitsky, T.; Kessler, R.C.; Finkelstein, S.N.; Berndt, E.R.; Davidson, J.R.; Fyer, A.J. The economic burden of anxiety disorders in the 1990s. J. Clin. Psychiatry 1999, 60, 427–435. [Google Scholar] [CrossRef]
- Martin, P. The epidemiology of anxiety disorders: A review. Dialogues Clin. Neurosci. 2003, 5, 281–298. [Google Scholar]
- Kessler, R.C.; Ruscio, A.M.; Shear, K.; Wittchen, H.U. Epidemiology of anxiety disorders. Curr. Top. Behav. Neurosci. 2010, 2, 21–35. [Google Scholar]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef]
- Cryan, J.F.; Sweeney, F.F. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 2011, 164, 1129–1161. [Google Scholar] [CrossRef]
- Casarrubea, M.; Sorbera, F.; Crescimanno, G. Structure of rat behavior in holeboard: II multivariate analysis of modifications induced by diazepam. Physiol. Behav. 2009, 96, 683–692. [Google Scholar] [CrossRef]
- Casarrubea, M.; Faulisi, F.; Pensabene, M.; Mendola, C.; Dell’utri, R.; Cardaci, M.; Santangelo, A.; Crescimanno, G. Effects of the benzodiazepine inverse agonist FG7142 on the structure of anxiety-related behavior of male Wistar rats tested in hole board. Psychopharmacology 2017, 234, 381–391. [Google Scholar] [CrossRef]
- Casarrubea, M.; Jonsson, G.K.; Faulisi, F.; Sorbera, F.; Di Giovanni, G.; Benigno, A.; Crescimanno, G.; Magnusson, M.S. T-pattern analysis for the study of temporal structure of animal and human behavior: A comprehensive review. J. Neurosci. Methods 2015, 239, 34–46. [Google Scholar] [CrossRef]
- Magnusson, M.S.; Burgoon, J.K.; Casarrubea, M. Discovering Hidden Temporal Patterns in Behavior and Interaction; Springer: New York, NY, USA, 2016; Volume 111. [Google Scholar]
- Magnusson, M.S. Understanding social interaction: Discovering hidden structure with model and algorithms. In The Hidden Structure of Interaction—From Neurons to Culture Patterns; Anolli, L., Duncan, S., Jr., Magnusson, M.S., Riva, G., Eds.; IOS Press: Amsterdam, The Netherlands, 2005; pp. 3–22. [Google Scholar]
- Magnusson, M.S. Hidden Real-Time Patterns in Intra- and Inter-Individual Behavior: Description and Detection. Eur. J. Psychol. Assess. 1996, 12, 112–123. [Google Scholar] [CrossRef]
- Magnusson, M.S. Discovering hidden time patterns in behavior: T-patterns and their detection. Behav. Res. Methods Instrum. Comput. 2000, 32, 93–110. [Google Scholar] [CrossRef]
- Magnusson, M.S. Repeated Patterns in Behavior and Other Biological Phenomena; Oller, D.K., Griebel, U., Eds.; The MIT Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Magnusson, M.S. Time and Self-Similar Structure in Behavior and Interactions: From Sequences to Symmetry and Fractals. In Discovering Hidden Temporal Patterns in Behavior and Interaction; Magnusson, M.S., Burgoon, J.K., Casarrubea, M., Eds.; Springer: New York, NY, USA, 2016; Volume 111. [Google Scholar]
- Magnusson, M.S. T-Patterns, external memory and mass-societies in proteins and humans: In an eye-blink the naked ape became a string-controlled citizen. Physiol. Behav. 2020, 227, 113146. [Google Scholar] [CrossRef]
- Magnusson, M.S. Why Search for Hidden Repeated Temporal Behavior Patterns: T-Pattern Analysis with Theme. Int. J. Clin. Pharmacol. Pharmacother. 2017, 2, 128. [Google Scholar] [CrossRef]
- Lyon, M.; Kemp, A.S. Increased temporal patterns in choice responding andaltered cognitive processes in schizophrenia and mania. Psychophamacology 2004, 172, 211–219. [Google Scholar]
- Kemp, A.S.; Fillmore, P.T.; Lenjavi, M.R.; Lyon, M.; Chicz-DeMet, A.; Touchette, P.E.; Sandman, C.A. Temporal patterns of self-injurious behavior correlate with stress hormone levels in the developmentally disabled. Psychiatry Res. 2008, 157, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Sandman, C.A.; Kemp, A.S.; Mabini, C.; Pincus, D.; Magnusson, M. The role of self-injury in the organisation of behaviour. J. Intellect. Disabil. Res. 2012, 56, 516–526. [Google Scholar] [CrossRef]
- Kemp, A.S.; Lenjavi, M.R.; Touchette, P.E.; Pincus, D.; Magnusson, M.S.; Sandman, C.A. The Self-Organization of Self-Injurious Behavior as Revealed through Temporal Pattern Analyses. In Discovering Hidden Temporal Patterns in Behavior and Interaction; Magnusson, M.S., Burgoon, J.K., Casarrubea, M., Eds.; Springer: New York, NY, USA, 2016; Volume 111. [Google Scholar]
- Bonasera, S.J.; Schenk, A.K.; Luxenberg, E.J.; Tecott, L.H. A novel method for automatic quantification of psychostimulant-evoked route-tracing stereotypy: Application to Mus musculus. Psychopharmacology 2008, 196, 591–602. [Google Scholar] [CrossRef]
- Kerepesi, A.; Jonsson, G.K.; Emiklósi, Á.; Topál, J.; Csányi, V.; Magnusson, M.S. Detection of temporal patterns in dog-human interaction. Behav. Process. 2005, 70, 69–79. [Google Scholar] [CrossRef]
- Kerepesi, A.; Kubinyi, E.; Jonsson, G.K.; Magnusson, M.S.; Emiklósi, A. Behavioural comparison of human-animal (dog) and human-robot (AIBO) interactions. Behav. Process. 2006, 73, 92–99. [Google Scholar] [CrossRef]
- Hirschenhauser, K.; Frigerio, D.; Grammer, K.; Magnusson, M.S. Monthly Patterns of Testosterone and Behavior in Prospective Fathers. Horm. Behav. 2002, 42, 172–181. [Google Scholar] [CrossRef]
- Casarrubea, M.; Aiello, S.; Di Giovanni, G.; Santangelo, A.; Palacino, M.; Crescimanno, G. Combining Quantitative and Qualitative Data in the Study of Feeding Behavior in Male Wistar Rats. Front. Psychol. 2019, 10, 881. [Google Scholar] [CrossRef]
- Santangelo, A.; Bortolato, M.; Mosher, L.J.; Crescimanno, G.; Di Giovanni, G.; Cassioli, E.; Ricca, V.; Casarrubea, M. Behavioral fragmentation in the D1CT-7 mouse model of Tourette’s syndrome. CNS Neurosci. Ther. 2018, 24, 703–711. [Google Scholar] [CrossRef]
- Casarrubea, M.; Di Giovanni, G.; Crescimanno, G.; Rosa, I.; Aiello, S.; Di Censo, D.; Ranieri, B.; Santangelo, A.; Busatta, D.; Cassioli, E.; et al. Effects of Substantia Nigra pars compacta lesion on the behavioral sequencing in the 6-OHDA model of Parkinson’s disease. Behav. Brain Res. 2019, 362, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Crescimanno, G.; Di Giovanni, G.; Casarrubea, M. T-patterns in the study of movement and behavioral disorders. Physiol. Behav. 2020, 215, 112790. [Google Scholar] [CrossRef]
- Casarrubea, M.; Sorbera, F.; Magnusson, M.; Crescimanno, G. Temporal patterns analysis of rat behavior in hole-board. Behav. Brain Res. 2010, 208, 124–131. [Google Scholar] [CrossRef]
- Casarrubea, M.; Sorbera, F.; Magnusson, M.S.; Crescimanno, G. T-pattern analysis of diazepam-induced modifications on the temporal organization of rat behavioral response to anxiety in hole board. Psychopharmacology 2011, 215, 177–189. [Google Scholar] [CrossRef]
- Casarrubea, M.; Roy, V.; Sorbera, F.; Magnusson, M.S.; Santangelo, A.; Arabo, A.; Crescimanno, G. Temporal structure of the rat’s behavior in elevated plus maze test. Behav. Brain Res. 2013, 237, 290–299. [Google Scholar] [CrossRef]
- Casarrubea, M.; Roy, V.; Sorbera, F.; Magnusson, M.S.; Santangelo, A.; Arabo, A.; Crescimanno, G. Significant divergences between the temporal structure of the behavior in Wistar and in the spontaneously more anxious DA/Han strain of rats tested in elevated plus maze. Behav. Brain Res. 2013, 250, 166–173. [Google Scholar] [CrossRef]
- Casarrubea, M.; Magnusson, M.S.; Roy, V.; Arabo, A.; Sorbera, F.; Santangelo, A.; Faulisi, F.; Crescimanno, G. Multivariate temporal pattern analysis applied to the study of rat behavior in the elevated plus maze: Methodological and conceptual highlights. J. Neurosci. Methods 2014, 234, 116–126. [Google Scholar] [CrossRef]
- Casarrubea, M.; Davies, C.; Faulisi, F.; Pierucci, M.; Colangeli, R.; Partridge, L.; Chambers, S.; Cassar, D.; Valentino, M.; Muscat, R.; et al. Acute nicotine induces anxiety and disrupts temporal pattern organization of rat exploratory behavior in hole-board: A potential role for the lateral habenula. Front. Cell. Neurosci. 2015, 9, 197. [Google Scholar] [CrossRef]
- Casarrubea, M.; Faulisi, F.; Caternicchia, F.; Santangelo, A.; Di Giovanni, G.; Benigno, A.; Magnusson, M.S.; Crescimanno, G. Temporal patterns of rat behaviour in the central platform of the elevated plus maze. Comparative analysis between male subjects of strains with different basal levels of emotionality. J. Neurosci. Methods 2016, 268, 155–162. [Google Scholar] [CrossRef]
- Casarrubea, M.; Pierucci, M.; Aiello, S.; Cassar, D.; Deidda, G.; Crescimanno, G.; Di Giovanni, G. Effects of chronic nicotine on the temporal structure of anxiety-related behavior in rats tested in hole-board. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 96, 109731. [Google Scholar] [CrossRef]
- Casarrubea, M.; Davies, C.; Pierucci, M.; Colangeli, R.; Deidda, G.; Santangelo, A.; Aiello, S.; Crescimanno, G.; Di Giovanni, G. The impact of chronic daily nicotine exposure and its overnight withdrawal on the structure of anxiety-related behaviors in rats: Role of the lateral Habenula. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110131. [Google Scholar] [CrossRef]
- Evans, A.K.; Lowry, C.A. Pharmacology of the β-carboline FG-7142, a partial inverse agonist at the benzodiazepine allosteric site of the GABAA receptor: Neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007, 13, 475–501. [Google Scholar] [CrossRef]
- Tang, H.H.; McNally, G.P.; Richardson, R. The effects of FG7142 on two types of forgetting in 18-day-old rats. Behav. Neurosci. 2007, 121, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- La-Vu, M.; Tobias, B.C.; Schuette, P.J.; Adhikari, A. To approach or avoid: An introductory overview of the study of anxiety using rodent assays. Front. Behav. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Matsumiya, T. Changes in head-dipping behavior in the holeboard test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998, 350, 21–29. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Wheaton, M.; Murphy, D.L. What’s wrong with my mouse model? Advances and strategies in animal modeling of anxiety and depression. Behav. Brain Res. 2007, 179, 1–18. [Google Scholar] [CrossRef]
- Anguera, M.T.; Blanco-Villaseñor, A.; Losada, J.L.; Sánchez-Algarra, P.; Onwuegbuzie, A.J. Revisiting the difference between mixed methods and multimethods: Is it all in the name? Qual. Quant. 2018, 52, 2757–2770. [Google Scholar] [CrossRef]
- Onwuegbuzie, A.J.; Leech, N.L. Generalization practices in qualitative research: A mixed methods case study. Qual. Quant. 2010, 44, 881–892. [Google Scholar] [CrossRef]



| Saline | Diazepam | FG7142 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ETP | Occs | % | Occs | % | χ2 | Vs | Occs | % | χ2 | Vs |
| Wa | 993 | 18.81 | 911 | 58.59 | <0.01 | ↑ | 0 | 0 | ↓ | |
| Cl | 2033 | 38.52 | 129 | 8.30 | <0.01 | ↓ | 626 | 29.05 | <0.01 | ↓ |
| IS | 4248 | 80.49 | 871 | 56.01 | <0.01 | ↓ | 684 | 31.74 | <0.01 | ↓ |
| Re | 0 | 0 | 0 | 0 | ─ | 0 | 0 | ─ | ||
| ES | 1130 | 21.41 | 52 | 3.34 | <0.01 | ↓ | 1036 | 48.07 | <0.01 | ↑ |
| HD | 3003 | 56.90 | 660 | 42.44 | <0.01 | ↓ | 834 | 38.70 | <0.01 | ↓ |
| ES→HD | 84 | 1.59 | 0 | 0 | ↓ | 486 | 22.50 | <0.01 | ↑ | |
| FPL | 213 | 4.04 | 0 | 0 | ↓ | 0 | 0 | ↓ | ||
| HPL | 0 | 0 | 0 | 0 | ─ | 0 | 0 | ─ | ||
| FG | 95 | 1.80 | 0 | 0 | ↓ | 0 | 0 | ↓ | ||
| BG | 0 | 0 | 0 | 0 | ─ | 0 | 0 | ─ | ||
| Im | 910 | 17.24 | 0 | 0 | ↓ | 359 | 16.67 | 0.54 | ─ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarrubea, M.; Di Giovanni, G.; Crescimanno, G. Effects of Different Anxiety Levels on the Behavioral Patternings Investigated through T-pattern Analysis in Wistar Rats Tested in the Hole-Board Apparatus. Brain Sci. 2021, 11, 714. https://doi.org/10.3390/brainsci11060714
Casarrubea M, Di Giovanni G, Crescimanno G. Effects of Different Anxiety Levels on the Behavioral Patternings Investigated through T-pattern Analysis in Wistar Rats Tested in the Hole-Board Apparatus. Brain Sciences. 2021; 11(6):714. https://doi.org/10.3390/brainsci11060714
Chicago/Turabian StyleCasarrubea, Maurizio, Giuseppe Di Giovanni, and Giuseppe Crescimanno. 2021. "Effects of Different Anxiety Levels on the Behavioral Patternings Investigated through T-pattern Analysis in Wistar Rats Tested in the Hole-Board Apparatus" Brain Sciences 11, no. 6: 714. https://doi.org/10.3390/brainsci11060714
APA StyleCasarrubea, M., Di Giovanni, G., & Crescimanno, G. (2021). Effects of Different Anxiety Levels on the Behavioral Patternings Investigated through T-pattern Analysis in Wistar Rats Tested in the Hole-Board Apparatus. Brain Sciences, 11(6), 714. https://doi.org/10.3390/brainsci11060714








