Cortical Excitability across the ALS Clinical Motor Phenotypes
Abstract
1. Introduction
2. Archetypical Clinical Motor Phenotypes
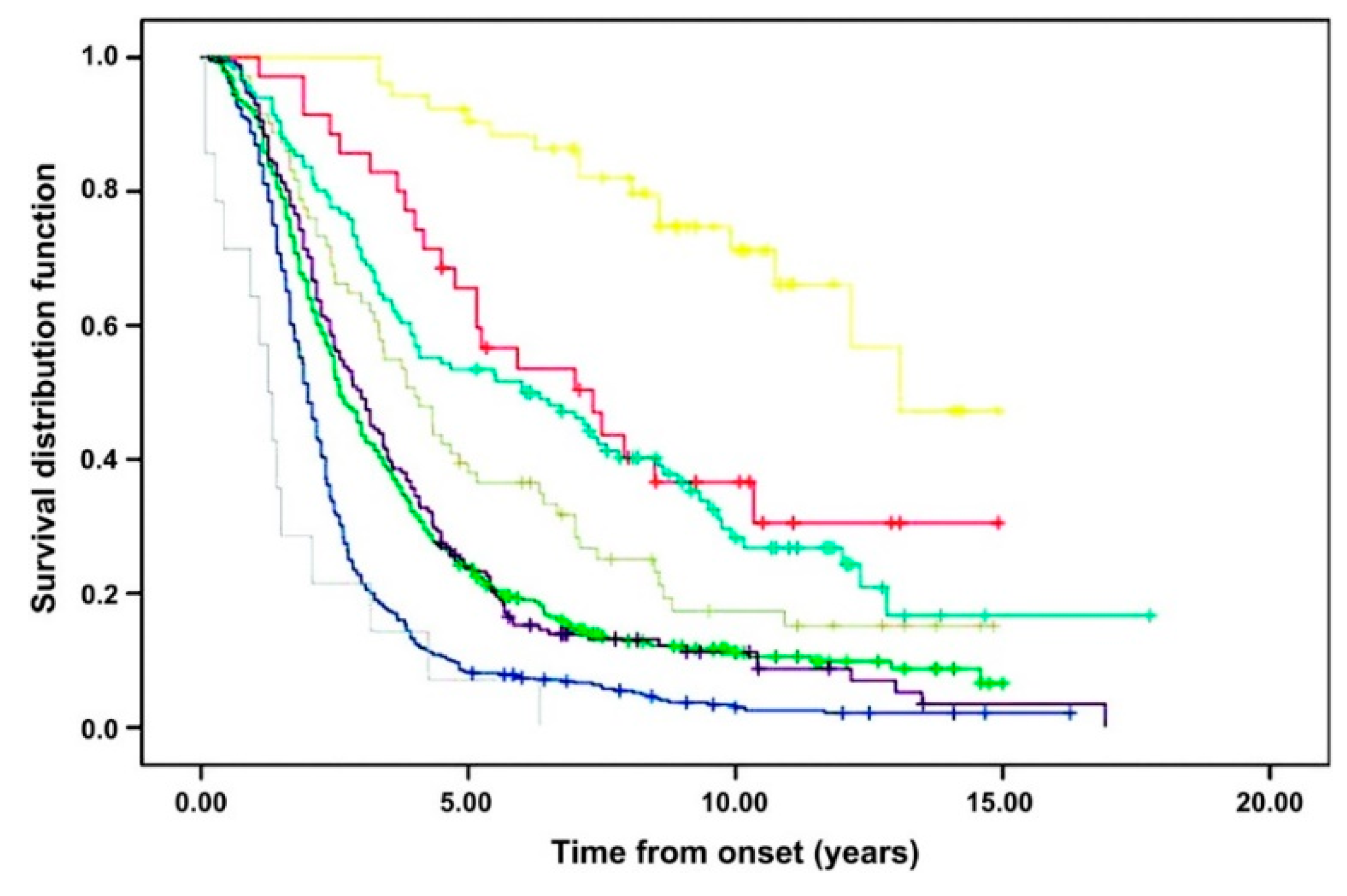
- The definitive clinical characteristics of this typical ALS phenotype are the presence of a relatively equal burden of UMN and LMN signs coexisting within the same symptomatic area (Figure 2d) [4,21]. Clinical disease can initially manifest in one of four main regions (upper-limb, lower-limb, bulbar, respiratory/truncal), but weakness usually begins in a limb, while respiratory onset is rare (1–5%) [3,4]. Bulbar-onset occurs in 20% of this group and increases in frequency with increasing age, which may explain the reported predominance in females [22]. These patients have a worse prognosis than their limb-onset counterparts [4,23]. Median survival is approximately 3 years [21], while UMN- or LMN-predominant variants of this form usually have a slower rate of progression [4].
- Patients with a pure UMN syndrome that has progressed for at least 4 years in the absence of LMN signs are diagnostically termed primary lateral sclerosis (PLS) (Figure 2g) [24]. PLS uniquely represents a selective loss of precentral pyramidal (upper) motor neurons [24,25], and in some cases, this is sharply delineated by ‘knife edge’ focal atrophy on structural MRI and a ‘stripe’ of fluorodeoxyglucose hypometabolism in the precentral gyrus on PET studies [26,27]. Whether this is a separate disorder or a forme fruste of classical ALS continues to be debated [24,28,29]. PLS is rare, representing 2–5% of all cases [3,4,29,30]. These patients are consistently younger and have a predilection for symmetrical lower-limb disease onset, although a very rare asymmetrical subtype of progressive hemiplegia has been described by Mills (1% of cases) (Figure 2f) [31]. Spinobulbar spasticity emerges insidiously as a rule, and a slow rate of progression gives this the most favorable prognosis of all the clinical motor phenotypes, with some reports of normal life expectancy [4,30].
- Patients with a clinically pure LMN phenotype represent 5% of all cases, and this was first reported as ‘progressive muscular atrophy’ (PMA) by Aran in 1850 (Figure 2c) [32]. This phenotype has a higher occurrence in males [4,20]. When occurring in a more generalised form (i.e., when more than 50% of limb regions are affected), it follows a similar prognostic course to classic ALS, and approximately 30% of patients develop UMN symptoms within 18 months [33].
- In patients with LMN-only symptoms (i.e., ‘PMA’), a ‘flail limb’ subgroup develops a clinical syndrome that remains restricted to either the upper limbs (flail arm syndrome, 5–6% cases; Figure 2b) or less commonly, to the lower limbs (flail leg syndrome, 3–5% of cases; Figure 2a) for at least 12 months [34]. The flail arm phenotype was first described as the ‘scapulohumeral variant of progressive muscular atrophy’, and it is represented by proximal wasting and weakness in the upper limbs, which typically evolves to involve both limbs symmetrically [34,35]. The flail leg syndrome, recognised by Pierre Marie and first described by his student Patrikios (the ‘Marie–Patrikios’ form), describes a distal onset of weakness that usually starts asymmetrically in the lower limbs. The flail limb subgroups are more common in men, particularly the flail arm phenotype (4:1) [34]. The natural history of these syndromes is better than for classical ALS: time to spread to a second region is longer (at least 18 months, with 27% of cases still confined to the onset limb(s) after 36 months), and overall prognosis is more favorable, with longer median survivals (Supplementary Table S1) [34,35].
- Isolated bulbar palsy (IBP) occurs in 1–4% of cases (Figure 2e) [36]. By definition, this differs from bulbar-onset ALS due to the restriction of progressive deficits to the bulbar area for 6 months or more, while limb strength remains preserved. Contention exists over whether this warrants nosological separation, as most eventually progress to the classical form of the disease [37], but the clinical pattern appears different [36]. Patients are commonly older and female. UMN bulbar symptoms predominate (e.g., spastic dysarthria, emotional lability), and survival in this group may be improved by at least 12 months compared to bulbar-onset ALS [36].
3. Cortical Excitability across ALS Motor Phenotypes
3.1. Classical ALS Phenotypes and ‘Cortical Focality’
3.2. Atypical Phenotypes
4. Implications
4.1. Clinical Implications
4.2. Prognostic Implications
5. Discussion
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Ravits, J.M.; La Spada, A.R. ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology 2009, 73, 805–811. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Henderson, R.D.; Talman, P.S.; Al Macdonell, R.; Mathers, S.; Schultz, D.W.; Needham, M.; Zoing, M.; Vucic, S.; Kiernan, M.C. Motor neurone disease: Progress and challenges. Med. J. Aust. 2017, 206, 357–362. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef]
- Chiò, A.; Calvo, A.; Moglia, C.; Mazzini, L.; Mora, G.; PARALS study group. Phenotypic heterogeneity of amyotrophic lateral sclerosis: A population based study. J. Neurol. Neurosurg. Psychiatry 2011, 82, 740–746. [Google Scholar] [CrossRef]
- Eisen, A.; Braak, H.; Del Tredici, K.; Lemon, R.; Ludolph, A.C.; Kiernan, M.C. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Lemon, F.; Grifithis, J. Comparing the function of the corticospinal system in different species: Organisational differences for motor specialisation? Muscle Nerve 2005, 32, 261–279. [Google Scholar] [CrossRef]
- Henderson, R.D.; Garton, F.C.; Kiernan, M.C.; Turner, M.R.; Eisen, A. Human cerebral evolution and the clinical syndrome of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 90, 570–575. [Google Scholar] [CrossRef]
- Ravits, J.; Paul, P.; Jorg, C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 2007, 68, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Huynh, W.; Simon, N.G.; Grosskreutz, J.; Turner, M.R.; Vucic, S.; Kiernan, M.C. Assessment of the upper motor neuron in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2016, 127, 2643–2660. [Google Scholar] [CrossRef] [PubMed]
- Swash, M.; Burke, D.; Turner, M.R.; Grosskreutz, J.; Leigh, P.N.; Decarvalho, M.; Kiernan, M.C. Occasional essay: Upper motor neuron syndrome in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 227–234. [Google Scholar] [CrossRef]
- Kaufmann, P.; Pullman, S.L.; Shungu, D.C.; Chan, S.; Hays, A.P.; Del Bene, M.L.; Dover, M.A.; Vukic, M.; Rowland, L.P.; Mitsumoto, H. Objective tests for upper motor neuron involvement in amyotrophic lateral sclerosis (ALS). Neurology 2004, 62, 1753–1757. [Google Scholar] [CrossRef]
- Vucic, S.; Ziemann, U.; Eisen, A.; Hallett, M.; Kiernan, M.C. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: Pathophysiological insights. J. Neurol. Neurosurg. Psychiatry 2012, 84, 1161–1170. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Matamala, J.M.; Howells, J.; Vucic, S.; Kiernan, M.C. Early focality and spread of cortical dysfunction in amyotrophic lateral sclerosis: A regional study across the motor cortices. Clin. Neurophysiol. 2020, 131, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.; Geevasinga, N.; Bos, M.V.D.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Cortical hyperexcitability and disease spread in amyotrophic lateral sclerosis. Eur. J. Neurol. 2017, 24, 816–824. [Google Scholar] [CrossRef]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, L.; Cohen, V.; Mall, A.; Kaelin-Lang, T.; Mima, S.; Rossi, G.; Thickbroom, P.; et al. A practical guide to diagnostic transcranial magnetic sitmulation: Report of an I.F.C.N. Committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef]
- Zanette, G.; Tamburin, S.; Manganotti, P.; Refatti, N.; Forgione, A.; Rizzuto, N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2002, 113, 1688–1697. [Google Scholar] [CrossRef]
- Ziemann, U.; Winter, M.; Reimers, C.D.; Reimers, K.; Tergau, F.; Paulus, W. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology 1997, 49, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Dharmadasa, T.; Howells, J.; Matamala, J.M.; Simon, N.G.; Burke, D.; Vucic, S.; Kiernan, M.C. Cortical inexcitability defines an adverse clinical profile in amyotrophic lateral sclerosis. Eur. J. Neurol. 2021, 28, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Park, S.B.; Geevasinga, N.; Menon, P.; Howells, J.; Simon, N.G.; Huynh, W.; Noto, Y.-I.; Götz, J.; Kril, J.J.; et al. Motor cortical function determines prognosis in sporadic ALS. Neurology 2016, 87, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Talman, P.; Duong, T.; Vucic, S.; Mathers, S.; Venkatesh, S.; Henderson, R.; Rowe, D.; Schultz, D.; Edis, R.; Needham, M.; et al. Identification and outcomes of clinical phenotypes in amyotrophic lateral sclerosis/motor neuron disease: Australian National Motor Neuron Disease observational cohort. BMJ Open 2016, 6, e012054. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.; Vucic, S.; Cheah, B.; Turner, M.; Eisen, A.; Hardiman, O.; Burrell, J.; Zoing, M. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef]
- Chiò, A.; Moglia, C.; Canosa, A.; Manera, U.; D’Ovidio, F.; Vasta, R.; Grassano, M.; Brunetti, M.; Barberis, M.; Corrado, L.; et al. ALS phenotype is influenced by age, sex, and genetics. Neurology 2020, 94, e802–e810. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Scaber, J.; Goodfellow, J.A.; Lord, M.E.; Marsden, R.; Talbot, K. The diagnostic pathway and prognosis in bulbar-onset amyotrophic lateral sclerosis. J. Neurol. Sci. 2010, 294, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Barohn, R.J.; Corcia, P.; Fink, J.K.; Harms, M.B.; Kiernan, M.C.; Ravits, J.; Silani, V.; Simmons, Z.; Statland, J.; et al. Primary lateral sclerosis: Consensus diagnostic criteria. J. Neurol. Neurosurg. Psychiatry 2020, 91, 373–377. [Google Scholar] [CrossRef]
- Mitsumoto, H.; Nagy, P.; Gennings, C.; Murphy, J.; Andrews, H.; Goetz, R.; Floeter, M.; Hupf, J.; Singleton, J.; Barohn, R.; et al. Phenotypic and molecular analysis of primary lateral sclerosis. Neurol. Genet. 2015, 1, e3. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Huynh, W.; Tsugawa, J.; Shimatani, Y.; Ma, Y.; Kiernan, M.C. Implications of structural and functional brain changes in amyotrophic lateral sclerosis. Expert Rev. Neurother. 2018, 18, 407–419. [Google Scholar] [CrossRef]
- Claassen, D.; Josephs, K.; Peller, P. The stripe of primary lateral sclerosis: Focal primary motor cortex hypometabolism seen on fluorodeoxyglucose F18 positron emission tomography. Arch. Neurol. 2010, 67, 122–125. [Google Scholar] [CrossRef]
- Rowland, L.P. Primary lateral sclerosis: Disease, syndrome, both or neither? J. Neurol. Sci. 1999, 170, 1–4. [Google Scholar] [CrossRef]
- Le Forestier, N.; Maisonobe, T.; Piquard, A.; Rivaud, S.; Crevier-Buchman, L.; Salachas, F.; Pradat, P.-F.; Lacomblez, L.; Meininger, V. Does primary lateral sclerosis exist? A study of 20 patients and a review of the literature. Brain 2001, 124, 1989–1999. [Google Scholar] [CrossRef]
- Gordon, P.H.; Cheng, B.; Katz, I.B.; Pinto, M.; Hays, A.P.; Mitsumoto, H.; Rowland, L.P. The natural history of primary lateral sclerosis. Neurology 2006, 66, 647–653. [Google Scholar] [CrossRef]
- Mills, C. Unilateral ascending paralysis and unilateral descending paralysis: Their clinical varieties and their pathological causes. J. Am. Med. Assoc. 1906, 20, 1638–1645. [Google Scholar] [CrossRef]
- Aran, F. Recherches sur une maladie non encore decrite du systeme musculaire (atrophie musculaire progressive). Arch. Gen. Med. 1850, 24, 5–35. [Google Scholar]
- Visser, J.; Berg-Vos, R.M.V.D.; Franssen, H.; Berg, L.H.V.D.; Wokke, J.H.; De Jong, J.M.V.; Holman, R.; De Haan, R.J.; De Visser, M. Disease Course and Prognostic Factors of Progressive Muscular Atrophy. Arch. Neurol. 2007, 64, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Wijesekera, L.C.; Mathers, S.; Talman, P.; Galtrey, C.; Parkinson, M.H.; Ganesalingam, J.; Willey, E.; Ampong, M.A.; Ellis, C.M.; Shaw, C.; et al. Natural history and clinical features of the flail arm and flail leg ALS variants. Neurology 2009, 72, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Hübers, A.; Hildebrandt, V.; Petri, S.; Kollewe, K.; Hermann, A.; Storch, A.; Hanisch, F.; Zierz, S.; Rosenbohm, A.; Ludolph, A.C.; et al. Clinical features and differential diagnosis of flail arm syndrome. J. Neurol. 2015, 263, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.R.; Vucic, S.; Kiernan, M.C. Isolated bulbar phenotype of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2011, 12, 283–289. [Google Scholar] [CrossRef]
- Karam, C.; Scelsa, S.N.; MacGowan, D.J.L. The clinical course of progressive bulbar palsy. Amyotroph. Lateral Scler. 2010, 11, 364–368. [Google Scholar] [CrossRef]
- Turner, M.R.; Kiernan, M.C. Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. 2012, 13, 245–250. [Google Scholar] [CrossRef]
- Vucic, S.; Bos, M.V.D.; Menon, P.; Howells, J.; Dharmadasa, T.; Kiernan, M.C. Utility of threshold tracking transcranial magnetic stimulation in ALS. Clin. Neurophysiol. Pract. 2018, 3, 164–172. [Google Scholar] [CrossRef]
- Vucic, S.; Nicholson, G.A.; Kiernan, M.C. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain 2008, 131, 1540–1550. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Fisher, R.J.; Nakamura, Y.; Bestmann, S.; Rothwell, J.C.; Bostock, H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp. Brain Res. 2002, 143, 240–248. [Google Scholar] [CrossRef]
- Nihei, K.; McKee, A.C.; Kowall, N.W. Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol. 1993, 86, 55–64. [Google Scholar] [CrossRef]
- Foerster, B.; Callaghan, B.; Petrou, M.; Edden, R.; Chenevert, T.; Feldmen, E. Decreased motor cortex y-aminobutyric acid in amyotrophic lateral sclerosis. Neurology 2012, 78, 1596–1600. [Google Scholar] [CrossRef]
- Mills, K.R.; Nithi, K.A. Corticomotor threshold is reduced in early sporadic amyotrophic lateral sclerosis. Muscle Nerve 1997, 20, 1137–1141. [Google Scholar] [CrossRef]
- Cantello, R.; Gianelli, M.; Civardi, C.; Mutani, R. Magnetic brain stimulation: The silent period after the motor evoked potential. Neurology 1992, 42, 1951–1959. [Google Scholar]
- Rossini, P.; Burke, D.; Chen, R.; Cohen, L.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.; George, M.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Stauffer, J.E.; Schulte, D.J.; Ravits, J. Neuropathology of Amyotrophic Lateral Sclerosis and Its Variants. Neurol. Clin. 2015, 33, 855–876. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Kiernan, M. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain 2006, 129, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.; Geevasinga, N.; Yiannikas, C.; Howells, J.; Kiernan, M.C.; Vucic, S. Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: A prospective study. Lancet Neurol. 2015, 14, 478–484. [Google Scholar] [CrossRef]
- Menon, P.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Regional motor cortex dysfunction in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Bede, P.; Bokde, A.; Elamin, M.; Byrne, S.; McLaughlin, R.; Jordan, N.; Hampel, H.; Gallagher, L.; Lynch, C.; Fagan, A.J.; et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): A neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J. Neurol. Neurosurg. Psychiatry 2012, 84, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.; Kasper, E.; Machts, J.; Bittner, D.; Kaufmann, J.; Benecke, R.; Teipel, S.; Vielhaber, S.; Prudlo, J. Focal thinning of the motor cortex mirrors clinical features of amyotrophic lateral sclerosis and their phenotypes: A neuroimaging study. J. Neurol. 2013, 260, 2856–2864. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Simmons, A.; Andrews, C.; Dawson, J.; Williams, S.; Leigh, P. A proton magnetic resonance spectroscopic study in ALS: Correlation with clinical findings. Neurology 1998, 51, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Simmons, A.; Jones, D.K.; Bland, J.; Dawson, J.M.; Horsfield, M.A.; Williams, S.C.R.; Leigh, P.N. Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 1999, 53, 1051. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Kiernan, M.C. Abnormalities in cortical and peripheral excitability in flail arm variant amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2007, 78, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Menon, P.; Geevasinga, N.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Cortical contributions to the flail leg syndrome: Pathophysiological insights. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 389–396. [Google Scholar] [CrossRef]
- Weber, M.; Stewart, H.; Hirota, N.; Eisen, A. Corticomotneuronal connections in primary lateral sclerosis (PLS). Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2002, 3, 190–198. [Google Scholar] [CrossRef]
- Kuipers-Upmeijer, J.; Jager, A.E.J.D.; Hew, J.M.; Snoek, J.W.; Van Weerden, T.W. Primary lateral sclerosis: Clinical, neurophysiological, and magnetic resonance findings. J. Neurol. Neurosurg. Psychiatry 2001, 71, 615–620. [Google Scholar] [CrossRef]
- Geevasinga, N.; Menon, P.; Sue, C.M.; Kumar, K.R.; Ng, K.; Yiannikas, C.; Kiernan, M.C.; Vucic, S. Cortical excitability changes distinguish the motor neuron disease phenotypes from hereditary spastic paraplegia. Eur. J. Neurol. 2015, 22, 826–831. [Google Scholar] [CrossRef]
- Takeda, T.; Kitagawa, K.; Arai, K. Phenotypic variability and its pathological basis in amyotrophic lateral sclerosis. Neuropathology 2019, 40, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Hammers, A.; Al-Chalabi, A.; Shaw, C.; Andersen, P.M.; Brooks, D.J.; Leigh, P.N. Cortical involvement in four cases of primary lateral sclerosis using [11C]-flumazenil PET. J. Neurol. 2007, 254, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Ince, P.; Evans, J.; Knopp, M.; Forster, G.; Hamdalla, H.; Wharton, S.; Shaw, P. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 2003, 60, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.A. Neuropathology of primary lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U. TMS and drugs. Clin. Neurophysiol. 2004, 115, 1717–1729. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Hammers, A.; Al-Chalabi, A.; Shaw, C.; Andersen, P.M.; Brooks, D.J.; Leigh, P.N. Distinct cerebral lesions in sporadic and ‘D90A’ SOD1 ALS: Studies with [11C]flumazenil PET. Brain 2005, 128, 1323–1329. [Google Scholar] [CrossRef]
- Cwik, V.A.; Hanstock, C.C.; Allen, P.S.; Martin, W.R.W. Estimation of brainstem neuronal loss in amyotrophic lateral sclerosis with in vivo proton magnetic resonance spectroscopy. Neurology 1998, 50, 72–77. [Google Scholar] [CrossRef]
- Maekawa, S.; Al-Sarraj, S.; Kibble, M.; Landau, S.; Parnavelas, J.; Cotter, D.; Everall, I.; Leigh, P.N. Cortical selective vulnerability in motor neuron disease: A morphometric study. Brain 2004, 127, 1237–1251. [Google Scholar] [CrossRef]
- Dharmadasa, T.; Matamala, J.M.; Kiernan, M.C. Treatment approaches in motor neurone disease. Curr. Opin. Neurol. 2016, 29, 581–591. [Google Scholar] [CrossRef]
- Ravits, J.; Appel, S.; Baloh, R.H.; Barohn, R.; Brooks, B.R.; Elman, L.; Floeter, M.K.; Henderson, C.; Lomen-Hoerth, C.; Macklis, J.D.; et al. Deciphering amyotrophic lateral sclerosis: What phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral Scler. Front. Degener. 2013, 14, 5–18. [Google Scholar] [CrossRef]
- Matamala, J.M.; Geevasinga, N.; Huynh, W.; Dharmadasa, T.; Howells, J.; Simon, N.G.; Menon, P.; Vucic, S.; Kiernan, M.C. Cortical function and corticomotoneuronal adaptation in monomelic amyotrophy. Clin. Neurophysiol. 2017, 128, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Talbot, K.; McDermott, C.J.; Hardiman, O.; Shefner, J.M.; Al-Chalabi, A.; Huynh, W.; Cudkowicz, M.; Talman, P.; et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2021, 17, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Agosta, F.; Pagani, E.; Petrolini, M.; Caputo, D.; Perini, M.; Prelle, A.; Salvi, F.; Filippi, M. Assessment of White Matter Tract Damage in Patients with Amyotrophic Lateral Sclerosis: A Diffusion Tensor MR Imaging Tractography Study. Am. J. Neuroradiol. 2010, 31, 1457–1461. [Google Scholar] [CrossRef] [PubMed]

| Cortical Parameters Using TMS | Typical Phenotype | Atypical Phenotype | |||
|---|---|---|---|---|---|
| Classical ALS | PLS | Flail Leg | Flail Arm | IBP | |
| Single Pulse | |||||
| RMT (%) | N or ↓; inexcitable (10–20%) | ↑↑ or inexcitable (71%) | N | N or ↓ | N |
| CSP (ms) | N or ↓ | ↓ | N or ↓ * | ↓ | N |
| CMCT (ms) | N or ↑ | N →↑↑ | ↑ | N or ↑ | ↑ |
| Paired-Pulse | |||||
| Averaged SICI, 1–7 ms (%) | ↓ or ↓↓ | ↓ | N or ↓ * | ↓ | N ** |
| ICF, 10–30 ms (%) | N or ↑ | ↑ | N | ↑ | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmadasa, T. Cortical Excitability across the ALS Clinical Motor Phenotypes. Brain Sci. 2021, 11, 715. https://doi.org/10.3390/brainsci11060715
Dharmadasa T. Cortical Excitability across the ALS Clinical Motor Phenotypes. Brain Sciences. 2021; 11(6):715. https://doi.org/10.3390/brainsci11060715
Chicago/Turabian StyleDharmadasa, Thanuja. 2021. "Cortical Excitability across the ALS Clinical Motor Phenotypes" Brain Sciences 11, no. 6: 715. https://doi.org/10.3390/brainsci11060715
APA StyleDharmadasa, T. (2021). Cortical Excitability across the ALS Clinical Motor Phenotypes. Brain Sciences, 11(6), 715. https://doi.org/10.3390/brainsci11060715






