Association between Temporomandibular Joint Disorder and Parkinson’s Disease
Abstract
:1. Introduction
2. Methods
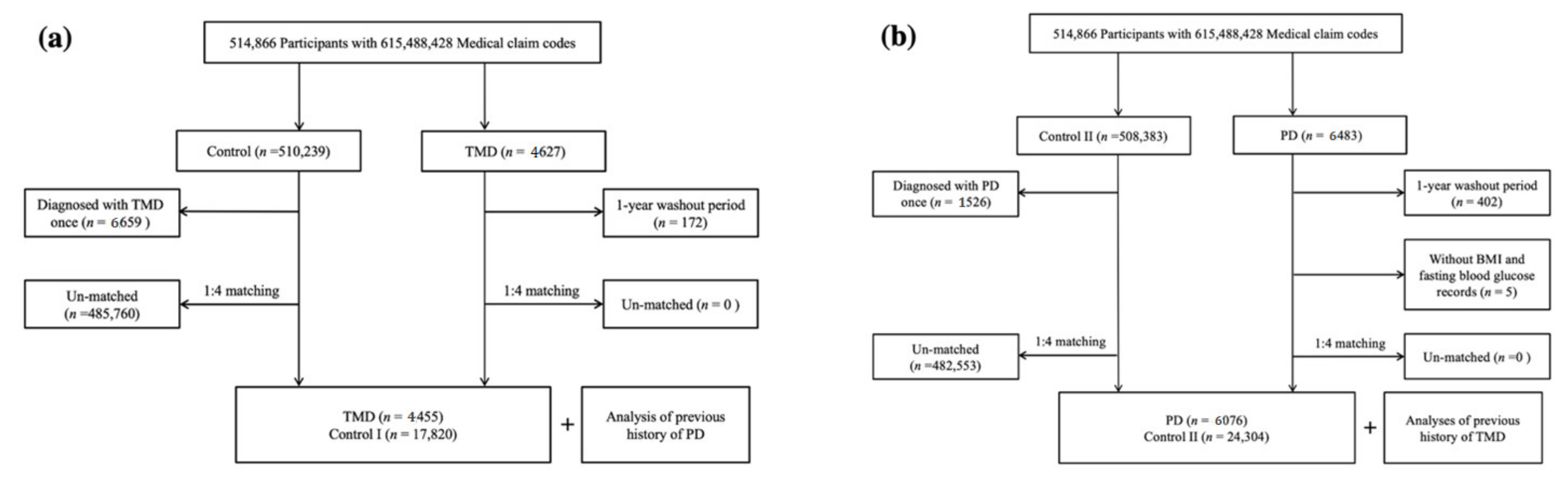
2.1. Study Population
2.2. Definition of Temporomandibular Joint Disorder
2.3. Definition of Parkinson’s Disease
2.4. Participant Selection
2.4.1. Study I
2.4.2. Study II
2.5. Covariates
2.6. Statistical Analyses
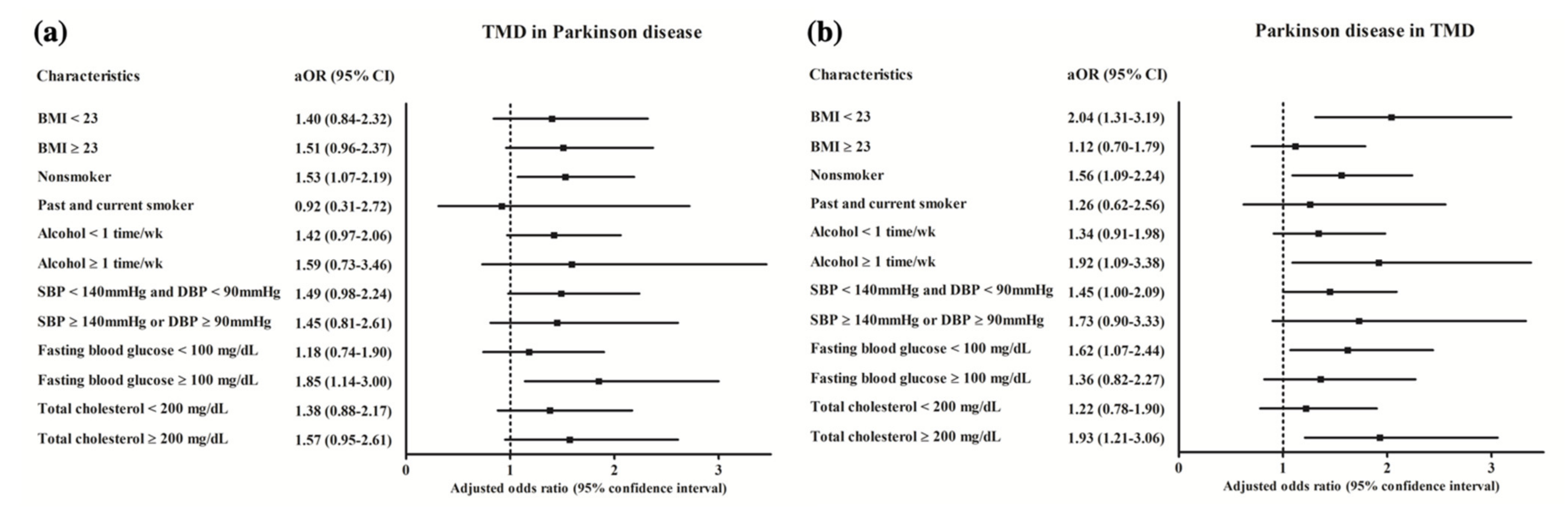
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Academy of Orofacial Pain. 22nd Meeting on Orofacial Pain and Temporomandibular Disorders. San Diego, California, February 14–16, 1997. Abstracts. J. Orofac. Pain 1997, 11, 178–186. [Google Scholar]
- Speciali, J.G.; Dach, F. Temporomandibular dysfunction and headache disorder. J. Head Face Pain 2015, 55, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, D.S.; Pal, U.S.; Jurel, S.K. Etiological factors of temporomandibular joint disorders. Natl. J. Maxillofac. Surg. 2011, 2, 116–119. [Google Scholar] [CrossRef]
- Shaffer, S.M.; Brismee, J.M.; Sizer, P.S.; Courtney, C.A. Temporomandibular disorders. Part 1: Anatomy and examination/diagnosis. J. Man. Manip. Ther. 2014, 22, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kanter, R.J.; Truin, G.J.; Burgersdijk, R.C.; Van‘t Hof, M.A.; Battistuzzi, P.G.; Kalsbeek, H.; Kayser, A.F. Prevalence in the Dutch adult population and a meta-analysis of signs and symptoms of temporomandibular disorder. J. Dent. Res. 1993, 72, 1509–1518. [Google Scholar] [CrossRef]
- Dworkin, S.F.; Huggins, K.H.; LeResche, L.; Von Korff, M.; Howard, J.; Truelove, E.; Sommers, E. Epidemiology of signs and symptoms in temporomandibular disorders: Clinical signs in cases and controls. J. Am. Dent. Assoc. 1990, 120, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, D.A.; Dal Fabbro, A.L.; Campos, J.A.; Bigal, M.E.; Speciali, J.G. Symptoms of temporomandibular disorders in the population: An epidemiological study. J. Orofac. Pain 2010, 24, 270–278. [Google Scholar]
- Franco, A.L.; Goncalves, D.A.; Castanharo, S.M.; Speciali, J.G.; Bigal, M.E.; Camparis, C.M. Migraine is the most prevalent primary headache in individuals with temporomandibular disorders. J. Orofac. Pain 2010, 24, 287–292. [Google Scholar]
- Lee, S.C.; Kim, J.H.; Kim, C.H.; Kim, B.J. Temporomandibular joint disorder from skull-base osteomyelitis: A case report. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 39. [Google Scholar] [CrossRef] [Green Version]
- Maeda, M.; Chaki, T.; Kawaguchi, R.; Kimijima, T.; Yamakage, M. Difficult airway management of a patient with limited range of motion in the temporomandibular joint and cervical extension caused by psoriatic arthritis: A case report. JA Clin. Rep. 2020, 6, 44. [Google Scholar] [CrossRef]
- Byun, S.H.; Min, C.; Choi, H.G.; Hong, S.J. Increased Risk of Temporomandibular Joint Disorder in Patients with Rheumatoid Arthritis: A Longitudinal Follow-Up Study. J. Clin. Med. 2020, 9, 3005. [Google Scholar] [CrossRef]
- Byun, S.H.; Min, C.; Yoo, D.M.; Yang, B.E.; Choi, H.G. Increased Risk of Migraine in Patients with Temporomandibular Disorder: A Longitudinal Follow-Up Study Using a National Health Screening Cohort. Diagnostics 2020, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.F.; LeResche, L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications, critique. J. Craniomandib. Disord. 1992, 6, 301–355. [Google Scholar]
- Sessle, B.J. Evolution of the Research Diagnostic Criteria for Temporomandibular Disorders. J. Orofac. Pain 2010, 24, 5. [Google Scholar] [PubMed]
- Dworkin, S.F. Research Diagnostic criteria for Temporomandibular Disorders: Current status & future relevance. J. Oral Rehabil. 2010, 37, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.L.; Truelove, E.L.; Ohrbach, R.; Anderson, G.C.; John, M.T.; List, T.; Look, J.O. The Research Diagnostic Criteria for Temporomandibular Disorders. I: Overview and methodology for assessment of validity. J. Orofac. Pain 2010, 24, 7–24. [Google Scholar] [PubMed]
- Bae, S.; Park, M.S.; Han, J.W.; Kim, Y.J. Correlation between pain and degenerative bony changes on cone-beam computed tomography images of temporomandibular joints. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 19. [Google Scholar] [CrossRef] [Green Version]
- Cairns, B.E. Pathophysiology of TMD pain--basic mechanisms and their implications for pharmacotherapy. J. Oral Rehabil. 2010, 37, 391–410. [Google Scholar] [CrossRef]
- De Lau, L.M.; Breteler, M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Sauerbier, A.; Qamar, M.A.; Rajah, T.; Chaudhuri, K.R. New concepts in the pathogenesis and presentation of Parkinson’s disease. Clin. Med. (Lond.) 2016, 16, 365–370. [Google Scholar] [CrossRef]
- Chagraoui, A.; Boukhzar, L.; Thibaut, F.; Anouar, Y.; Maltete, D. The pathophysiological mechanisms of motivational deficits in Parkinson’s disease. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2018, 81, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Haikal, C.; Li, W.; Li, J.Y. Gut Inflammation in Association With Pathogenesis of Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Fan, H.C.; Tung, M.C.; Chang, Y.K. The association between Parkinson’s disease and temporomandibular disorder. PLoS ONE 2019, 14, e0217763. [Google Scholar] [CrossRef] [Green Version]
- Palinkas, M.; Pagnano, L.; Da Siva, G.P.; Hallak, J.E.C.; Da Silva, N.S.; Regalo, I.H.; Verri, E.D.; Regalo, S.C.H.; Siessere, S. Does Parkinson’s disease interfere with electromyographic fatigue of masticatory muscles? Arch. Ital. Biol. 2019, 157, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, Y.; Balash, Y.; Korczyn, A.D.; Giladi, N.; Gurevich, T. Disorders of the oral cavity in Parkinson’s disease and parkinsonian syndromes. Park. Dis. 2015, 2015, 379482. [Google Scholar] [CrossRef] [Green Version]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Watts, M.W.; Tan, E.K.; Jankovic, J. Bruxism and cranial-cervical dystonia: Is there a relationship? Cranio 1999, 17, 196–201. [Google Scholar] [CrossRef]
- Silva, P.F.; Biasotto-Gonzalez, D.A.; Motta, L.J.; Silva, S.M.; Ferrari, R.A.; Fernandes, K.P.; Bussadori, S.K. Impact in oral health and the prevalence of temporomandibular disorder in individuals with Parkinson’s disease. J. Phys. Ther. Sci. 2015, 27, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Byun, S.H.; Min, C.; Park, I.S.; Kim, H.; Kim, S.K.; Park, B.J.; Choi, H.G.; Hong, S.J. Increased Risk of Chronic Periodontitis in Chronic Rhinosinusitis Patients: A Longitudinal Follow-Up Study Using a National Health-Screening Cohort. J. Clin. Med. 2020, 9, 1170. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.L.; Cho, J.J.; Park, K.M.; Noh, H.M.; Park, Y.S. Diagnostic performance of body mass index using the Western Pacific Regional Office of World Health Organization reference standards for body fat percentage. J. Korean Med. Sci. 2015, 30, 162–166. [Google Scholar] [CrossRef]
- Anuurad, E.; Shiwaku, K.; Nogi, A.; Kitajima, K.; Enkhmaa, B.; Shimono, K.; Yamane, Y. The new BMI criteria for asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J. Occup. Health 2003, 45, 335–343. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Verhoeff, M.C.; Lobbezoo, F.; Wetselaar, P.; Aarab, G.; Koutris, M. Parkinson’s disease, temporomandibular disorders and bruxism: A pilot study. J. Oral Rehabil. 2018, 45, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Laine, C.M.; Yavuz, S.U.; D’Amico, J.M.; Gorassini, M.A.; Turker, K.S.; Farina, D. Jaw tremor as a physiological biomarker of bruxism. Clin. Neurophysiol. 2015, 126, 1746–1753. [Google Scholar] [CrossRef]
- List, T.; Axelsson, S. Management of TMD: Evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2010, 37, 430–451. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Mantyvaara, J.; Sjoholm, T.; Kirjavainen, T.; Waltimo, A.; Iivonen, M.; Kemppainen, P.; Pertovaara, A. Altered control of submaximal bite force during bruxism in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, M.C.; Koutris, M.; van Selms, M.K.A.; Brandwijk, A.N.; Heres, M.S.; Berendse, H.W.; van Dijk, K.D.; Lobbezoo, F. Is dopaminergic medication dose associated with self-reported bruxism in Parkinson’s disease? A cross-sectional, questionnaire-based study. Clin. Oral Investig. 2021, 25, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.K.; Jankovic, J. Hemifacial spasm and hypertension: How strong is the association? Mov. Disord. 2000, 15, 363–365. [Google Scholar] [CrossRef]
- Abe, S.; Gagnon, J.F.; Montplaisir, J.Y.; Postuma, R.B.; Rompre, P.H.; Huynh, N.T.; Kato, T.; Kawano, F.; Lavigne, G.J. Sleep bruxism and oromandibular myoclonus in rapid eye movement sleep behavior disorder: A preliminary report. Sleep Med. 2013, 14, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Erro, R.; Stamelou, M.; Saifee, T.A.; Ganos, C.; Antelmi, E.; Balint, B.; Cordivari, C.; Bhatia, K.P. Facial tremor in dystonia. Park. Relat. Disord. 2014, 20, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Wilken, M.; Morisset, P.; Farina, S.; Cerquetti, D.; Merello, M. Facial tremors in patients with and without parkinsonism. Neurol. Sci. 2016, 37, 1999–2002. [Google Scholar] [CrossRef]
- Udagedara, T.B.; Gooneratne, I.K. Jaw tremor: A manifestation of vascular parkinsonism?—A case report. BMC Neurol. 2018, 18, 92. [Google Scholar] [CrossRef] [Green Version]
- Byun, S.H.; Min, C.; Hong, S.J.; Choi, H.G.; Koh, D.H. Analysis of the Relation between Periodontitis and Chronic Gastritis/Peptic Ulcer: A Cross-Sectional Study Using KoGES HEXA Data. Int. J. Environ. Res. Public Health 2020, 17, 4387. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.H.M., C.; Kim, Y.B.; Kim, H.; Kang, S.H.; Park, B.J.; Wee, J.H.; Choi, H.G.; Hong, S.J. Analysis of Chronic Periodontitis in Tonsillectomy Patients: A Longitudinal Follow-Up Study Using a National Health Screening Cohort. Appl. Sci. 2020, 10, 3663. [Google Scholar] [CrossRef]
- Byun, S.H.; Yoo, D.M.; Lee, J.W.; Choi, H.G. Analyzing the Association between Hyperuricemia and Periodontitis: A Cross-Sectional Study Using KoGES HEXA Data. Int. J. Environ. Res. Public Health 2020, 17, 777. [Google Scholar] [CrossRef]
| Characteristics | Study I | Study II | |||||
|---|---|---|---|---|---|---|---|
| TMD | Control I | p-Value | PD | Control II | p-Value | ||
| Age (n, %) | 1.000 | 1.000 | |||||
| 40–44 | 130 (2.9) | 520 (2.9) | 8 (0.1) | 32 (0.1) | |||
| 45–49 | 437 (9.8) | 1748 (9.8) | 75 (1.2) | 300 (1.2) | |||
| 50–54 | 697 (15.7) | 2788 (15.7) | 234 (3.9) | 936 (3.9) | |||
| 55–59 | 718 (16.1) | 2872 (16.1) | 361 (5.9) | 1444 (5.9) | |||
| 60–64 | 634 (14.2) | 2536 (14.2) | 654 (10.8) | 2616 (10.8) | |||
| 65–69 | 699 (15.7) | 2796 (15.7) | 1014 (16.7) | 4056 (16.7) | |||
| 70–74 | 603 (13.5) | 2412 (13.5) | 1431 (23.6) | 5724 (23.6) | |||
| 75–79 | 376 (8.4) | 1504 (8.4) | 1396 (23.0) | 5584 (23.0) | |||
| 80–84 | 130 (2.9) | 520 (2.9) | 721 (11.9) | 2884 (11.9) | |||
| 85+ | 31 (0.7) | 124 (0.7) | 182 (3.0) | 728 (3.0) | |||
| Sex (n, %) | 1.000 | 1.000 | |||||
| Males | 1903 (42.7) | 7612 (42.7) | 2858 (47.0) | 11,432 (47.0) | |||
| Females | 2552 (57.3) | 10,208 (57.3) | 3218 (53.0) | 12,872 (53.0) | |||
| Income (n, %) | 1.000 | 1.000 | |||||
| 1 (lowest) | 699 (15.7) | 2796 (15.7) | 1151 (18.9) | 4604 (18.9) | |||
| 2 | 572 (12.8) | 2288 (12.8) | 672 (11.1) | 2688 (11.1) | |||
| 3 | 717 (16.1) | 2868 (16.1) | 825 (13.6) | 3300 (13.6) | |||
| 4 | 929 (20.9) | 3716 (20.9) | 1148 (18.9) | 4592 (18.9) | |||
| 5 (highest) | 1538 (34.5) | 6152 (34.5) | 2280 (37.5) | 9120 (37.5) | |||
| Region of residence (n, %) | 1.000 | 1.000 | |||||
| Urban | 1822 (40.9) | 7288 (40.9) | 2252 (37.1) | 9008 (37.1) | |||
| Rural | 2633 (59.1) | 10,532 (59.1) | 3824 (62.9) | 15,296 (62.9) | |||
| Obesity † (n, %) | <0.001 * | 0.651 | |||||
| Underweight | 130 (2.9) | 469 (2.6) | 254 (4.2) | 922 (3.8) | |||
| Normal | 1731 (38.9) | 6248 (35.1) | 2165 (35.6) | 8771 (36.1) | |||
| Overweight | 1280 (28.7) | 4808 (27.0) | 1581 (26.0) | 6389 (26.3) | |||
| Obese I | 1219 (27.4) | 5710 (32.0) | 1883 (31.0) | 7452 (30.7) | |||
| Obese II | 95 (2.1) | 585 (3.3) | 193 (3.2) | 770 (3.2) | |||
| Smoking status (n, %) | <0.001 * | <0.001 * | |||||
| Non-smoker | 3416 (76.7) | 13,410 (75.3) | 4789 (78.8) | 18,269 (75.2) | |||
| Past smoker | 526 (11.8) | 1900 (10.7) | 670 (11.0) | 2897 (11.9) | |||
| Current smoker | 513 (11.5) | 2510 (14.1) | 617 (10.2) | 3138 (12.9) | |||
| Alcohol consumption (n, %) | 0.605 | <0.001 * | |||||
| <1 time a week | 3180 (71.4) | 12,650 (71.0) | 4746 (78.1) | 17,613 (72.5) | |||
| ≥1 time a week | 1275 (28.6) | 5170 (29.0) | 1330 (21.9) | 6691 (27.5) | |||
| Systolic blood pressure (n, %) | 0.194 | ||||||
| <120 mmHg | 1480 (33.2) | 5296 (29.7) | <0.001 * | 1384 (22.8) | 5458 (22.5) | ||
| 120–139 mmHg | 2168 (48.7) | 8566 (48.1) | 2877 (47.4) | 11,814 (48.6) | |||
| ≥140 mmHg | 807 (18.1) | 3958 (22.2) | 1815 (29.9) | 7032 (28.9) | |||
| Diastolic blood pressure (n, %) | <0.001 * | 0.396 | |||||
| <80 mmHg | 2264 (50.8) | 8377 (47.0) | 2656 (43.7) | 10,659 (43.9) | |||
| 80–89 mmHg | 1542 (34.6) | 6331 (35.5) | 2188 (36.0) | 8897 (36.6) | |||
| ≥90 mmHg | 649 (14.6) | 3112 (17.5) | 1232 (20.3) | 4748 (19.5) | |||
| Fasting blood glucose (n, %) | <0.001 * | <0.001 * | |||||
| <100 mg/dL | 2928 (65.7) | 11,222 (63.0) | 3292 (54.2) | 14,289 (58.8) | |||
| 100–125 mg/dL | 1201 (27.0) | 5025 (28.2) | 1918 (31.6) | 7338 (30.2) | |||
| ≥126 mg/dL | 326 (7.3) | 1573 (8.8) | 866 (14.3) | 2677 (11.0) | |||
| Total cholesterol (n, %) | 0.006 * | 0.003 * | |||||
| <200 mg/dL | 2387 (53.6) | 9317 (52.3) | 3414 (56.2) | 13,189 (54.3) | |||
| 200–239 mg/dL | 1503 (33.7) | 5912 (33.2) | 1805 (29.7) | 7774 (32.0) | |||
| ≥240 mg/dL | 565 (12.7) | 2591 (14.5) | 857 (14.1) | 3341 (13.8) | |||
| CCI score (n, %) | 0.003 * | <0.001 * | |||||
| 0 | 2988 (67.1) | 12,106 (67.9) | 2110 (34.7) | 13,659 (56.2) | |||
| 1 | 684 (15.4) | 2599 (14.6) | 1377 (22.7) | 4383 (18.0) | |||
| 2 | 410 (9.2) | 1400 (7.9) | 989 (16.3) | 2652 (10.9) | |||
| 3 | 169 (3.8) | 766 (4.3) | 652 (10.7) | 1555 (6.4) | |||
| ≥4 | 204 (4.6) | 949 (5.3) | 948 (15.6) | 2055 (8.5) | |||
| PD | 47 (1.1) | 132 (0.7) | 0.036 * | 6076 (100.0) | 0 (0.0) | <0.001 * | |
| TMD | 4445 (100.0) | 0 (0.0) | <0.001 * | 53 (0.9) | 145 (0.6) | 0.017 * | |
| Characteristics | Odd Ratios (95% Confidence Interval) for TMD | |||
|---|---|---|---|---|
| Crude † | p-Value | Adjusted †,‡ | p-Value | |
| Total participants (n = 22,275) | ||||
| PD | 1.43 (1.02–2.01) | 0.036 * | 1.43 (1.02–2.00) | 0.040 * |
| Non-PD | 1.00 | 1.00 | ||
| Age < 60 years old, males (n = 4275) | ||||
| PD | 6.00 (1.00–35.91) | 0.050 * | 5.68 (0.94–34.37) | 0.059 |
| Non-PD | 1.00 | 1.00 | ||
| Age < 60 years old, females (n = 5635) | ||||
| PD | 2.67 (0.45–16.06) | 0.282 | 3.03 (0.50–18.40) | 0.228 |
| Non-PD | 1.00 | 1.00 | ||
| Age ≥ 60 years old, males (n = 5240) | ||||
| PD | 1.17 (0.64–2.14) | 0.609 | 1.15 (0.63–2.10) | 0.658 |
| Non-PD | 1.00 | 1.00 | ||
| Age ≥ 60 years old, females (n = 7125) | ||||
| PD | 1.43 (0.92–2.21) | 0.109 | 1.40 (0.90–2.17) | 0.138 |
| Non-PD | 1.00 | 1.00 | ||
| High income (n = 9940) | ||||
| PD | 1.85 (1.11–3.08) | 0.018 * | 1.80 (1.08–3.00) | 0.025 * |
| Non-PD | 1.00 | 1.00 | ||
| Low income (n = 12,335) | ||||
| PD | 1.20 (0.76–1.88) | 0.440 | 1.20 (0.76–1.89) | 0.429 |
| Non-PD | 1.00 | 1.00 | ||
| Urban living (n = 9110) | ||||
| PD | 1.58 (0.91–2.73) | 0.105 | 1.56 (0.90–2.72) | 0.114 |
| Non-PD | 1.00 | 1.00 | ||
| Rural living (n = 13,165) | ||||
| PD | 1.36 (0.89–2.08) | 0.160 | 1.35 (0.88–2.07) | 0.167 |
| Non-PD | 1.00 | 1.00 | ||
| Characteristics | Odd Ratios (95% Confidence Interval) for PD | |||
|---|---|---|---|---|
| Crude † | p-Value | Adjusted †,‡ | p-Value | |
| Total participants (n = 30,380) | ||||
| TMD | 1.47 (1.07–2.01) | 0.018 * | 1.56 (1.13–2.15) | 0.007 * |
| Non-TMD | 1.00 | 1.00 | ||
| Age < 70 years old, males (n = 5770) | ||||
| TMD | 1.60 (0.50–5.12) | 0.426 | 2.16 (0.67–7.00) | 0.198 |
| Non-TMD | 1.00 | 1.00 | ||
| Age < 70 years old, females (n = 5960) | ||||
| TMD | 1.34 (0.63–2.85) | 0.454 | 1.33 (0.61–2.86) | 0.472 |
| Non-TMD | 1.00 | 1.00 | ||
| Age ≥ 70 years old, males (n = 8520) | ||||
| TMD | 1.37 (0.76–2.46) | 0.298 | 1.40 (0.77–2.54) | 0.266 |
| Non-TMD | 1.00 | 1.00 | ||
| Age ≥ 70 years old, females (n = 10,130) | ||||
| TMD | 1.57 (0.99-2.50) | 0.057 | 1.68 (1.04–2.70) | 0.033 * |
| Non-TMD | 1.00 | 1.00 | ||
| High income (n = 13,240) | ||||
| TMD | 1.59 (0.98–2.59) | 0.060 | 1.66 (1.01–2.73) | 0.046 * |
| Non-TMD | 1.00 | 1.00 | ||
| Low income (n = 17,140) | ||||
| TMD | 1.38 (0.91–2.10) | 0.127 | 1.49 (0.98–2.28) | 0.062 |
| Non-TMD | 1.00 | 1.00 | ||
| Urban living (n = 11,260) | ||||
| TMD | 1.31 (0.74–2.31) | 0.352 | 1.40 (0.78–2.48) | 0.257 |
| Non-TMD | 1.00 | 1.00 | ||
| Rural living (n = 19,120) | ||||
| TMD | 1.55 (1.06–2.27) | 0.025 * | 1.65 (1.12–2.43) | 0.011 * |
| Non-TMD | 1.00 | 1.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-G.; Yoon, J.-H.; Chung, T.-H.; Min, C.; Yoo, D.-M.; Wee, J.-H.; Kang, S.-Y.; Choi, Y.; Hong, S.-J.; Byun, S.-H. Association between Temporomandibular Joint Disorder and Parkinson’s Disease. Brain Sci. 2021, 11, 747. https://doi.org/10.3390/brainsci11060747
Choi H-G, Yoon J-H, Chung T-H, Min C, Yoo D-M, Wee J-H, Kang S-Y, Choi Y, Hong S-J, Byun S-H. Association between Temporomandibular Joint Disorder and Parkinson’s Disease. Brain Sciences. 2021; 11(6):747. https://doi.org/10.3390/brainsci11060747
Chicago/Turabian StyleChoi, Hyo-Geun, Joo-Heung Yoon, Tae-Hwan Chung, Chanyang Min, Dae-Myoung Yoo, Jee-Hye Wee, Suk-Yun Kang, Yeso Choi, Seok-Jin Hong, and Soo-Hwan Byun. 2021. "Association between Temporomandibular Joint Disorder and Parkinson’s Disease" Brain Sciences 11, no. 6: 747. https://doi.org/10.3390/brainsci11060747






