Prevalence and Characteristics of Polyneuropathy in Atypical Parkinsonian Syndromes: An Explorative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Assessments
2.2. Nerve Conduction Studies, HRUS and Diagnosis Criteria of PNP
2.3. Laboratory Assessment
2.4. Statistical Analysis
3. Results
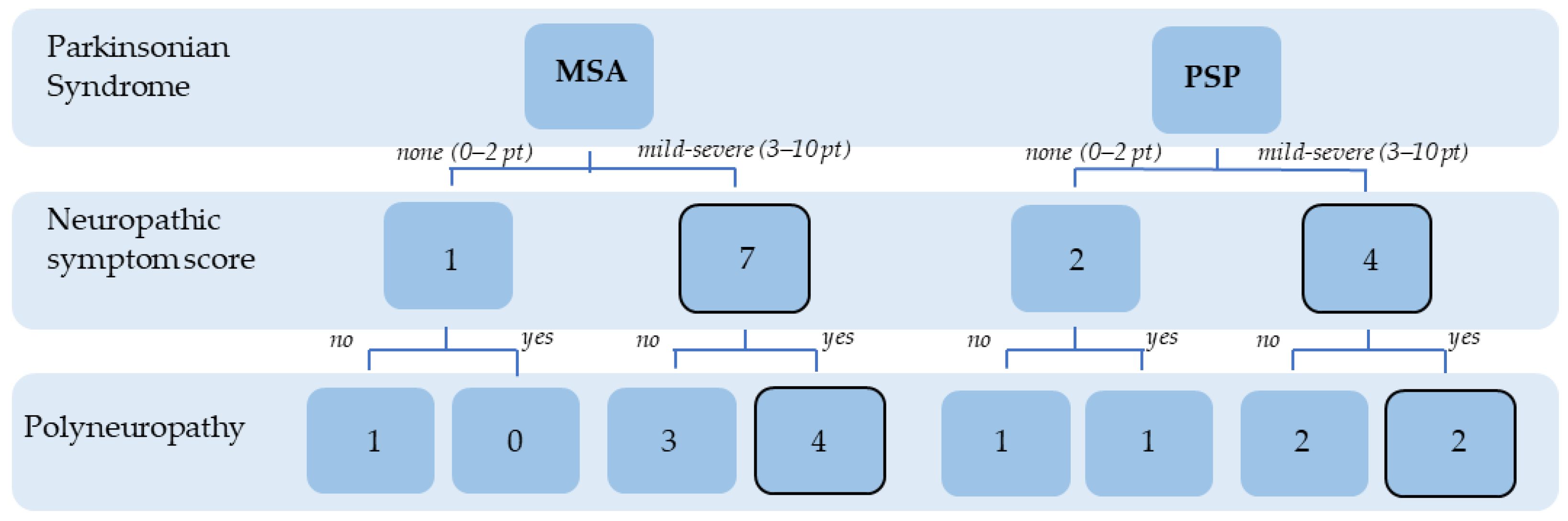
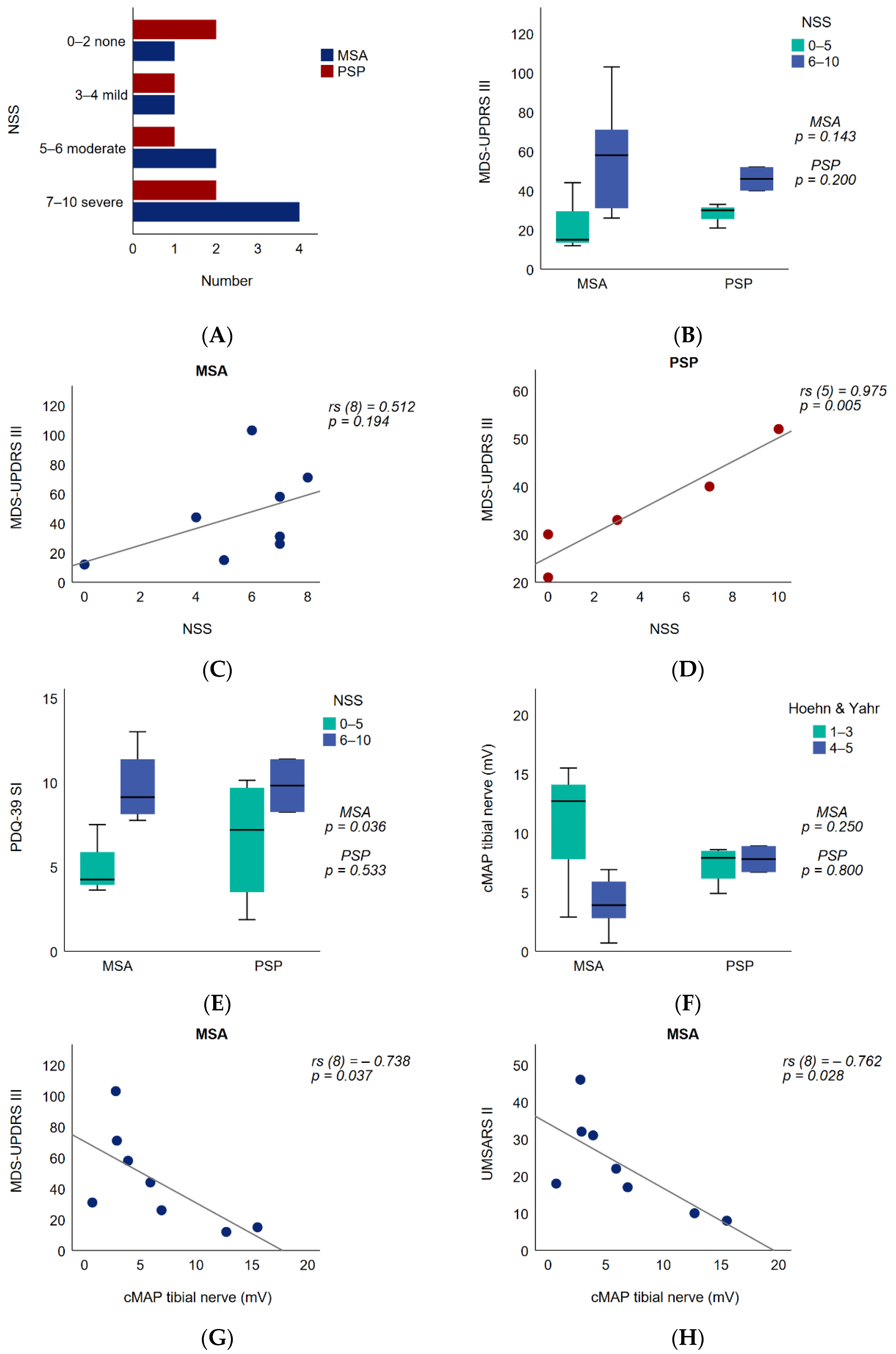
3.1. Demographic and Clinical Data
3.2. Patient-Reported Neuropathy Symptoms and Functional Impact
3.3. Peripheral Nerve Motor Amplitude and Functional Impact
3.4. Nerve Alterations in High-Resolution Ultrasound of Peripheral Nerves
3.5. Laboratory Assessment of PNP in APS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Dürr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Multiple System Atrophy: An Oligodendroglioneural Synucleinopathy1. J. Alzheimer’s Dis. 2018, 62, 1141–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Boxer, A.L.; Yu, J.-T.; Golbe, L.I.; Litvan, I.; Lang, A.E.; Höglinger, G.U. Advances in progressive supranuclear palsy: New diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017, 16, 552–563. [Google Scholar] [CrossRef] [Green Version]
- Colosimo, C.; Morgante, L.; Antonini, A.; Barone, P.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, M.G.; Ceravolo, R.; Cicarelli, G.; et al. Non-motor symptoms in atypical and secondary parkinsonism: The PRIAMO study. J. Neurol. 2010, 257, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Chaithra, S.P.; Prasad, S.; Holla, V.V.; Stezin, A.; Kamble, N.; Yadav, R.; Pal, P.K. The Non-Motor Symptom Profile of Progressive Supranuclear Palsy. J. Mov. Disord. 2020, 13, 118–126. [Google Scholar] [CrossRef]
- Toth, C.; Breithaupt, K.; Ge, S.; Duan, Y.; Terris, J.M.; Thiessen, A.; Wiebe, S.; Zochodne, D.W.; Suchowersky, O. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann. Neurol. 2010, 68, 28–36. [Google Scholar] [CrossRef]
- Merola, A.; Rosso, M.; Romagnolo, A.; Comi, C.; Fasano, A.; Zibetti, M.; Lopez-Castellanos, J.R.; Cocito, D.; Lopiano, L.; Espay, A.J. Peripheral neuropathy as marker of severe Parkinson’s disease phenotype. Mov. Disord. 2017, 32, 1256–1258. [Google Scholar] [CrossRef]
- Kühn, E.; Averdunk, P.; Huckemann, S.; Müller, K.; Biesalski, A.-S.; Zum Hof Berge, F.; Motte, J.; Fisse, A.L.; Schneider-Gold, C.; Gold, R.; et al. Correlates of polyneuropathy in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 1898–1907. [Google Scholar] [CrossRef]
- Doppler, K.; Jentschke, H.-M.; Schulmeyer, L.; Vadasz, D.; Janzen, A.; Luster, M.; Höffken, H.; Mayer, G.; Brumberg, J.; Booij, J.; et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 2017, 133, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Pramstaller, P.P.; Wenning, G.K.; Smith, S.J.; Beck, R.O.; Quinn, N.P.; Fowler, C.J. Nerve conduction studies, skeletal muscle EMG, and sphincter EMG in multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 1995, 58, 618–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawel, M.; Jamrozik, Z.; Szmidt-Salkowska, E.; Slawek, J.; Rowinska-Marcinska, K. Is peripheral neuron degeneration involved in multiple system atrophy? A clinical and electrophysiological study. J. Neurol. Sci. 2012, 319, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Abele, M.; Schulz, J.B.; Bürk, K.; Topka, H.; Dickgans, J.; Klockgether, T. Nerve conduction studies in multiple system atrophy. Eur. Neurol. 2000, 43, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Yoshida, M.; Hashizume, Y.; Hishikawa, N.; Sobue, G. alpha-Synuclein-immunoreactive structure formation is enhanced in sympathetic ganglia of patients with multiple system atrophy. Acta Neuropathol. 2005, 110, 19–26. [Google Scholar] [CrossRef]
- Doppler, K.; Weis, J.; Karl, K.; Ebert, S.; Ebentheuer, J.; Trenkwalder, C.; Klebe, S.; Volkmann, J.; Sommer, C. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov. Disord. 2015, 30, 1688–1692. [Google Scholar] [CrossRef]
- Nakamura, K.; Mori, F.; Kon, T.; Tanji, K.; Miki, Y.; Tomiyama, M.; Kurotaki, H.; Toyoshima, Y.; Kakita, A.; Takahashi, H.; et al. Filamentous aggregations of phosphorylated α-synuclein in Schwann cells (Schwann cell cytoplasmic inclusions) in multiple system atrophy. Acta Neuropathol. Commun. 2015, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Gawel, M.; Jamrozik, Z.; Szmidt-Salkowska, E.; Slawek, J.; Gawel, D.; Rowińska-Marcińska, K.; Kaminska, A. Electrophysiological features of lower motor neuron involvement in progressive supranuclear palsy. J. Neurol. Sci. 2013, 324, 136–139. [Google Scholar] [CrossRef]
- Dubbioso, R.; Provitera, V.; Vitale, F.; Stancanelli, A.; Borreca, I.; Caporaso, G.; de Michele, G.; de Rosa, A.; Picillo, M.; Barone, P.; et al. Cutaneous sensory and autonomic denervation in Progressive Supranuclear Palsy. Neuropathol. Appl. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Dyck, P.J.; Sherman, W.R.; Hallcher, L.M.; Service, F.J.; O’Brien, P.C.; Grina, L.A.; Palumbo, P.J.; Swanson, C.J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann. Neurol. 1980, 8, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Lu, B.; Ye, H.; Wu, X.; Zhang, T.; Li, Y. The Diagnostic Value of Neuropathy Symptom and Change Score, Neuropathy Impairment Score and Michigan Neuropathy Screening Instrument for Diabetic Peripheral Neuropathy. Eur. Neurol. 2015, 74, 323–327. [Google Scholar] [CrossRef]
- Wenning, G.K.; Tison, F.; Seppi, K.; Sampaio, C.; Diem, A.; Yekhlef, F.; Ghorayeb, I.; Ory, F.; Galitzky, M.; Scaravilli, T.; et al. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov. Disord. 2004, 19, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Golbe, L.I.; Ohman-Strickland, P.A. A clinical rating scale for progressive supranuclear palsy. Brain 2007, 130, 1552–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Marinus, J.; Stiggelbout, A.M.; van Hilten, J.J. Assessment of autonomic dysfunction in Parkinson’s disease: The SCOPA-AUT. Mov. Disord. 2004, 19, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.V.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Jenkinson, C.; Fitzpatrick, R.; Peto, V.; Greenhall, R.; Hyman, N. The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Hagell, P.; Nilsson, M.H. The 39-Item Parkinson’s Disease Questionnaire (PDQ-39): Is it a Unidimensional Construct? Ther. Adv. Neurol. Disord. 2009, 2, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöhr, M.; Pfister, R. Klinische Elektromyographie und Neurographie-Lehrbuch und Atlas, 6th ed.; Kohlhammer: Stuttgart, Germany, 2014; ISBN 9783170214736. [Google Scholar]
- Kerasnoudis, A.; Pitarokoili, K.; Behrendt, V.; Gold, R.; Yoon, M.-S. Cross sectional area reference values for sonography of peripheral nerves and brachial plexus. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2013, 124, 1881–1888. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Statist. 1979, 6, 65–70. [Google Scholar]
- Beghi, E.; Monticelli, M.L.; Amoruso, L.; Apollo, F.; Delodovici, M.L.; Grampa, G.; Perini, M.; Porazzi, D.; Simone, P.; Tonali, P.; et al. Chronic symmetric symptomatic polyneuropathy in the elderly: A field screening investigation in two Italian regions. I. Prevalence and general characteristics of the sample. Italian General Practitioner Study Group (IGPSG). Neurologie 1995, 45, 1832–1836. [Google Scholar] [CrossRef]
- Hanewinckel, R.; Drenthen, J.; van Oijen, M.; Hofman, A.; van Doorn, P.A.; Ikram, M.A. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurologie 2016, 87, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Zis, P.; Grünewald, R.A.; Chaudhuri, R.K.; Hadjivassiliou, M. Peripheral neuropathy in idiopathic Parkinson’s disease: A systematic review. J. Neurol. Sci. 2017, 378, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Visser, N.A.; Notermans, N.C.; Linssen, R.S.N.; van den Berg, L.H.; Vrancken, A.F.J.E. Incidence of polyneuropathy in Utrecht, the Netherlands. Neurologie 2015, 84, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Vitaliani, R.; Scaravilli, T.; Egarter-Vigl, E.; Giometto, B.; Klein, C.; Scaravilli, F.; An, S.F.; Pramstaller, P.P. The pathology of the spinal cord in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2002, 61, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.; Yoshida, M.; Hashizume, Y.; Hattori, M.; Aiba, I.; Sobue, G. Widespread spinal cord involvement in progressive supranuclear palsy. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2007, 27, 331–340. [Google Scholar] [CrossRef]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.E.; Kulkarni, J.; Boulton, A.J.M. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanta, O.M.; Pintea, S.; Perju-Dumbrava, L. The impact of associated large-fiber peripheral neuropathy on health-related quality of life in Parkinson’s disease—Results from a Romanian cohort. Rom. J. Neurol. 2019, 18, 177–183. [Google Scholar] [CrossRef]
- Carpenter, M.G.; Bloem, B.R. Postural control in Parkinson patients: A proprioceptive problem? Exp. Neurol. 2011, 227, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Radicati, F.G.; Martinez Martin, P.; Fossati, C.; Chaudhuri, K.R.; Torti, M.; Rodriguez Blazquez, C.; Vacca, L.; Stocchi, F. Non motor symptoms in progressive supranuclear palsy: Prevalence and severity. NPJ Parkinson’s Dis. 2017, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Avenali, M.; Tassorelli, C.; de Icco, R.; Perrotta, A.; Serrao, M.; Fresia, M.; Pacchetti, C.; Sandrini, G. Pain processing in atypical Parkinsonisms and Parkinson disease: A comparative neurophysiological study. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017, 128, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, F.M.; Murray, B.; Coen, R.F.; Dockree, P.M.; Bellgrove, M.A.; Garavan, H.; Lynch, T.; Robertson, I.H. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain 2007, 130, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Melis, M. The peripheral nerve involvement in Parkinson Disease: A multifaceted phenomenon. Parkinsonism Relat. Disord. 2016, 25, 17–20. [Google Scholar] [CrossRef]
- Rodolico, C.; Toscano, A.; De Luca, G.; Mazzeo, A.; Di Leo, R.; Baldari, S.; Girlanda, P.; Vita, G. Peripheral neuropathy as the presenting feature of multiple system atrophy. Clin. Auton. Res. 2001, 11, 119–121. [Google Scholar] [CrossRef]
- Kanda, T.; Tsukagoshi, H.; Oda, M.; Miyamoto, K.; Tanabe, H. Changes of unmyelinated nerve fibers in sural nerve in amyotrophic lateral sclerosis, Parkinson’s disease and multiple system atrophy. Acta Neuropathol. 1996, 91, 145–154. [Google Scholar] [CrossRef]
- Galassi, G.; Nemni, R.; Baraldi, A.; Gibertoni, M.; Colombo, A. Peripheral neuropathy in multiple system atrophy with autonomic failure. Neurologie 1982, 32, 1116–1121. [Google Scholar] [CrossRef]
- Kuzdas-Wood, D.; Irschick, R.; Theurl, M.; Malsch, P.; Mair, N.; Mantinger, C.; Wanschitz, J.; Klimaschewski, L.; Poewe, W.; Stefanova, N.; et al. Involvement of Peripheral Nerves in the Transgenic PLP-α-Syn Model of Multiple System Atrophy: Extending the Phenotype. PLoS ONE 2015, 10, e0136575. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Namba, Y.; Ikeda, K.; Akiguchi, I.; Oda, M. Neurofibrillary tangles in the neurons of spinal dorsal root ganglia of patients with progressive supranuclear palsy. Acta Neuropathol. 1993, 85, 453–457. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Hayashi, S.; Morita, T.; Shibasaki, Y.; Watanabe, Y.; Takahashi, H. Neurofibrillary tangles in the peripheral sympathetic ganglia of non-Alzheimer elderly individuals. Clin. Neuropathol. 1999, 18, 171–175. [Google Scholar]
- Rodríguez-Leyva, I.; Chi-Ahumada, E.G.; Carrizales, J.; Rodríguez-Violante, M.; Velázquez-Osuna, S.; Medina-Mier, V.; Martel-Gallegos, M.G.; Zarazúa, S.; Enríquez-Macías, L.; Castro, A.; et al. Parkinson disease and progressive supranuclear palsy: Protein expression in skin. Ann. Clin. Transl. Neurol. 2016, 3, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Pitarokoili, K.; Fels, M.; Kerasnoudis, A.; Tönges, L.; Gold, R.; Yoon, M.-S. High-Resolution Nerve Ultrasound and Electrophysiological Findings in Restless Legs Syndrome. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2018, 28, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Pitarokoili, K.; Kerasnoudis, A.; Behrendt, V.; Labedi, A.; Ayzenberg, I.; Gold, R.; Yoon, M.-S. Facing the diagnostic challenge: Nerve ultrasound in diabetic patients with neuropathic symptoms. Muscle Nerve 2016, 54, 18–24. [Google Scholar] [CrossRef] [PubMed]

| Total MSA Patients | MSA Patients without PNP (A) | MSA Patients with PNP (B) | Median Difference | 95% CI | p Value | ||
|---|---|---|---|---|---|---|---|
| (n = 8) | (n = 4) | (n = 4) | LL | UL | (A vs. B) | ||
| Age at evaluation (years) | 68.9 ± 9.2 | 63.3 ± 8.2 | 74.5 ± 6.8 | −11.0 | −25.0 | 2.0 | 0.078 |
| Female | 4 | 1 | 3 | n.a. | n.a. | n.a. | 0.486 |
| Disease duration (years) | 3.3 ± 2.3 | 2.3 ± 1.5 | 4.3 ± 2.6 | −1.5 | −7.0 | 1.0 | 0.235 |
| Age at diagnosis (years) | 65.5 ± 9.6 | 60.8 ± 9.1 | 70.3 ± 8.5 | −9.5 | −24.0 | 9.0 | 0.176 |
| Hoehn & Yahr, Median (IQR) | 4 ± 1.4 | 3.3 ± 2.6 | 4 ± 0.8 | −0.3 | −2.0 | 1.0 | 0.686 |
| Levodopa equivalence dose | 705 ± 327.7 | 486.8 ± 207.3 | 923.3 ± 283.9 | −464.5 | −928.0 | −62.0 | 0.048 |
| MDS-UPDRS III Score | 45 ± 31.1 | 39 ± 43.1 | 51 ± 17.3 | −24.0 | −56.0 | 59.0 | 0.624 |
| UMSARS II Score | 23 ± 12.7 | 20.3 ± 17.6 | 25.8 ± 6.8 | −11.0 | −23.0 | 24.0 | 0.581 |
| NSS Score | 5.5 ± 2.6 | 4.5 ± 3.1 | 6.5 ± 1.7 | −1.5 | −7.0 | 2.0 | 0.304 |
| NDS Score 1 | 2.3 ± 1.8 | 1.5 ± 1.9 | 3.3 ± 1.2 | −2.0 | −4.0 | 2.0 | 0.206 |
| PDQ-39 SI | 8.1 ± 3.2 | 7.5 ± 4.4 | 8.7 ± 1.8 | −2.8 | −7.1 | 5.3 | 0.636 |
| NCS: Amplitude (mV) | |||||||
| N. suralis 2 | 2.8 ± 2.9 | 4 ± 3.5 | 1.9 ± 2.5 | 2.2 | −5.2 | 6.7 | 0.393 |
| N. tibialis | 6.4 ± 5.2 | 9.5 ± 5.7 | 3.4 ± 2.2 | 6.5 | −1.1 | 12.6 | 0.118 |
| N. medianus (motor) 1 | 6.1 ± 3.4 | 6.3 ± 3.2 | 5.8 ± 4.3 | 0.7 | −6.3 | 9.1 | 0.875 |
| N. medianus (sensory) 1 | 6.8 ± 4.9 | 9.1 ± 4.8 | 3.7 ± 3.6 | 4.5 | −1.8 | 16.0 | 0.168 |
| HRUS: CSA of Entrapment Sites (mm2) | |||||||
| N. medianus (Carpal tunnel) 3 | 10.9 ± 2 | 11 ± 2.3 | 10.7 ± 0 | 0.3 | −2.3 | 3.2 | 0.900 |
| N. fibularis (Caput fibularis) 4 | 13.4 ± 5.9 | 11.3 ± 1.6 | 17.6 ± 10.7 | −6.2 | −15.8 | 3.4 | 0.800 |
| HRUS: CSA of Non-Entrapment Sites (mm2) | |||||||
| N. medianus (upper arm) 4 | 9 ± 1.3 | 9.3 ± 1.3 | 8.2 ± 1.3 | 1.4 | −0.5 | 4.1 | 0.385 |
| N. fibularis (fossa tibialis) 4 | 9.2 ± 3.8 | 7.4 ± 1.5 | 12.8 ± 5.1 | −5.4 | −10.7 | −0.1 | 0.370 |
| Total PSP Patients | PSP Patients without PNP (C) | PSP Patients with PNP (D) | Median Difference | 95% CI | p Value | ||
| (n = 6) | (n = 3) | (n = 3) | LL | UL | (C vs. D) | ||
| M ± SD | M ± SD | M ± SD | |||||
| Age at evaluation (years) | 72.7 ± 10.2 | 74 ± 5.2 | 71.3 ± 15 | 5.0 | −18.0 | 21.0 | 0.786 |
| Female | 1 | 0 | 1 | n.a. | n.a. | n.a. | 1.000 |
| Disease duration (years) | 4.5 ± 3.4 | 3.3 ± 1.5 | 5.7 ± 4.7 | −1.0 | −9.0 | 3.0 | 0.700 |
| Age at diagnosis (years) | 67.7 ± 13.6 | 70.3 ± 7.2 | 65 ± 19.7 | 6.0 | −21.0 | 31.0 | 0.682 |
| Hoehn & Yahr, Median (IQR) | 3 ± 1 | 3 ± 0 | 3 ± 0 | 0.0 | −1.0 | 1.0 | 1.000 |
| Levodopa equivalence dose | 410.8 ± 392.9 | 475 ± 532.1 | 346.7 ± 300.9 | 0.0 | −540.0 | 1050.0 | 0.735 |
| MDS−UPDRS III Score 5 | 35.2 ± 11.6 | 36.5 ± 21.9 | 34.3 ± 5.1 | 1.5 | −19.0 | 22.0 | 0.912 |
| PSPRS V + VI Score 6 | 14 ± 4.7 | 14.5 ± 6.4 | 13.5 ± 4.9 | 1.0 | −7.0 | 9.0 | 0.877 |
| NSS Score | 4.2 ± 4 | 5 ± 5 | 3.3 ± 3.5 | 2.0 | −7.0 | 10.0 | 0.661 |
| NDS Score | 4 ± 1.8 | 4.7 ± 2.3 | 3.3 ± 1.2 | 2.0 | −2.0 | 4.0 | 0.422 |
| PDQ-39 SI | 7.7 ± 3.5 | 8.6 ± 3.2 | 6.8 ± 4.3 | 1.3 | −5.0 | 9.5 | 0.586 |
| NCS: Amplitude (mV) | |||||||
| N. suralis | 2.7 ± 2.2 | 4.2 ± 1 | 1.2 ± 2.1 | 3.3 | −0.4 | 5.2 | 0.096 |
| N. tibialis | 7.5 ± 1.5 | 8.3 ± 0.8 | 6.7 ± 1.8 | 1.9 | −1.0 | 4.0 | 0.215 |
| N. medianus (motor) | 5.4 ± 2.2 | 6.6 ± 2.5 | 4.1 ± 0.5 | 3.6 | −0.8 | 4.8 | 0.400 |
| N. medianus (sensory) | 7.2 ± 4.8 | 6.3 ± 2.4 | 8 ± 7 | −4.4 | −9.2 | 8.7 | 0.707 |
| HRUS: CSA of Entrapment Sites (mm2) | |||||||
| N. medianus (Carpal tunnel) | 9 ± 2.1 | 10.4 ± 2 | 7.7 ± 0.9 | 2.4 | 0.7 | 6.1 | 0.102 |
| N. fibularis (Caput fibularis) | 19.1 ± 9.1 | 16.7 ± 6.7 | 21.5 ± 12.1 | −2.6 | −23.6 | 10.0 | 0.400 |
| HRUS: CSA of Non-Entrapment Sites (mm2) | |||||||
| N. medianus (upper arm) | 8.9 ± 1.4 | 9.4 ± 1.1 | 8.4 ± 1.7 | 0.8 | −1.6 | 3.9 | 0.425 |
| N. fibularis (fossa tibialis) | 11.3 ± 3.8 | 10 ± 5.4 | 12.6 ± 1.5 | −4.4 | −7.2 | 5.3 | 0.466 |
| mild, sensory PNP | amplitude of the sural sNAP <5 mV for patients <50 years or <3.6 mV for patients >50 years amplitude of the tibial cMAP >5 mV amplitude of the median cMAP >5 mV |
| moderate, sensorimotor PNP | amplitude of the sural sNAP <5 mV for patients <50 years and <3.6 mV for patients >50 years amplitude of the tibial cMAP <5 mV amplitude of the median cMAP >5 mV |
| severe, sensorimotor PNP | amplitude of the sural sNAP <5 mV for patients <50 years and <3.6 mV for patients >50 years amplitude of the tibial cMAP <5 mV amplitude of the median cMAP <5 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohmann, R.; Kühn, E.; Scherbaum, R.; Hilker, L.; Kools, S.; Scholz, L.; Müller, K.; Huckemann, S.; Schneider-Gold, C.; Gold, R.; et al. Prevalence and Characteristics of Polyneuropathy in Atypical Parkinsonian Syndromes: An Explorative Study. Brain Sci. 2021, 11, 879. https://doi.org/10.3390/brainsci11070879
Rohmann R, Kühn E, Scherbaum R, Hilker L, Kools S, Scholz L, Müller K, Huckemann S, Schneider-Gold C, Gold R, et al. Prevalence and Characteristics of Polyneuropathy in Atypical Parkinsonian Syndromes: An Explorative Study. Brain Sciences. 2021; 11(7):879. https://doi.org/10.3390/brainsci11070879
Chicago/Turabian StyleRohmann, Rachel, Eva Kühn, Raphael Scherbaum, Lovis Hilker, Saskia Kools, Leonard Scholz, Katharina Müller, Sophie Huckemann, Christiane Schneider-Gold, Ralf Gold, and et al. 2021. "Prevalence and Characteristics of Polyneuropathy in Atypical Parkinsonian Syndromes: An Explorative Study" Brain Sciences 11, no. 7: 879. https://doi.org/10.3390/brainsci11070879
APA StyleRohmann, R., Kühn, E., Scherbaum, R., Hilker, L., Kools, S., Scholz, L., Müller, K., Huckemann, S., Schneider-Gold, C., Gold, R., Pitarokoili, K., Tönges, L., & Kwon, E. H. (2021). Prevalence and Characteristics of Polyneuropathy in Atypical Parkinsonian Syndromes: An Explorative Study. Brain Sciences, 11(7), 879. https://doi.org/10.3390/brainsci11070879







