The Effect of Comorbid Attention-Deficit/Hyperactivity Disorder Symptoms on Face Memory in Children with Autism Spectrum Disorder: Insights from Transdiagnostic Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Characteristics
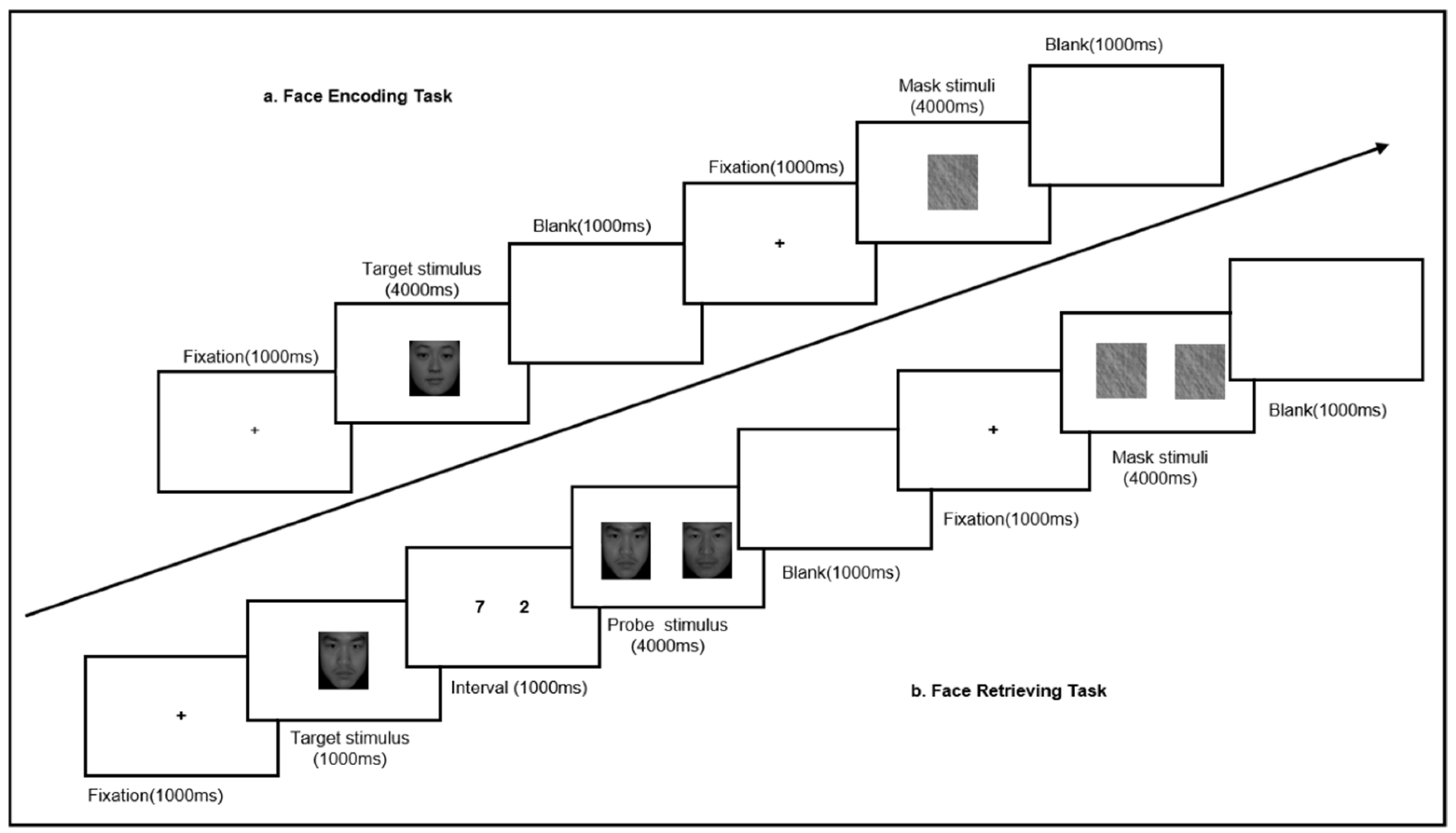
2.3. Face Memory
2.4. Executive Function
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Ability of Face Memory
3.3. Correlation between Face Encoding and Retrieving and the Symptoms of ASD and ADHD
3.4. Association between Face Memory and Executive Function
4. Discussion
4.1. Transdiagnostic Features of Face Memory in Children with ASD and ADHD
4.2. Characteristic of Face Memory in Children with ASD Comorbid ADHD Symptoms
4.3. Relationship between EF and Face Memory
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collin, L.; Bindra, J.; Raju, M.; Gillberg, C.; Minnis, H. Facial emotion recognition in child psychiatry: A systematic review. Res. Dev. Disabil. 2013, 34, 1505–1520. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, E.; Kresse, A.; Faja, S.; Bernier, R.A.; Webb, S.J. Face processing among twins with and without autism: Social correlates and twin concordance. Soc. Cogn. Affect. Neurosci. 2016, 11, 44–54. [Google Scholar] [CrossRef]
- Lewis, G.J.; Shakeshaft, N.G.; Plomin, R. Face Identity Recognition and the Social Difficulties Component of the Autism-Like Phenotype: Evidence for Phenotypic and Genetic Links. J. Autism Dev. Disord. 2018, 48, 2758–2765. [Google Scholar] [CrossRef] [Green Version]
- Minio-Paluello, I.; Porciello, G.; Pascual-Leone, A.; Baron-Cohen, S. Face individual identity recognition: A potential endophenotype in autism. Mol. Autism 2020, 11, 81. [Google Scholar] [CrossRef]
- Corbett, B.A.; Newsom, C.; Key, A.P.; Qualls, L.R.; Edmiston, E.K. Examining the relationship between face processing and social interaction behavior in children with and without autism spectrum disorder. J. Neurodev. Disord. 2014, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Webb, S.J.; Neuhaus, E.; Faja, S. Face perception and learning in autism spectrum disorders. Q. J. Exp. Psychol. 2017, 70, 970–986. [Google Scholar] [CrossRef]
- Lord, C.; Bishop, S.; Anderson, D. Developmental trajectories as autism phenotypes. Am. J. Med. Genet. Part. C Semin. Med. Genet. 2015, 169, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Lai, M.C.; Baron-Cohen, S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry 2019, 24, 1435–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avni, E.; Ben-Itzchak, E.; Zachoro, D.A. The Presence of Comorbid ADHD and Anxiety Symptoms in Autism Spectrum Disorder: Clinical Presentation and Predictors. Front. Psychiatry 2018, 9, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llanes, E.; Blacher, J.; Stavropoulos, K.; Eisenhower, A. Parent and Teacher Reports of Comorbid Anxiety and ADHD Symptoms in Children with ASD. J. Autism Dev. Disord. 2020, 50, 1520–1531. [Google Scholar] [CrossRef]
- Kentrou, V.; De Veld, D.M.J.; Mataw, K.J.K.; Begeer, S. Delayed autism spectrum disorder recognition in children and adolescents previously diagnosed with attention-deficit/hyperactivity disorder. Autism 2019, 23, 1065–1072. [Google Scholar] [CrossRef]
- Ameis, S.H. Heterogeneity Within and Between Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder Challenge or Opportunity? JAMA Psychiatry 2017, 74, 1093–1094. [Google Scholar] [CrossRef]
- Carta, A.; Fuca, E.; Guerrera, S.; Napoli, E.; Valeri, G.; Vicari, S. Characterization of Clinical Manifestations in the Co-occurring Phenotype of Attention Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Front. Psychol. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Hollingdale, J.; Absoue, M.; Bolton, P.; Branney, P.; Colley, W.; Craze, E.; Dave, M.; Deeley, Q.; Farrag, E.; et al. Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus. BMC Med. 2020, 18, 146. [Google Scholar] [CrossRef]
- Locke, J.; Shih, W.; Kretzmann, M.; Kasari, C. Examining playground engagement between elementary school children with and without autism spectrum disorder. Autism 2016, 20, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Mikami, A.Y.; Miller, M.; Lerner, M.D. Social functioning in youth with attention-deficit/hyperactivity disorder and autism spectrum disorder: Transdiagnostic commonalities and differences. Clin. Psychol. Rev. 2019, 68, 54–70. [Google Scholar] [CrossRef]
- Zaidman-Zait, A.; Mirenda, P.; Szatmari, P.; Duku, E.; Smith, I.M.; Zwaigenbaum, L.; Vaillancourt, T.; Kerns, C.; Volden, J.; Waddell, C.; et al. Profiles and Predictors of Academic and Social School Functioning among Children with Autism Spectrum Disorder. J. Clin. Child. Adolesc. Psychol. 2020, 1–13. [Google Scholar] [CrossRef]
- Abikoff, H.; Hechtman, L.; Klein, R.G.; Gallagher, R.; Fleiss, K.; Etcovitch, J.; Cousins, L.; Greenfield, B.; Martin, D.; Pollalck, S. Social functioning in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J. Am. Acad. Child. Adolesc. Psychiatry 2004, 43, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, N.M.; King, S.L.; Hilton, D.C.; Rondon, A.T.; Jarrett, M.A. Social Functioning in Youth with Attention-Deficit/Hyperactivity Disorder and Sluggish Cognitive Tempo. Yale J. Biol. Med. 2019, 92, 29–35. [Google Scholar] [PubMed]
- Berggren, S.; Engstrom, A.-C.; Bolte, S. Facial affect recognition in autism, ADHD and typical development. Cogn. Neuropsychiatry 2016, 21, 213–227. [Google Scholar] [CrossRef]
- Suri, K.; Lewis, M.; Minar, N.; Willson, E.; Ace, J. Face Memory Deficits in Children and Adolescents with Autism Spectrum Disorder. J. Psychopathol. Behav. Assess. 2021, 43, 108–118. [Google Scholar] [CrossRef]
- Field, T.M.; Woodson, R.; Greenberg, R.; Cohen, D. Discrimination and imitation of facial expression by neonates. Science 1982, 218, 179–181. [Google Scholar] [CrossRef]
- Strauss, M.S.; Newell, L.C.; Best, C.A.; Hannigen, S.F.; Gastgeb, H.Z.; Giovannelli, J.L. The Development of Facial Gender Categorization in Individuals with and without Autism: The Impact of Typicality. J. Autism Dev. Disord. 2012, 42, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Gaigg, S.B.; Bowler, D.M.; Ecker, C.; Calvo-Merino, B.; Murphy, D.G. Episodic Recollection Difficulties in ASD Result from Atypical Relational Encoding: Behavioral and Neural Evidence. Autism Res. 2015, 8, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanmarcke, S.; Wagemans, J. Priming Facial Gender and Emotional Valence: The Influence of Spatial Frequency on Face Perception in ASD. J. Autism Dev. Disord. 2017, 47, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Richter, F.R.; Bays, P.M.; Plaisted-Grant, K.C.; Baron-Cohen, S.; Simons, J.S. Reduced Hippocampal Functional Connectivity during Episodic Memory Retrieval in Autism. Cereb Cortex 2017, 27, 888–902. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.C.; Padmanabhan, A.; Simmonds, D.; Foran, W.; Hallquist, M.N.; Luna, B.; O’Hearn, K. Functional connectivity differences in autism during face and car recognition: Underconnectivity and atypical age-related changes. Dev. Sci. 2018, 21, e12508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krauel, K.; Duzel, E.; Hinrichs, H.; Rellum, T.; Santel, S.; Baving, L. Emotional memory in ADHD patients with and without comorbid ODD/CD. J. Neural Transm. 2009, 116, 117–120. [Google Scholar] [CrossRef]
- Kim, S.; Liu, Z.X.; Glizer, D.; Tannock, R.; Woltering, S. Adult ADHD and working memory: Neural evidence of impaired encoding. Clin. Neurophysiol. 2014, 125, 1596–1603. [Google Scholar] [CrossRef]
- Tye, C.; Battaglia, M.; Bertoletti, E.; Ashwood, K.L.; Azadi, B.; Asherson, P.; Bolton, P.; McLoughlin, G. Altered neurophysiological responses to emotional faces discriminate children with ASD, ADHD and ASD+ADHD. Biol. Psychol. 2014, 103, 125–134. [Google Scholar] [CrossRef]
- McVey, A.J.; Schiltz, H.K.; Haendel, A.D.; Dolan, B.K.; Willar, K.S.; Pleiss, S.S.; Karst, J.; Carlson, M.; Krueger, W.; Murphy, C.C.; et al. Social Difficulties in Youth With Autism With and Without Anxiety and ADHD Symptoms. Autism Res. 2018, 11, 1679–1689. [Google Scholar] [CrossRef]
- Zachor, D.A.; Ben-Itzchak, E. From Toddlerhood to Adolescence: Which Characteristics among Toddlers with Autism Spectrum Disorder Predict Adolescent Attention Deficit/Hyperactivity Symptom Severity? A Long-Term Follow-Up Study. J. Autism Dev. Disord. 2019, 49, 3191–3202. [Google Scholar] [CrossRef]
- Antshel, K.M.; Zhang-James, Y.; Wagner, K.E.; Ledesma, A.; Faraone, S.V. An update on the comorbidity of ADHD and ASD: A focus on clinical management. Expert Rev. Neurother. 2016, 16, 279–293. [Google Scholar] [CrossRef]
- Van Hulst, B.M.; de Zeeuw, P.; Vlaskamp, C.; Rijks, Y.; Zandbelt, B.B.; Durston, S. Children with ADHD symptoms show deficits in reactive but not proactive inhibition, irrespective of their formal diagnosis. Psychol. Med. 2018, 48, 2515–2521. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.L. Evaluating the theory of executive dysfunction in autism. Dev. Rev. 2004, 24, 189–233. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.E. A New Understanding of ADHD in Children and Adults: Executive Function Impairments; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Demetriou, E.A.; Lampit, A.; Quintana, D.S.; Naismith, S.L.; Song, Y.J.C.; Pye, J.E.; Hickie, I.; Guastella, A.J. Autism spectrum disorders: A meta-analysis of executive function. Mol. Psychiatry 2018, 23, 1198–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleminshaw, C.L.; DuPaul, G.J.; Kipperman, K.L.; Evans, S.W.; Owens, J.S. Social Deficits in High School Students With Attention-Deficit/Hyperactivity Disorder and the Role of Emotion Dysregulation. Sch. Psychol. 2020, 35, 233–242. [Google Scholar] [CrossRef]
- Fong, V.C.; Iarocci, G. The Role of Executive Functioning in Predicting Social Competence in Children with and without Autism Spectrum Disorder. Autism Res. 2020, 13, 1856–1866. [Google Scholar] [CrossRef]
- Kercood, S.; Grskovic, J.A.; Banda, D.; Begeske, J. Working memory and autism: A review of literature. Res. Autism Spectr. Disord. 2014, 8, 1316–1332. [Google Scholar] [CrossRef]
- De Vries, M.; Prins, P.J.; Schmand, B.A.; Geurts, H.M. Working memory and cognitive flexibility-training for children with an autism spectrum disorder: A randomized controlled trial. J. Child. Psychol. Psychiatry 2015, 56, 566–576. [Google Scholar] [CrossRef]
- Zinke, K.; Fries, E.; Altgassen, M.; Kirschbaum, C.; Dettenborn, L.; Kliegel, M. Visuospatial Short-Term Memory Explains Deficits in Tower Task Planning in High-Functioning Children with Autism Spectrum Disorder. Child. Neuropsychol. 2010, 16, 229–241. [Google Scholar] [CrossRef]
- Van Ewijk, H.; Heslenfeld, D.J.; Luman, M.; Rommelse, N.N.; Hartman, C.A.; Hoekstra, P.; Franke, B.; Buitelaar, J.K.; Oosterlaan, J. Visuospatial working memory in ADHD patients, unaffected siblings, and healthy controls. J. Atten. Disord. 2014, 18, 369–378. [Google Scholar] [CrossRef]
- Roselló, B.; Berenguer, C.; Navío, P.; Baixauli, I.; Miranda, A. Executive Functioning, Social Cognition, Pragmatics, and Social Interaction in Attention Deficit Hyperactivity Disorder and Autism Spectrum Disorder. Curr. Dev. Disord. Rep. 2017, 4, 72–77. [Google Scholar] [CrossRef]
- Doyle, A.E.; Vuijk, P.J.; Doty, N.D.; McGrath, L.M.; Willoughby, B.L.; O’Donnell, E.H.; Wilson, H.K.; Colvin, M.K.; Toner, D.C.; Hudson, K.E.; et al. Cross-Disorder Cognitive Impairments in Youth Referred for Neuropsychiatric Evaluation. J. Int. Neuropsychol. Soc. 2018, 24, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Karalunas, S.L.; Hawkey, E.; Gustafsson, H.; Miller, M.; Langhorst, M.; Cordova, M.; Fair, D.; Nigg, J.T. Overlapping and Distinct Cognitive Impairments in Attention-Deficit/Hyperactivity and Autism Spectrum Disorder without Intellectual Disability. J. Abnorm. Child. Psychol. 2018, 46, 1705–1716. [Google Scholar] [CrossRef]
- Vaidya, C.J.; You, X.Z.; Mostofsky, S.; Pereira, F.; Berl, M.M.; Kenworthy, L. Data-driven identification of subtypes of executive function across typical development, attention deficit hyperactivity disorder, and autism spectrum disorders. J. Child. Psychol. Psychiatry 2020, 61, 51–61. [Google Scholar] [CrossRef]
- Wang, Z.; Jing, J.; Igarashi, K.; Fan, L.; Yang, S.; Li, Y.; Jin, Y. Executive function predicts the visuospatial working memory in autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 2018, 11, 1148–1156. [Google Scholar] [CrossRef]
- Neely, R.J.; Green, J.L.; Sciberras, E.; Hazell, P.; Anderson, V. Relationship Between Executive Functioning and Symptoms of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in 6–8 Year Old Children. J. Autism Dev. Disord. 2016, 46, 3270–3280. [Google Scholar] [CrossRef]
- Salunkhe, G.; Weissbrodt, K.; Feige, B.; Saville, C.W.N.; Berger, A.; Dundon, N.M.; Bender, S.; Smyrnis, N.; Beauducel, A.; Biscaldi, M.; et al. Examining the Overlap Between ADHD and Autism Spectrum Disorder (ASD) Using Candidate Endophenotypes of ADHD. J. Atten. Disord. 2018, 25, 217–232. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale, 2nd ed.; Western Psychological Services: Los Angeles, CA, USA, 2012. [Google Scholar]
- Gau, S.S.; Shang, C.Y.; Liu, S.K.; Lin, C.H.; Swanson, J.M.; Liu, Y.C.; Tu, C.L. Psychometric properties of the Chinese version of the Swanson, Nolan, and Pelham, version IV scale—parent form. Int. J. Methods Psychiatr. Res. 2008, 17, 35–44. [Google Scholar] [CrossRef]
- Thimm, M.; Krug, A.; Markov, V.; Krach, S.; Jansen, A.; Zerres, K.; Eggermann, T.; Stöcker, T.; Shah, N.J.; Nöthen, M.M.; et al. The impact of dystrobrevin-binding protein 1 (DTNBP1) on neural correlates of episodic memory encoding and retrieval. Hum. Brain Mapp. 2010, 31, 203–209. [Google Scholar] [CrossRef]
- Krug, A.; Krach, S.; Jansen, A.; Nieratschker, V.; Witt, S.H.; Shah, N.J.; Nöthen, M.M.; Rietschel, M.; Kircher, T. The effect of neurogranin on neural correlates of episodic memory encoding and retrieval. Schizophr Bull. 2013, 39, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietsche, B.; Backes, H.; Stratmann, M.; Konrad, C.; Kircher, T.; Krug, A. Altered Neural Function During Episodic Memory Encoding and Retrieval in Major Depression. Hum. Brain Mapp. 2014, 35, 4293–4302. [Google Scholar] [CrossRef]
- Landry, O.; Al-Taie, S. A Meta-analysis of the Wisconsin Card Sort Task in Autism. J. Autism Dev. Disord. 2016, 46, 1220–1235. [Google Scholar] [CrossRef]
- Xu, C.J.; Zhang, L.; Pan, N.; Lin, Q.X.; Ye, J.; Jing, J.; Jin, Y. Event-related potential of working memory on emotional faces in children with autism spectrum disorder. Zhongguo Dang Dai Er Ke Za Zhi 2017, 19, 280–285. (In Chinese) [Google Scholar]
- Dwyer, P.; Xu, B.Y.; Tanaka, J.W. Investigating the perception of face identity in adults on the autism spectrum using behavioural and electrophysiological measures. Vis. Res. 2019, 157, 132–141. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, K.; Larsen, B.; Fedor, J.; Luna, B.; Lynn, A. Representational similarity analysis reveals atypical age-related changes in brain regions supporting face and car recognition in autism. Neuroimage 2020, 209, 116322. [Google Scholar] [CrossRef]
- Koshino, H.; Kana, R.K.; Keller, T.A.; Cherkassky, V.L.; Minshew, N.J.; Just, M.A. FMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb. Cortex 2008, 18, 289–300. [Google Scholar] [CrossRef]
- Seng, G.-J.; Tseng, W.-L.; Chiu, Y.-N.; Tsai, W.-C.; Wu, Y.-Y.; Gau, S.S.-F. Executive functions in youths with autism spectrum disorder and their unaffected siblings. Psychol. Med. 2020, 1–10. [Google Scholar] [CrossRef]
- Lee, S.E.; Kibby, M.Y.; Cohen, M.J.; Stanford, L.; Park, Y.; Strickland, S. Differences in memory functioning between children with attention-deficit/hyperactivity disorder and/or focal epilepsy. Child. Neuropsychol. 2016, 22, 979–1000. [Google Scholar] [CrossRef]
- Romani, M.; Vigliante, M.; Faedda, N.; Rossetti, S.; Pezzuti, L.; Guidetti, V.; Cardona, F. Face memory and face recognition in children and adolescents with attention deficit hyperactivity disorder: A systematic review. Neurosci. Biobehav. Rev. 2018, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, C.; Seernani, D.; Stefanou, M.E.; Riedel, A.; Tebartz van Elst, L.; Smyrnis, N.; Fleischhaker, C.; Biscaldi-Schaefer, M.; Boccignone, G.; Klein, C. Comorbidity Matters: Social Visual Attention in a Comparative Study of Autism Spectrum Disorder, Attention-Deficit/Hyperactivity Disorder and Their Comorbidity. Front. Psychiatry 2020, 11, 545567. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Tsuchiya, K.J.; Saito, M.; Hirano, Y.; Matsuo, M.; Kikuchi, M.; Maegaki, Y.; Choi, D.; Kato, S.; Yoshida, T.; et al. Developmental changes in attention to social information from childhood to adolescence in autism spectrum disorders: A comparative study. Mol. Autism 2020, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Song, D.-Y.; Kim, Y.A.; Bong, G.; Kim, J.-M.; Kim, J.H.; Yoo, H.J. How Do Children with Autism Spectrum Disorder Encode and Reproduce Visuospatial Stimuli?: Investigation into Visuospatial Processing Abilities and Styles. Psychiatry Investig. 2020, 17, 1105–1107. [Google Scholar] [CrossRef]
- Li, S.Z.; Hu, J.S.; Chang, R.S.; Li, Q.; Wan, P.; Liu, S.Q. Eye Movements of Spatial Working Memory Encoding in Children with and without Autism: Chunking Processing and Reference Preference. Autism Res. 2021, 14, 897–910. [Google Scholar] [CrossRef]
- Desaunay, P.; Briant, A.R.; Bowler, D.M.; Ring, M.; Gerardin, P.; Baleyte, J.M.; Guénolé, F.; Eustache, F.; Parienti, J.-J.; Guillery-Girard, B. Memory in Autism Spectrum Disorder: A Meta-Analysis of Experimental Studies. Psychol. Bull. 2020, 146, 377–410. [Google Scholar] [CrossRef]
- Tye, C.; Asherson, P.; Ashwood, K.L.; Azadi, B.; Bolton, P.; McLoughlin, G. Attention and inhibition in children with ASD, ADHD and co-morbid ASD plus ADHD: An event-related potential study. Psychol. Med. 2014, 44, 1101–1116. [Google Scholar] [CrossRef]
- Cowan, N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol. Bull. 1988, 104, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Engle, R.W. Working memory capacity as executive attention. Curr. Dir. Psychol. Sci. 2002, 11, 19–23. [Google Scholar] [CrossRef]
- Sinzig, J.; Morsch, D.; Lehmkuhl, G. Do hyperactivity, impulsivity and inattention have an impact on the ability of facial affect recognition in children with autism and ADHD? Eur. Child. Adoles. Psy. 2008, 17, 63–72. [Google Scholar] [CrossRef]
- Livingston, L.A.; Happe, F. Conceptualising compensation in neurodevelopmental disorders: Reflections from autism spectrum disorder. Neurosci. Biobehav. Rev. 2017, 80, 729–742. [Google Scholar] [CrossRef] [Green Version]
- Livingston, L.A.; Colvert, E.; Bolton, P.; Happe, F.; Social Relationships Study Team. Good social skills despite poor theory of mind: Exploring compensation in autism spectrum disorder. J. Child. Psychol. Psychiatry 2019, 60, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Livingston, L.A.; Shah, P.; Happe, F. Compensatory strategies below the behavioural surface in autism: A qualitative study. Lancet Psychiatry 2019, 6, 766–777. [Google Scholar] [CrossRef] [Green Version]
- Herrington, J.D.; Riley, M.E.; Grupe, D.W.; Schultz, R.T. Successful face recognition is associated with increased prefrontal cortex activation in autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Hogeveen, J.; Krug, M.K.; Geddert, R.M.; Ragland, J.D.; Solomon, M. Compensatory Hippocampal Recruitment Supports Preserved Episodic Memory in Autism Spectrum Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 97–109. [Google Scholar] [CrossRef]
- Lukito, S.; Jones, C.R.G.; Pickles, A.; Baird, G.; Happe, F.; Charman, T.; Simonoff, E. Specificity of executive function and theory of mind performance in relation to attention-deficit/hyperactivity symptoms in autism spectrum disorders. Mol. Autism 2017, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Holland, L.; Low, J. Do children with autism use inner speech and visuospatial resources for the service of executive control? Evidence from suppression in dual tasks. Br. J. Dev. Psychol. 2010, 28, 369–391. [Google Scholar] [CrossRef]
- Macoun, S.J.; Schneider, I.; Bedir, B.; Sheehan, J.; Sung, A. Pilot Study of an Attention and Executive Function Cognitive Intervention in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2020. [Google Scholar] [CrossRef]
| ASD− | ADHD | ASD+ | NTC | F/H | p | Post hoc | |
|---|---|---|---|---|---|---|---|
| Male (%) | 21 (87.5%) | 21 (91.3%) | 20 (87%) | 22 (78.6%) | 1.708 | 0.662 | |
| Age | 7.8 ± 1.8 | 8.3 ± 1.4 | 7.9 ± 1.8 | 8.2 ± 1.8 | 0.412 | 0.745 | |
| FIQ | 97.8 ± 17.7 | 98.6 ± 15.8 | 92.1 ± 17.4 | 108.7 ± 9.5 | 5.395 | 0.002 | 3 < 4 |
| VCI | 97.2 ± 16.4 | 103.7 ± 13.5 | 93.1 ± 18.3 | 111.3 ± 10.5 | 7.429 | <0.001 | 1, 3 < 4 |
| PRI | 104.1 ± 18.2 | 104.0 ± 17.6 | 98.4 ± 17.5 | 110.2 ± 10.9 | 2.276 | 0.085 | - |
| WMI | 100.8 ± 18.7 | 95.4 ± 14.2 | 94.8 ± 15.2 | 101.8 ± 13.9 | 5.536 | 0.136 | - |
| PSI | 87.5 ± 13.6 ^ | 88.6 ± 14.1 | 86.8 ± 19.2 ^ | 100.7 ± 9.8 | 21.954 | <0.001 | 1, 2, 3 < 4 |
| CARS # | 31.4 ± 2.2 | 20.0 ± 1.8 | 31.4 ± 2.6 | 16.7 ± 1.5 | 80.265 | <0.001 | 1, 3 > 2 > 4 |
| SRS | |||||||
| Total score | 69.4 ± 22.1 | 62.5 ± 18.2 | 96.8 ± 18.7 | 45.6 ± 17.4 | 30.821 | <0.001 | 3 > 1, 2 > 4 |
| Awareness | 9.5 ± 2.9 | 9.8 ± 2.5 | 12.4 ± 2.2 | 8.0 ± 1.9 | 14.148 | <0.001 | 3 > 1, 2, 4 |
| Cognition | 13.1 ± 4.8 | 12.8 ± 4.2 | 19.6 ± 4.1 | 8.8 ± 4.2 | 26.515 | <0.001 | 3 > 1, 2 > 4 |
| Communication | 23.9 ± 8.7 | 19.8 ± 6.9 | 31.5 ± 7.7 | 13.4 ± 7.3 ^ | 24.698 | <0.001 | 3 > 1, 2 > 4 |
| Motivation | 10.4 ± 3.4 | 9.9 ± 3.4 ^ | 13.5 ± 4.5 ^ | 9.4 ± 4.3 ^ | 5.171 | 0.002 | 3 > 2, 4 |
| Autistic behavior | 12.4 ± 6.3 | 10.1 ± 5.9 | 19.8 ± 5.5 | 6.0 ± 3.2 | 30.107 | <0.001 | 3 > 1, 2 > 4 |
| SNAP | |||||||
| Total score | 36.3 ± 5.1 | 46.7 ± 6.6 | 47.8 ± 6.6 | 33.0 ± 6.6 | 35.030 | <0.001 | 2, 3 > 1, 4 |
| Inattention | 20.5 ± 2.7 | 26.0 ± 4.3 | 26.1 ± 2.1 | 18.7 ± 3.9 | 31.226 | <0.001 | 2, 3 > 1, 4 |
| Hyperactivity | 15.2 ± 3.7 | 20.7 ± 4.5 | 21.7 ± 5.9 | 14.3 ± 3.6 | 16.232 | <0.001 | 2, 3 > 1, 4 |
| ASD− | ADHD | ASD+ | NTC | F1 | P1 | F2 | P2 | F3 | P3 | Post hoc | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FET | |||||||||||
| ACC | 0.61 ± 0.16 | 0.74 ± 0.20 | 0.67 ± 0.14 | 0.81 ± 0.10 | 7.928 | <0.001 | 5.145 | 0.002 | 5.323 | 0.002 | 1 < 2, 4 |
| RT | 1547.13 ± 359.04 | 1377.73 ± 239.38 | 1532.99 ± 437.97 | 1274.72 ± 283.12 | 3.868 | 0.012 | 2.024 | 0.116 | 2.775 | 0.046 | - |
| RT-mask | 813.82 ± 207.01 | 678.47 ± 129.67 | 862.73 ± 245.45 | 648.71 ± 243.09 | 5.442 | 0.002 | 2.998 | 0.035 | 3.362 | 0.022 | - |
| FRT | |||||||||||
| ACC | 0.60 ± 0.17 | 0.81 ± 0.14 | 0.64 ± 0.16 | 0.81 ± 0.17 | 12.118 | <0.001 | 9.641 | <0.001 | 9.131 | <0.001 | 1, 3 < 2, 4 |
| RT | 1283.16 ± 457.58 | 1263.17 ± 312.42 | 1175.17 ± 421.23 | 1058.83 ± 280.75 | 1.973 | 0.123 | 1.780 | 0.156 | 1.920 | 0.059 | - |
| RT-mask | 580.81 ± 173.31 | 539.03 ± 159.50 | 554.92 ± 143.52 | 485.75 ± 135.74 | 1.808 | 0.151 | 0.659 | 0.580 | 0.785 | 0.505 | - |
| ASD− (n = 24) | ADHD (n = 23) | ASD+ (n = 23) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Encoding | Retrieving | Encoding | Retrieving | Encoding | Retrieving | |||||||
| ACC | RT | ACC | RT | ACC | RT | ACC | RT | ACC | RT | ACC | RT | |
| SRS | ||||||||||||
| Total score | −0.271 | −0.411 * | −0.219 | −0.366 | −0.124 | 0.012 | −0.278 | 0.231 | −0.433 * | −0.108 | −0.403 | −0.313 |
| Awareness | −0.154 | −0.462 * | 0.045 | −0.245 | 0.036 | −0.273 | −0.135 | 0.196 | −0.129 | −0.314 | 0.151 | −0.593 ** |
| Cognition | −0.339 | −0.455 * | −0.418 * | −0.406 * | −0.118 | −0.032 | −0.061 | 0.101 | −0.372 | 0.000 | −0.317 | −0.243 |
| Communication | −0.194 | −0.294 | −0.227 | −0.324 | −0.072 | 0.000 | −0.263 | 0.174 | −0.286 | −0.183 | −0.395 | −0.210 |
| Motivation | −0.202 | −0.107 | −0.033 | −0.199 | 0.100 | 0.132 | −0.428 * | 0.412 | −0.521 * | 0.210 | −0.382 | −0.230 |
| Autistic behavior | −0.244 | −0.418 * | −0.138 | −0.307 | 0.290 | 0.097 | −0.200 | 0.113 | −0.337 | −0.157 | −0.331 | −0.164 |
| SNAP | ||||||||||||
| Total score | −0.266 | −0.199 | −0.254 | −0.260 | −0.345 | 0.129 | −0.077 | 0.423 | 0.167 | −0.497 * | 0.160 | −0.389 |
| Inattention | −0.089 | −0.131 | −0.317 | −0.295 | −0.280 | 0.016 | 0.014 | 0.298 | 0.317 | −0.002 | −0.011 | −0.197 |
| Hyperactivity | −0.307 | −0.183 | −0.122 | −0.147 | −0.239 | 0.174 | −0.127 | 0.337 | 0.071 | −0.550 ** | 0.181 | −0.360 |
| ASD− | ADHD | ASD+ | NTC | H | p | Post hoc | |
|---|---|---|---|---|---|---|---|
| CC | 4 (2.6) | 2 (2.6) | 2 (2.6) | 5 (2.6) | 18.781 | <0.001 | 1, 2, 3 < 4 |
| RE | 57 (16.82) | 75.5 (16.107) | 78 (27.95) | 42 (9.107) | 16.886 | 0.001 | 2, 3 < 4 |
| RPE | 6 (1.11) | 6 (2.12) | 4 (1.37) | 7 (2.30) | 1.753 | 0.188 | - |
| FMS | 2 (0.4) | 1 (0.4) | 1 (0.3) | 1 (0.5) | 2.158 | 0.540 | - |
| Group | Dependent | Independent | β (SE) | b’ | t | p | R2 |
|---|---|---|---|---|---|---|---|
| ASD− | ACC-Retrieving | CC | 0.043 (0.016) | 0.508 | 2.768 | 0.011 | 0.285 |
| ADHD | RT-Retrieving | RPE | −40.781 (14.724) | −0.517 | −2.770 | 0.011 | 0.268 |
| ASD+ | RT-Encoding | FMS | 163.741 (72.155) | 0.414 | 2.269 | 0.034 | 0.338 |
| RPE | 15.541 (7.435) | 0.381 | 2.090 | 0.050 | |||
| RT-Retrieving | RPE | 23.284 (6.855) | 0.594 | 3.382 | 0.003 | 0.353 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, Z.; Wan, B.; Chen, Q.; Zhai, K.; Jin, Y. The Effect of Comorbid Attention-Deficit/Hyperactivity Disorder Symptoms on Face Memory in Children with Autism Spectrum Disorder: Insights from Transdiagnostic Profiles. Brain Sci. 2021, 11, 859. https://doi.org/10.3390/brainsci11070859
Chen Q, Wang Z, Wan B, Chen Q, Zhai K, Jin Y. The Effect of Comorbid Attention-Deficit/Hyperactivity Disorder Symptoms on Face Memory in Children with Autism Spectrum Disorder: Insights from Transdiagnostic Profiles. Brain Sciences. 2021; 11(7):859. https://doi.org/10.3390/brainsci11070859
Chicago/Turabian StyleChen, Qi, Zengjian Wang, Bin Wan, Qingxin Chen, Kun Zhai, and Yu Jin. 2021. "The Effect of Comorbid Attention-Deficit/Hyperactivity Disorder Symptoms on Face Memory in Children with Autism Spectrum Disorder: Insights from Transdiagnostic Profiles" Brain Sciences 11, no. 7: 859. https://doi.org/10.3390/brainsci11070859






