Optimal Woven EndoBridge (WEB) Device Size Selection Using Automated Volumetric Software
Abstract
:1. Introduction
2. Methods
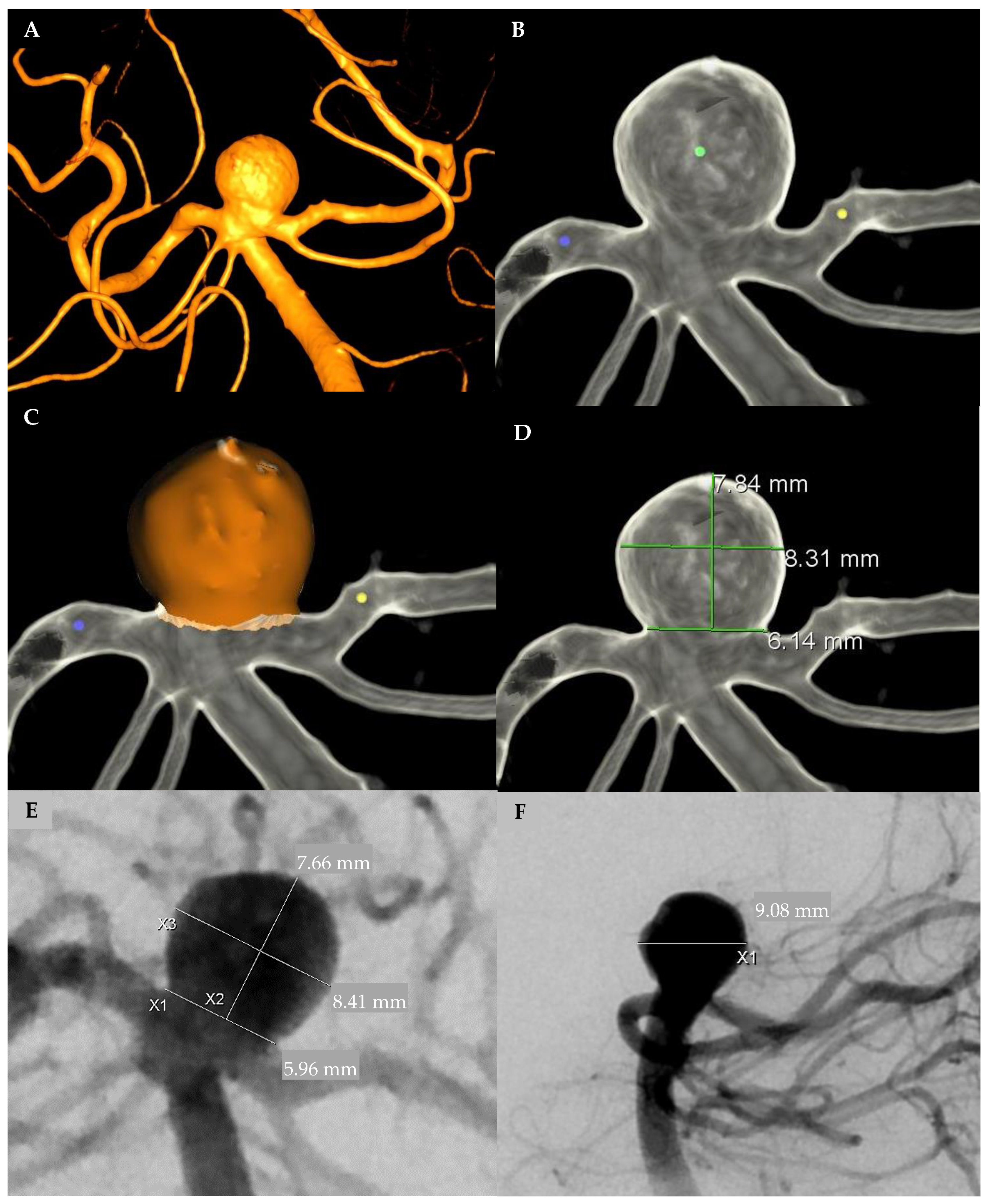
2.1. Aneurysm Measurement
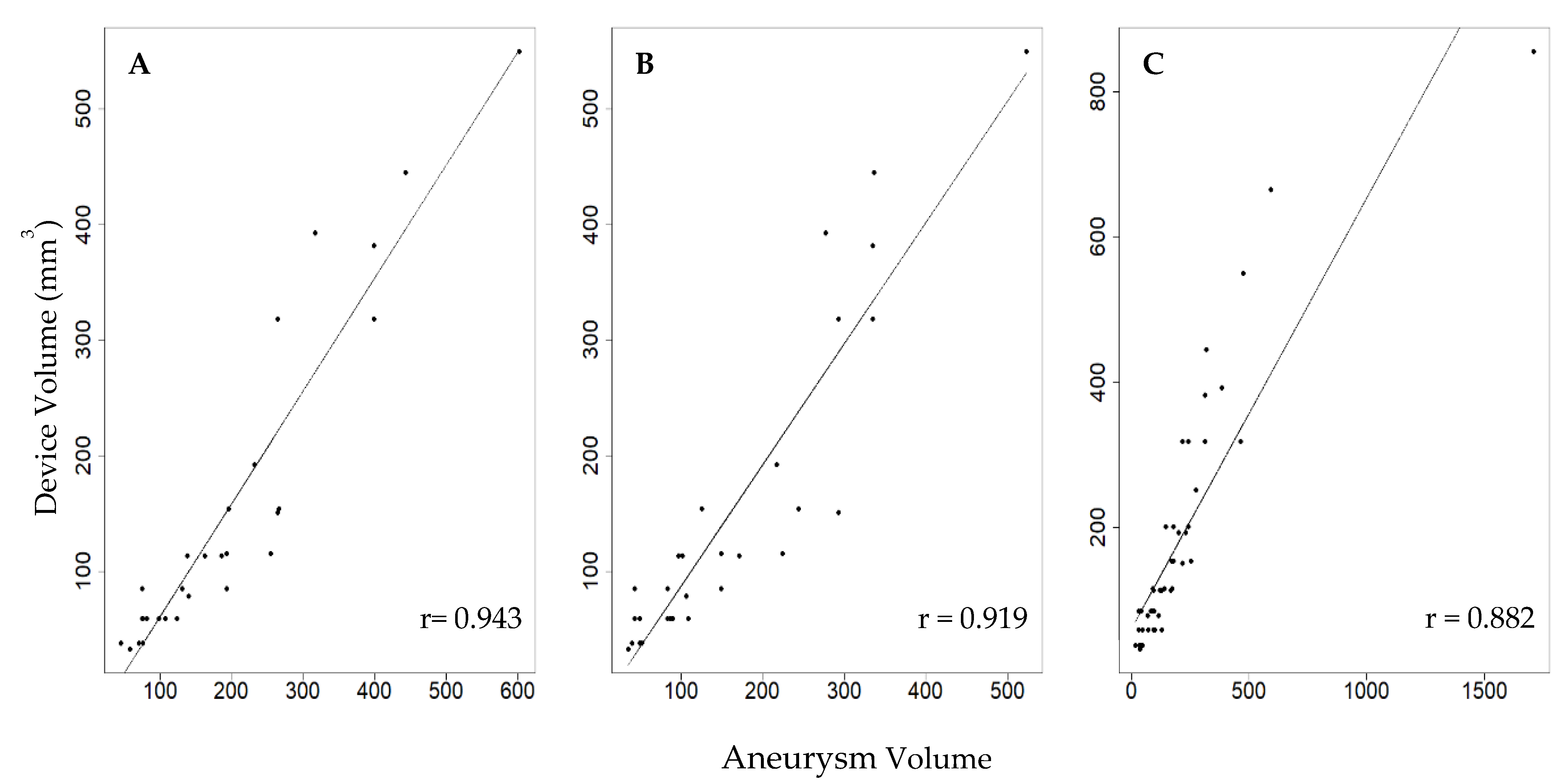
2.2. Device–Aneurysm Ratio
2.3. Radiological Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, N.; Hoit, D.; Di Nitto, J.; Elijovich, L.; Fiorella, D.; Pierot, L.; Lamin, S.; Spelle, L.; Saatci, I.; Cekirge, S.; et al. How to web: A practical review of methodology for the use of the Woven EndoBridge. J. Neurointerv. Surg. 2020, 12, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazareska, M.; Aliji, V.; Stojovska-Jovanovska, E.; Businovska, J.; Mircevski, V.; Kostov, M.; Papazova, M. Endovascular Treatment of Wide Neck Aneurysms. Open Access Maced. J. Med Sci. 2018, 6, 2316–2322. [Google Scholar] [CrossRef] [Green Version]
- Arthur, A.S.; Molyneux, A.; Coon, A.L.; Saatci, I.; Szikora, I.; Baltacioglu, F.; Sultan, A.; Hoit, D.; Almandoz, J.E.D.; Elijovich, L.; et al. The safety and effectiveness of the Woven EndoBridge (WEB) system for the treatment of wide-necked bifurcation aneurysms: Final 12-month results of the pivotal WEB Intrasaccular Therapy (WEB-IT) Study. J. Neurointerv. Surg. 2019, 11, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Behme, D.; Berlis, A.; Weber, W. Woven EndoBridge Intrasaccular Flow Disrupter for the Treatment of Ruptured and Unruptured Wide-Neck Cerebral Aneurysms: Report of 55 Cases. Am. J. Neuroradiol. 2015, 36, 1501–1506. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.T.; Turner, R.C.; Brotman, R.G.; Boo, S. Comparison of operating room variables, radiation exposure and implant costs for WEB versus stent assisted coiling for treatment of wide neck bifurcation aneurysms. Interv. Neuroradiol. 2020, 2020, 1591019920965392. [Google Scholar] [CrossRef] [PubMed]
- Cognard, C.; Januel, A.C. Remnants and recurrences after the use of the WEB intrasaccular device in large-neck bifurcation aneurysms. Neurosurgery 2015, 76, 522–530. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kida, S.; Nomura, M.; Hasegawa, M.; Yamashima, T.; Yamashita, J.; Matsui, O. Significance of volume embolization ratio as a predictor of recanalization on endovascular treatment of cerebral aneurysms with Guglielmi detachable coils. Interv. Neuroradiol. 2000, 6 (Suppl. 1), 59–63. [Google Scholar] [CrossRef] [PubMed]
- Misaki, K.; Uchiyama, N.; Nambu, I.; Aida, Y.; Kamide, T.; Mohri, M.; Ueda, F.; Nakada, M. Optimizing the volume of the initial framing coil to facilitate tight packing of intracranial aneurysms. World Neurosurg. 2016, 90, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neki, H.; Kohyama, S.; Otsuka, T.; Yonezawa, A.; Ishihara, S.; Yamane, F. Optimal first coil selection to avoid aneurysmal recanalization in endovascular intracranial aneurysmal coiling. J. Neurointerv. Surg. 2018, 10, 50–54. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Ruedinger, K.L.; Rutkowski, D.R.; Schafer, S.; Roldán-Alzate, A.; Oberstar, E.L.; Strother, C. Impact of image reconstruction parameters when using 3D DSA reconstructions to measure intracranial aneurysms. J. Neurointerv. Surg. 2017, 10, 285–289. [Google Scholar] [CrossRef]
- Escobar-de la Garma, V.H.; Zenteno, M.; Padilla-Vázquez, F.; San-Juan, D.; Cerón-Morales, A. Comparative analysis of aneurysm volume by different methods based on angiography and computed tomography angiography. Neurosurg. Rev. 2018, 41, 1013–1019. [Google Scholar] [CrossRef]
- Chan, S.-H.V.; Wong, K.-S.A.; Woo, Y.-M.P.; Chan, K.-Y.; Leung, K.-M. Volume measurement of the intracranial aneurysm: A discussion and comparison of the alternatives to manual segmentation. J. Cerebrovasc. Endovasc. Neurosurg. 2014, 16, 358. [Google Scholar] [CrossRef] [Green Version]
- Sadato, A.; Hayakawa, M.; Tanaka, T.; Hirose, Y. Comparison of cerebral aneurysm volumes as determined by digitally measured 3D rotational angiography and approximation from three diameters. Interv. Neuroradiol. 2011, 17, 154–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, D.; Milot, G.; Raymond, J. Endovascular Treatment of Unruptured Aneurysms. Stroke 2001, 32, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Varble, N.; Davies, J.M.; Rai, A.T.; Kono, K.; Sugiyama, S.I.; Binning, M.J.; Tawk, R.G.; Choi, H.; Ringer, A.J.; et al. Initial clinical experience with AView—A clinical computational platform for intracranial aneurysm morphology, hemodynamics, and treatment management. World Neurosurg. 2017, 108, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ospel, J.; Gascou, G.; Costalat, V.; Piergallini, L.; Blackham, K.; Zumofen, D. Comparison of Pipeline Embolization Device Sizing Based on Conventional 2D Measurements and Virtual Simulation Using the Sim & Size Software: An Agreement Study. Am. J. Neuroradiol. 2019, 40, 524–530. [Google Scholar] [PubMed] [Green Version]
- Cagnazzo, F.; Marnat, G.; Ferreira, I.; Daube, P.; Derraz, I.; Dargazanli, C.; Lefevre, P.H.; Gascou, G.; Riquelme, C.; Morganti, R.; et al. Comparison of Woven EndoBridge device sizing with conventional measurements and virtual simulation using the Sim&Size software: A multicenter experience. J. Neurointerv. Surg. 2020, 1–7. [Google Scholar] [CrossRef]
- Chueh, J.-Y.; Vedantham, S.; Wakhloo, A.K.; Carniato, S.L.; Puri, A.S.; Bzura, C.; Coffin, S.; Bogdanov, A.A.; Gounis, M.J. Aneurysm permeability following coil embolization: Packing density and coil distribution. J. Neurointerv. Surg. 2014, 7, 676–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosello, R.; Batista, U.; Pereira, B.; Piske, R. Packing density necessary to reach a high complete occlusion rate in circumferential Unruptured intracranial aneurysms treated with stent-assisted coil embolization. Am. J. Neuroradiol. 2017, 38, 1973–1977. [Google Scholar] [CrossRef] [Green Version]
- Cagnazzo, F.; Ahmed, R.; Zannoni, R.; Dargazanli, C.; Lefevre, P.-H.; Gascou, G.; Derraz, I.; Riquelme, C.; Bonafe, A.; Costalat, V.; et al. Predicting Factors of Angiographic Aneurysm Occlusion after Treatment with the Woven EndoBridge Device: A Single-Center Experience with Midterm Follow-Up. Am. J. Neuroradiol. 2019, 40, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Janot, K.; Herbreteau, D.; Amelot, A.; Charbonnier, G.; Boustia, F.; Narata, A.P.; Kerleroux, B.; Bibi, R.; Papagiannaki, C.; Rouchaud, A.; et al. Quantitative evaluation of WEB shape modification: A five-year follow-up study. J. Neuroradiol. 2020, 47, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Caroff, J.; Mihalea, C.; Da Ros, V.; Yagi, T.; Iacobucci, M.; Ikka, L.; Moret, J.; Spelle, L. A computational fluid dynamics (CFD) study of WEB-treated aneurysms: Can CFD predict WEB “compression” during follow-up? J. Neuroradiol. 2017, 44, 262–268. [Google Scholar] [CrossRef]
- Pierot, L.; Szikora, I.; Barreau, X.; Holtmannspoetter, M.; Spelle, L.; Herbreteau, D.; Fiehler, J.; Costalat, V.; Klisch, J.; Januel, A.-C.; et al. Aneurysm treatment with WEB in the cumulative population of two prospective, multicenter series: 3-year follow-up. J. Neurointerv. Surg. 2021, 13, 363–368. [Google Scholar] [CrossRef]

| Variable | Number |
|---|---|
| No. of Patients (aneurysms) | 41 (43) |
| Proportion of Women | 33/43 (77%) |
| Median Age | 66 years |
| Past Medical History | |
| Hypertension | 24/43 (56%) |
| Diabetes | 3/43 (7%) |
| Atrial Fibrillation | 3/43 (7%) |
| Heart Failure | 4/43 (10%) |
| Coronary Artery Disease | 5/43 (12%) |
| Hyperlipidemia | 20/43 (47%) |
| Smoker | 34/43 (79%) |
| Previous Stroke | 3/43 (7%) |
| Antiplatelet Use | 23/43 (53%) |
| Ruptured Aneurysm | 2/43 (5%) |
| Aneurysm Location | |
| MCA | 11/43 (26%) |
| ICA | 3/43 (7%) |
| BA | 12/43 (28%) |
| ACA | 2/43 (5%) |
| ACOMM | 13/43 (30%) |
| PCOMM | 1/43 (2%) |
| VA | 1/43 (2%) |
| Total WEBS Used | |
| SL | 49/50 (98%) |
| SLS | 1/50 (2%) |
| Number of Second Attempts | 7/50 (14%) |
| Median Device Volume * | 113.1 mm3 |
| Median Aneurysm Volumes | |
| 3D Automatic | 151.4 mm3 |
| 3D Manual ** | 108.2 mm3 |
| 2D DSA ** | 127.5 mm3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, S.; Zevallos, C.B.; Farooqui, M.; Dajles, A.; Schafer, S.; Quispe-Orozco, D.; Mendez-Ruiz, A.; Abdelkarim, S.; Dandapat, S.; Ortega-Gutierrez, S. Optimal Woven EndoBridge (WEB) Device Size Selection Using Automated Volumetric Software. Brain Sci. 2021, 11, 901. https://doi.org/10.3390/brainsci11070901
Ansari S, Zevallos CB, Farooqui M, Dajles A, Schafer S, Quispe-Orozco D, Mendez-Ruiz A, Abdelkarim S, Dandapat S, Ortega-Gutierrez S. Optimal Woven EndoBridge (WEB) Device Size Selection Using Automated Volumetric Software. Brain Sciences. 2021; 11(7):901. https://doi.org/10.3390/brainsci11070901
Chicago/Turabian StyleAnsari, Sameer, Cynthia B. Zevallos, Mudassir Farooqui, Andres Dajles, Sebastian Schafer, Darko Quispe-Orozco, Alan Mendez-Ruiz, Samir Abdelkarim, Sudeepta Dandapat, and Santiago Ortega-Gutierrez. 2021. "Optimal Woven EndoBridge (WEB) Device Size Selection Using Automated Volumetric Software" Brain Sciences 11, no. 7: 901. https://doi.org/10.3390/brainsci11070901
APA StyleAnsari, S., Zevallos, C. B., Farooqui, M., Dajles, A., Schafer, S., Quispe-Orozco, D., Mendez-Ruiz, A., Abdelkarim, S., Dandapat, S., & Ortega-Gutierrez, S. (2021). Optimal Woven EndoBridge (WEB) Device Size Selection Using Automated Volumetric Software. Brain Sciences, 11(7), 901. https://doi.org/10.3390/brainsci11070901






