Childhood Trauma, the Combination of MAO-A and COMT Genetic Polymorphisms and the Joy of Being Aggressive in Forensic Psychiatric Patients
Abstract
:1. Introduction
2. Materials and Method
2.1. Ethical Approval Statement
2.2. Participants
2.3. Data and Saliva Sample Acquisition
2.4. Questionnaires
2.4.1. Sociodemographic, Clinical, and Forensic Characteristics
2.4.2. Assessment of Adverse Childhood Experiences
2.4.3. Assessment of Appetitive and Reactive Aggression
2.5. Salvia Sampling and Genotyping
2.6. Statistics
3. Results
3.1. Frequency Distribution of MAO-A Variants across the Sample
3.2. Frequency Distribution of COMT Variants across the Sample
3.3. Distribution of Genotype Combination and Childhood Experiences amongst the Test Group
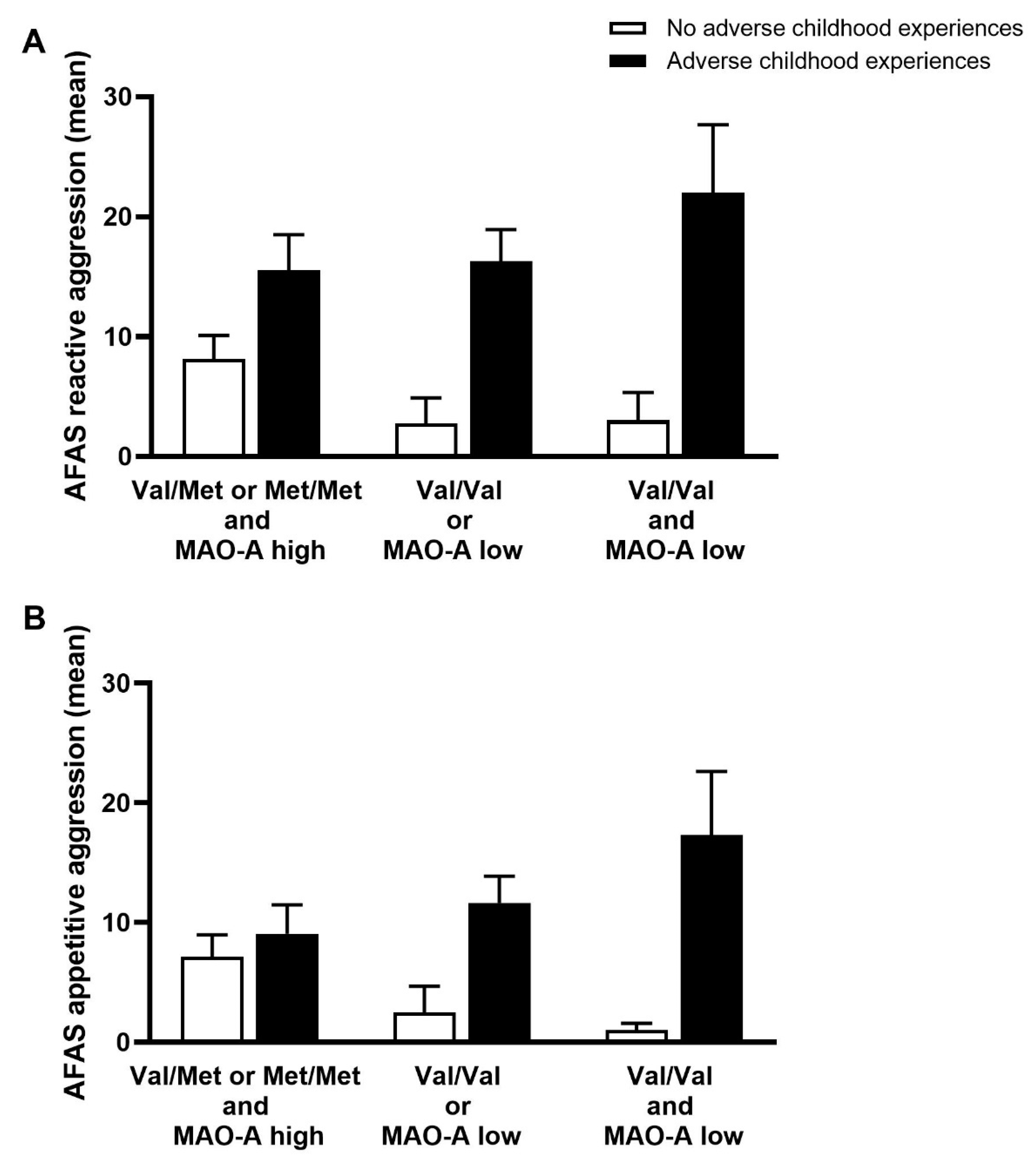
3.4. The Effect of Childhood Experiences and MAO-A/COMT Gene Variant Combination on Reactive and Appetitive Aggression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kempes, M.; Matthys, W.; De Vries, H.; Van Engeland, H. Reactive and proactive aggression in children A review of theory, findings and the relevance for child and adolescent psychiatry. Eur. Child Adolesc. Psychiatry 2005, 14, 11–19. [Google Scholar] [CrossRef]
- Ireland, C.A.; Ireland, J.L.; Birch, P. The Routledge International Handbook of Human Aggression, Current Issues and Perspectives. In Routledge International Handbooks, 1st ed.; Taylor and Francis: London, UK, 2018; Available online: https://www.routledgehandbooks.com/doi/10.4324/9781315618777 (accessed on 29 July 2021).
- Augsburger, M.; Meyer-Parlapanis, D.; Elbert, T.; Nandi, C.; Bambonye, M.; Crombach, A. Succumbing to the Call of Violence -Sex-Linked Development of Appetitive Aggression in Relation to Familial and Organized Violence. Front. Psychol. 2017, 8, 751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weierstall, R.; Elbert, T. The Appetitive Aggression Scale—Development of an instrument for the assessment of human’s attraction to violence. Eur. J. Psychotraumatol. 2011, 2, 8430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbert, T.; Moran, J.K.; Schauer, M. Lust for violence: Appetitive aggression as a fundamental part of human nature. e-Neuroforum 2017, 23, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Golden, S.A.; Shaham, Y. Aggression Addiction and Relapse: A New Frontier in Psychiatry. Neuropsychopharmacology 2017, 43, 224–225. [Google Scholar] [CrossRef] [Green Version]
- Caspi, A.; McClay, J.; Moffitt, T.E.; Mill, J.; Martin, J.; Craig, I.W.; Taylor, A.; Poulton, R. Role of Genotype in the Cycle of Violence in Maltreated Children. Science 2002, 297, 851–854. [Google Scholar] [CrossRef]
- Keiley, M.K.; Howe, T.R.; Dodge, K.A.; Bates, J.E.; Pettit, G.S. The timing of child physical maltreatment: A cross-domain growth analysis of impact on adolescent externalizing and internalizing problems. Dev. Psychopathol. 2001, 13, 891–912. [Google Scholar] [CrossRef]
- Widom, C.S. Child abuse, neglect, and adult behavior: Research design and findings on criminality, violence, and child abuse. Am. J. Orthopsychiatry 1989, 59, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.; Cicchetti, D. Reactive Aggression among Maltreated Children: The Contributions of Attention and Emotion Dysregulation. J. Clin. Child Psychol. 1998, 27, 381–395. [Google Scholar] [CrossRef]
- Kolla, N.J.; Malcolm, C.; Attard, S.; Arenovich, T.; Blackwood, N.; Hodgins, S. Childhood Maltreatment and Aggressive Behaviour in Violent Offenders with Psychopathy. Can. J. Psychiatry 2013, 58, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Dudeck, M.; Sosic-Vasic, Z.; Otte, S.; Rasche, K.; Leichauer, K.; Tippelt, S.; Shenar, R.; Klingner, S.; Vasic, N.; Streb, J. The association of adverse childhood experiences and appetitive aggression with suicide attempts and violent crimes in male forensic psychiatry inpatients. Psychiatry Res. 2016, 240, 352–357. [Google Scholar] [CrossRef]
- Fritz, M.; Shenar, R.; Cardenas-Morales, L.; Jäger, M.; Streb, J.; Dudeck, M.; Franke, I. Aggressive and Disruptive Behavior Among Psychiatric Patients With Major Depressive Disorder, Schizophrenia, or Alcohol Dependency and the Effect of Depression and Self-Esteem on Aggression. Front. Psychiatry 2020, 11, 599828. [Google Scholar] [CrossRef]
- Sabol, S.Z.; Hu, S.; Hamer, D. A functional polymorphism in the monoamine oxidase a gene promoter. Qual. Life Res. 1998, 103, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, S.; Zohsel, K.; Buchmann, A.F.; Blomeyer, R.; Holz, N.; Boecker-Schlier, R.; Jennen-Steinmetz, C.; Rietschel, M.; Witt, S.H.; Schmidt, M.H.; et al. Interacting effect of MAOA genotype and maternal prenatal smoking on aggressive behavior in young adulthood. J. Neural Transm. 2016, 123, 885–894. [Google Scholar] [CrossRef]
- McDermott, R.; Tingley, D.; Cowden, J.; Frazzetto, G.; Johnson, D.D.P. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proc. Natl. Acad. Sci. USA 2009, 106, 2118–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardle, M.C.; de Wit, H.; Penton-Voak, I.; Lewis, G.; Munafo, M. Lack of Association Between COMT and Working Memory in a Population-Based Cohort of Healthy Young Adults. Neuropsychopharmacology 2013, 38, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, A.; Zai, C.C.; Hirata, Y.; Tiwari, A.K.; Cheema, S.; Nowrouzi, B.; Beitchman, J.H.; Kennedy, J.L. The Role of the Catechol-o-Methyltransferase (COMT) GeneVal158Met in Aggressive Behavior, a Review of Genetic Studies. Curr. Neuropharmacol. 2015, 13, 802–814. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Lipska, B.K.; Halim, N.; Ma, Q.D.; Matsumoto, M.; Melhem, S.; Kolachana, B.S.; Hyde, T.M.; Herman, M.M.; Apud, J.; et al. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on mRNA, Protein, and Enzyme Activity in Postmortem Human Brain. Am. J. Hum. Genet. 2004, 75, 807–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baud, P.; Courtet, P.; Perroud, N.; Jollant, F.; Buresi, C.; Malafosse, A. Catechol-O-methyltransferase polymorphism (COMT) in suicide attempters: A possible gender effect on anger traits. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2007, 144B, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Monuteaux, M.C.; Biederman, J.; Doyle, A.E.; Mick, E.; Faraone, S. Genetic Risk for Conduct Disorder Symptom Subtypes in an ADHD Sample: Specificity to Aggressive Symptoms. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Langley, K.; Milne, B.; Moffitt, T.E.; O’Donovan, M.; Owen, M.J.; Tomas, M.P.; Poulton, R.; Rutter, M.; Taylor, A.; et al. A Replicated Molecular Genetic Basis for Subtyping Antisocial Behavior in Children with Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2008, 65, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, H.; Deater-Deckard, K.; Zhang, W. Interacting Effect of Catechol-O-Methyltransferase (COMT) and Monoamine Oxidase A (MAOA) Gene Polymorphisms, and Stressful Life Events on Aggressive Behavior in Chinese Male Adolescents. Front. Psychol. 2018, 9, 1079. [Google Scholar] [CrossRef] [Green Version]
- Isele, D.; Teicher, M.H.; Ruf-Leuschner, M.; Elbert, T.; Kolassa, I.-T.; Schury, K.; Schauer, M. KERF–Ein Instrument zur umfassenden Ermittlung belastender Kindheitserfahrungen. Z. Klin. Psychol. Psychother. 2014, 43, 121–130. [Google Scholar] [CrossRef]
- Teicher, M.H.; Parigger, A. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) Scale for the Retrospective Assessment of Abuse and Neglect during Development. PLoS ONE 2015, 10, e0117423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.P.; Stein, J.A.; Newcomb, M.D.; Walker, E.; Pogge, D.; Ahluvalia, T.; Stokes, J.; Handelsman, L.; Medrano, M.; Desmond, D.; et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus. Negl. 2003, 27, 169–190. [Google Scholar] [CrossRef]
- Schmidt, L.G.; Sander, T.; Kühn, S.; Smolka, M.; Rommelspacher, H.; Samochowiec, J.; Lesch, K.P. Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious-depressive alcoholics. J. Neural Transm. 2000, 107, 681–689. [Google Scholar] [CrossRef]
- Castro, T.B.G.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.; García, S.P.; Genis, A.; Nicolini, H.; Narváez, L.L. Distribution of the Val108/158Met polymorphism of the COMT gene in healthy Mexican population. Gene 2013, 526, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Bellis, M.; Hughes, K.; Leckenby, N.; Hardcastle, K.; Perkins, C.; Lowey, H. Measuring mortality and the burden of adult disease associated with adverse childhood experiences in England: A national survey. J. Public Health 2014, 37, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef] [Green Version]
- Aslund, C.; Nordquist, N.; Comasco, E.; Leppert, J.; Oreland, L.; Nilsson, K.W. Maltreatment, MAOA, and delinquency: Sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behav. Genet. 2011, 41, 262–272. [Google Scholar] [CrossRef]
- Fergusson, D.; Lynskey, M.T. Physical punishment/maltreatment during childhood and adjustment in young adulthood. Child Abus. Negl. 1997, 21, 617–630. [Google Scholar] [CrossRef]
- Fonseka, R.W.; Minnis, A.M.; Gomez, A.M. Impact of Adverse Childhood Experiences on Intimate Partner Violence Perpetration among Sri Lankan Men. PLoS ONE 2015, 10, e0136321. [Google Scholar] [CrossRef]
- Miller, E.; Breslau, J.; Chung, W.-J.J.; Green, J.; McLaughlin, K.; Kessler, R.C. Adverse childhood experiences and risk of physical violence in adolescent dating relationships. J. Epidemiol. Community Health 2011, 65, 1006–1013. [Google Scholar] [CrossRef] [Green Version]
- Stoltenborgh, M.; Bakermans-Kranenburg, M.J.; Alink, L.R.A.; van Ijzendoorn, M. The Prevalence of Child Maltreatment across the Globe: Review of a Series of Meta-Analyses. Child Abus. Rev. 2014, 24, 37–50. [Google Scholar] [CrossRef]
- Bhakta, S.G.; Zhang, J.-P.; Malhotra, A.K. The COMT Met158 allele and violence in schizophrenia: A meta-analysis. Schizophr. Res. 2012, 140, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.P.; Volavka, J.; Czobor, P.; Van Dorn, R.A. A Meta-Analysis of the Val158Met COMT Polymorphism and Violent Behavior in Schizophrenia. PLoS ONE 2012, 7, e43423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strous, R.D.; Nolan, K.A.; Lapidus, R.; Diaz, L.; Saito, T.; Lachman, H.M. Aggressive behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: A replication study. Am. J. Med Genet. 2003, 120, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, M.D.; Harder, V.S.; Althoff, R.R.; Rettew, D.C.; Ehli, E.A.; Lengyel-Nelson, T.; Davies, G.E.; Ayer, L.; Sulman, J.; Stanger, C.; et al. COMT Val158Met genotype as a risk factor for problem behaviors in youth. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazzetto, G.; Di Lorenzo, G.; Carola, V.; Proietti, L.; Sokolowska, E.; Siracusano, A.; Gross, C.; Troisi, A. Early Trauma and Increased Risk for Physical Aggression during Adulthood: The Moderating Role of MAOA Genotype. PLoS ONE 2007, 2, e486. [Google Scholar] [CrossRef] [Green Version]
- Kim-Cohen, J.; Caspi, A.; Taylor, A.; Williams, B.; Newcombe, R.; Craig, I.W.; Moffitt, T. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol. Psychiatry 2006, 11, 903–913. [Google Scholar] [CrossRef] [Green Version]
- Widom, C.S.; Brzustowicz, L.M. MAOA and the “Cycle of Violence”: Childhood Abuse and Neglect, MAOA Genotype, and Risk for Violent and Antisocial Behavior. Biol. Psychiatry 2006, 60, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.K.; Syagailo, Y.V.; Barr, C.S.; Wendland, J.R.; Champoux, M.; Graessle, M.; Suomi, S.J.; Higley, J.D.; Lesch, K.-P. Monoamine oxidase a gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol. Psychiatry 2005, 57, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Buckholtz, J.W.; Meyer-Lindenberg, A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008, 31, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, T.D.; Beaulieu, J.-M.; Espinoza, S.; Masri, B.; Zhang, X.; Salahpour, A.; Barak, L.S.; Caron, M.G.; Gainetdinov, R.R. The Dopamine Metabolite 3-Methoxytyramine is a Neuromodulator. PLoS ONE 2010, 5, e13452. [Google Scholar] [CrossRef]
- Lambert, G.; Eisenhofer, G.; Jennings, G.; Esler, M. Regional homovanillic acid production in humans. Life Sci. 1993, 53, 63–75. [Google Scholar] [CrossRef]
- Leo, D.; Mus, L.; Espinoza, S.; Hoener, M.; Sotnikova, T.; Gainetdinov, R. Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: Role of D2 dopamine autoreceptors. Neuropharmacology 2014, 81, 283–291. [Google Scholar] [CrossRef]
- Miller, G.M. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J. Neurochem. 2010, 116, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, C.J. Genetic Contributions to Antisocial Personality and Behavior: A Meta-Analytic Review From an Evolutionary Perspective. J. Soc. Psychol. 2010, 150, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Beaver, K.M.; Schutt, J.E.; Boutwell, B.B.; Ratchford, M.; Roberts, K.; Barnes, J.C. Genetic and Environmental Influences on Levels of Self-Control and Delinquent Peer Affiliation. Crim. Justice Behav. 2008, 36, 41–60. [Google Scholar] [CrossRef] [Green Version]
- Jackson Dylan, B.; Beaver, K.M. Candidate Genes for Criminal and Delinquent Behavior. Int. J. Psychol. Res. 2012, 7, 411. [Google Scholar]
- Stetler, D.A.; Davis, C.; Leavitt, K.; Schriger, I.; Benson, K.; Bhakta, S.; Wang, L.C.; Oben, C.; Watters, M.; Haghnegahdar, T.; et al. Association of low-activity MAOA allelic variants with violent crime in incarcerated offenders. J. Psychiatr. Res. 2014, 58, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mean (SD) | |
|---|---|
| Age (years) | 34.00 (9.70) |
| Length of treatment (Months) | 11.35 (12.24) |
| n (percentage) | |
| Level of education | |
| No formal Diploma | 12 (19%) |
| Middle School Diploma | 29 (45%) |
| Secondary School Diploma | 17 (27%) |
| High School Diploma | 6 (9%) |
| Main Diagnosis (ICD-10) | |
| F1 (all drug-related disorders) | 62 (97%) |
| F6 (personality disorders) | 2 (3%) |
| Secondary Diagnosis (ICD-10) | |
| F3 (affective disorders) | 5 (8%) |
| F6 (personality disorders) | 14 (22%) |
| Others | 3 (5%) |
| Index crime | |
| Murder/Manslaughter | 2 (3%) |
| Robbery | 7 (11%) |
| Aggravated Battery | 11 (17%) |
| Sexual Assault | 1 (2%) |
| Fraud/Theft | 11 (17%) |
| Violation of the Narcotics Act | 29 (45%) |
| Others | 3 (5%) |
| Actual Number | Expected Distribution | Expected Number | Residuum | |
|---|---|---|---|---|
| Low | 27 | 35% | 21.3 | 5.7 |
| High | 34 | 65% | 39.7 | −5.6 |
| Actual Number | Expected Distribution | Expected Number | Residuum | |
|---|---|---|---|---|
| Val/Val | 19 | 25% | 15.3 | 3.8 |
| Val/Met | 29 | 50% | 30.5 | −1.5 |
| Met/Met | 13 | 25% | 15.3 | −2.2 |
| Adverse Childhood Experiences | No Adverse Childhood Experiences | Total | |
|---|---|---|---|
| Val/Val and MAO-A low | 7 | 3 | 10 |
| Val/Val or MAO-A low | 19 | 7 | 26 |
| Val/Met or Met/Met and MAO-A high | 17 | 8 | 25 |
| Total | 43 | 18 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fritz, M.; Rösel, F.; Dobler, H.; Streb, J.; Dudeck, M. Childhood Trauma, the Combination of MAO-A and COMT Genetic Polymorphisms and the Joy of Being Aggressive in Forensic Psychiatric Patients. Brain Sci. 2021, 11, 1008. https://doi.org/10.3390/brainsci11081008
Fritz M, Rösel F, Dobler H, Streb J, Dudeck M. Childhood Trauma, the Combination of MAO-A and COMT Genetic Polymorphisms and the Joy of Being Aggressive in Forensic Psychiatric Patients. Brain Sciences. 2021; 11(8):1008. https://doi.org/10.3390/brainsci11081008
Chicago/Turabian StyleFritz, Michael, Franziska Rösel, Hannah Dobler, Judith Streb, and Manuela Dudeck. 2021. "Childhood Trauma, the Combination of MAO-A and COMT Genetic Polymorphisms and the Joy of Being Aggressive in Forensic Psychiatric Patients" Brain Sciences 11, no. 8: 1008. https://doi.org/10.3390/brainsci11081008




