Reorganization of the Social Brain in Individuals with Only One Intact Cerebral Hemisphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Psychological and Behavioral Measures
2.3. fMRI Stimulus
2.4. MRI Data Acquisition
2.5. MRI Preprocessing
2.6. Anatomical Data Preprocessing
2.7. fmri Preprocessing
2.8. fMRI Denoising
2.9. Head Motion
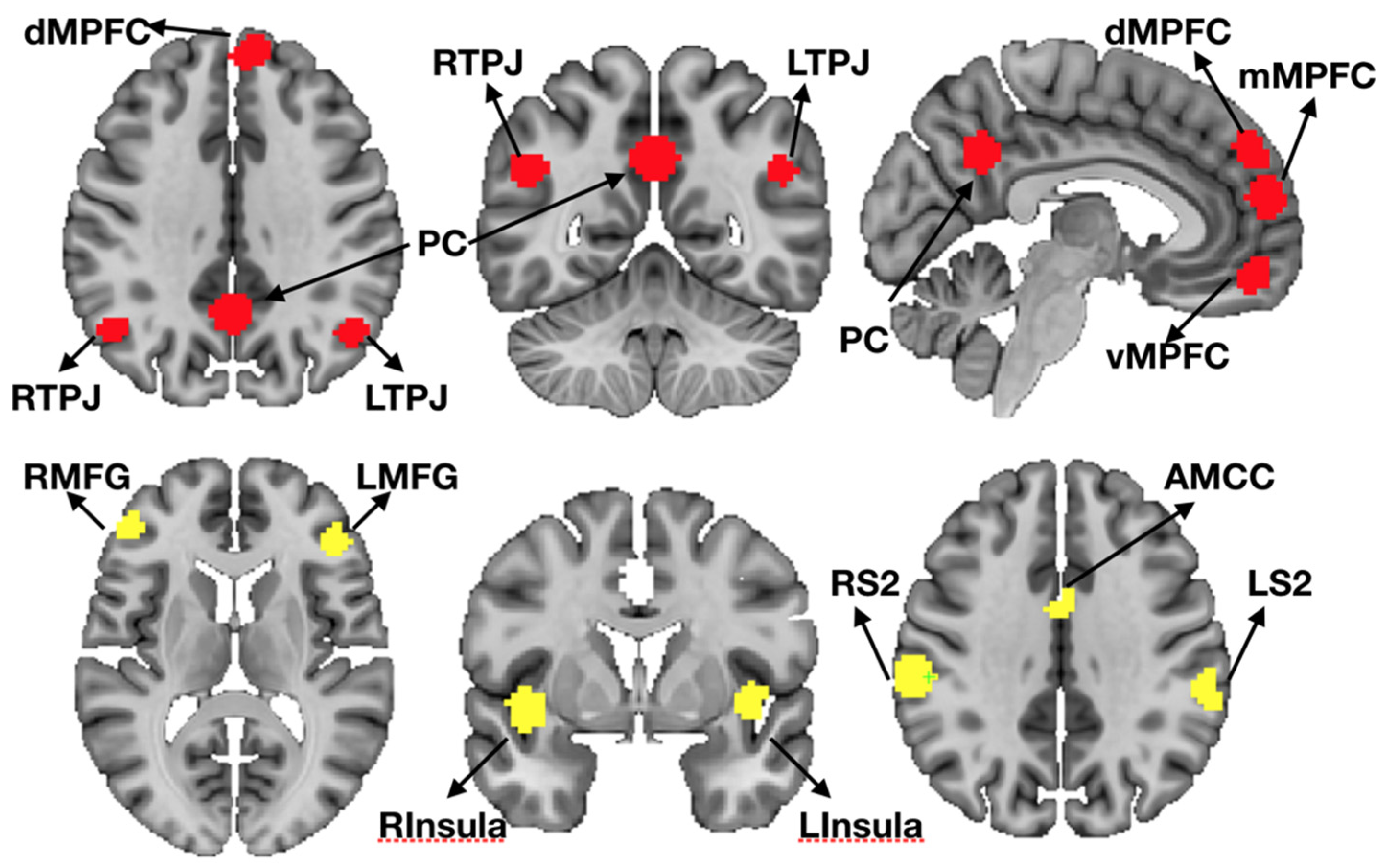
2.10. Region of Interest (ROI) Definition
2.11. ROI Registration Procedure
2.12. FMRI Inter-Region Correlation of Time Series Analysis
2.13. Comparison Data Previously Published in Richardson et al., 2018
2.14. Quantification of Results
3. Results
3.1. Behavioral Data
3.2. Functional MRI Data
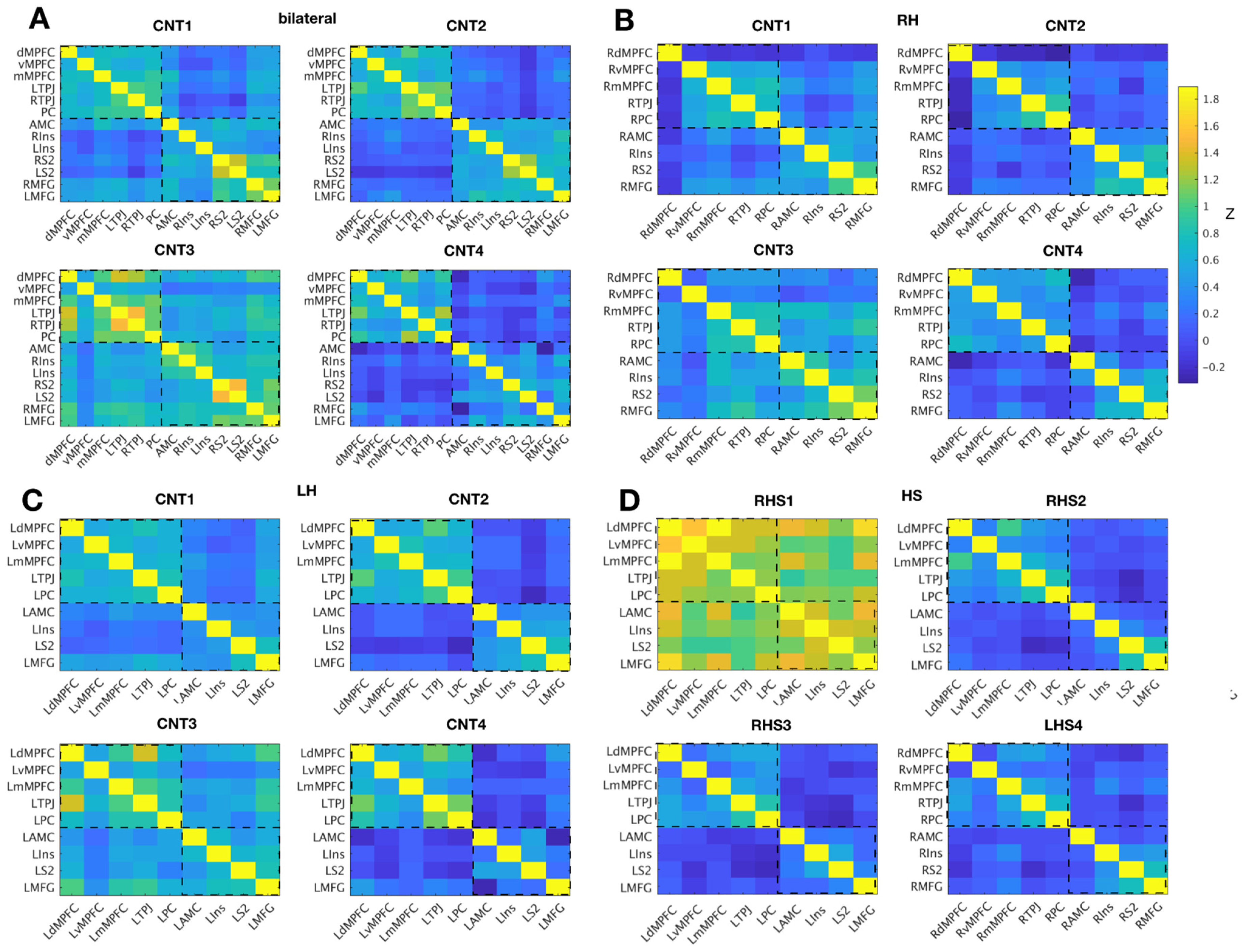
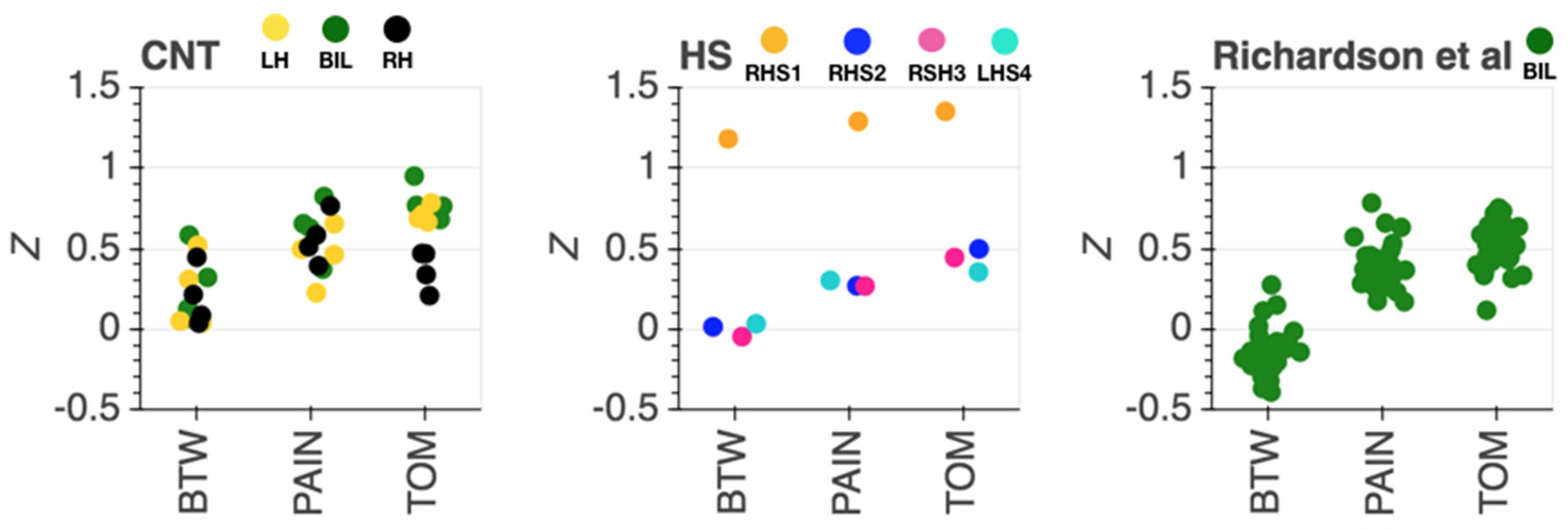
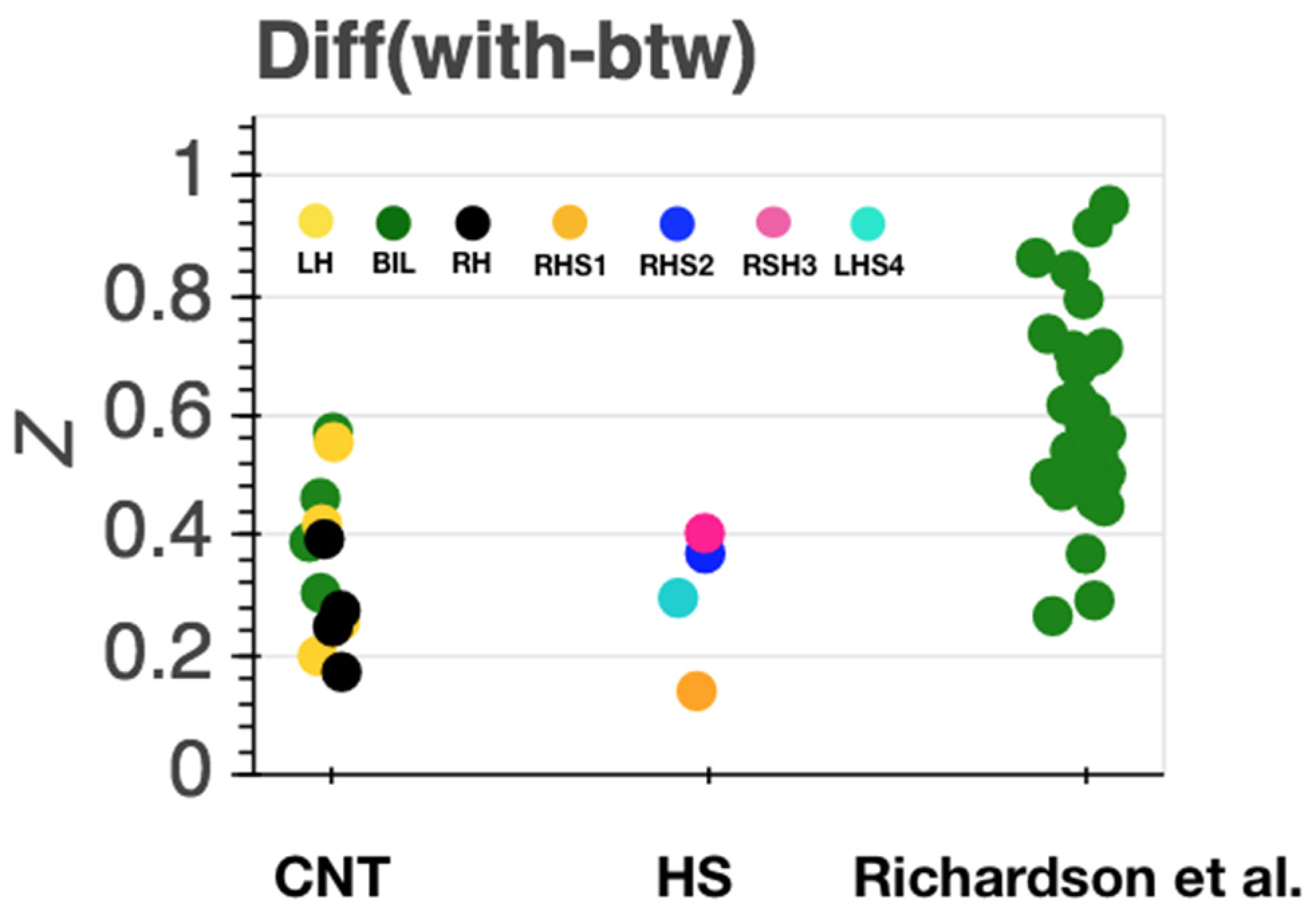
Inter-Region Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernald, A. Approval and disapproval: Infant responsiveness to vocal affect in familiar and unfamiliar languages. Child Dev. 1993, 64, 657–674. [Google Scholar] [CrossRef]
- Wu, Y.; Muentener, P.; Schulz, L.E. One- to four-year-olds connect diverse positive emotional vocalizations to their probable causes. Proc. Natl. Acad. Sci. USA 2017, 114, 11896–11901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, C.A. The recognition of facial expressions in the first two years of life: Mechanisms of development. Child Dev. 1987, 58, 889–909. [Google Scholar] [CrossRef]
- Perner, J.; Lang, B. Development of theory of mind and executive control. Trends Cogn. Sci. 1999, 3, 337–344. [Google Scholar] [CrossRef]
- Davidson, R.J.; Pizzagalli, D.; Nitschke, J.B.; Putnam, K. Depression: Perspectives from affective neuroscience. Annu. Rev. Psychol. 2002, 53, 545–574. [Google Scholar] [CrossRef] [PubMed]
- Pantelis, P.C.; Byrge, L.; Tyszka, J.M.; Adolphs, R.; Kennedy, D.P. A specific hypoactivation of right temporo-parietal junction/posterior superior temporal sulcus in response to socially awkward situations in autism. Soc. Cogn. Affect. Neurosci. 2015, 10, 1348–1356. [Google Scholar] [CrossRef] [Green Version]
- Richardson, H.; Lisandrelli, G.; Riobueno-Naylor, A.; Saxe, R. Development of the social brain from age three to twelve years. Nat. Commun. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.; Yacoub, E.; Ugurbil, K.; Consortium, W.U.-M.H. The WU-Minn Human Connectome Project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, L.K.; Brown, W.S.; Adolphs, R.; Tyszka, J.M.; Richards, L.J.; Mukherjee, P.; Sherr, E.H. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nat. Rev. Neurosci. 2007, 8, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Tyszka, J.M.; Kennedy, D.P.; Adolphs, R.; Paul, L.K. Intact bilateral resting-state networks in the absence of the corpus callosum. J. Neurosci. 2011, 31, 15154–15162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliemann, D.; Adolphs, R.; Tyszka, J.M.; Fischl, B.; Yeo, B.T.T.; Nair, R.; Dubois, J.; Paul, L.K. Intrinsic Functional Connectivity of the Brain in Adults with a Single Cerebral Hemisphere. Cell Rep. 2019, 29, 2398–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennard, M. Cortical reorganization of motor function. Arch. Neurol. 1942, 48, 227–240. [Google Scholar] [CrossRef]
- Kanwisher, N. Functional specificity in the human brain: A window into the functional architecture of the mind. Proc. Natl. Acad. Sci. USA 2010, 107, 11163–11170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downing, P.E.; Jiang, Y.; Shuman, M.; Kanwisher, N. A cortical area selective for visual processing of the human body. Science 2001, 293, 2470–2473. [Google Scholar] [CrossRef] [PubMed]
- Kanwisher, N.; Yovel, G. The fusiform face area: A cortical region specialized for the perception of faces. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 2109–2128. [Google Scholar] [CrossRef] [Green Version]
- Pitcher, D.; Walsh, V.; Duchaine, B. The role of the occipital face area in the cortical face perception network. Exp. Brain Res. 2011, 209, 481–493. [Google Scholar] [CrossRef]
- Schwarzlose, R.F.; Baker, C.I.; Kanwisher, N. Separate face and body selectivity on the fusiform gyrus. J. Neurosci. 2005, 25, 11055–11059. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Morris, J.P. Brain Mechanisms for Interpreting the Actions of Others from Biological-Motion Cues. Curr. Dir. Psychol. Sci. 2006, 15, 136–140. [Google Scholar] [CrossRef] [Green Version]
- Saxe, R.; Kanwisher, N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage 2003, 19, 1835–1842. [Google Scholar] [CrossRef]
- De Renzi, E.; Perani, D.; Carlesimo, G.A.; Silveri, M.C.; Fazio, F. Prosopagnosia can be associated with damage confined to the right hemisphere—An MRI and PET study and a review of the literature. Neuropsychologia 1994, 32, 893–902. [Google Scholar] [CrossRef]
- Rangarajan, V.; Parvizi, J. Functional asymmetry between the left and right human fusiform gyrus explored through electrical brain stimulation. Neuropsychologia 2016, 83, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.; Apperly, I.A.; Chiavarino, C.; Humphreys, G.W. Left temporoparietal junction is necessary for representing someone else’s belief. Nat. Neurosci. 2004, 7, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Rosenblau, G.; Kliemann, D.; Lemme, B.; Walter, H.; Heekeren, H.R.; Dziobek, I. The role of the amygdala in naturalistic mentalising in typical development and in autism spectrum disorder. Br. J. Psychiatry 2016, 208, 556–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spunt, R.P.; Elison, J.T.; Dufour, N.; Hurlemann, R.; Saxe, R.; Adolphs, R. Amygdala lesions do not compromise the cortical network for false-belief reasoning. Proc. Natl. Acad. Sci. USA 2015, 112, 4827–4832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frith, C.D.; Frith, U. The neural basis of mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurz, M.; Radua, J.; Tholen, M.G.; Maliske, L.; Margulies, D.S.; Mars, R.B.; Sallet, J.; Kanske, P. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol. Bull. 2021, 147, 293–327. [Google Scholar] [CrossRef]
- Spunt, R.P.; Kemmerer, D.; Adolphs, R. The neural basis of conceptualizing the same action at different levels of abstraction. Soc. Cogn. Affect. Neurosci. 2016, 11, 1141–1151. [Google Scholar] [CrossRef] [Green Version]
- Spunt, R.P.; Falk, E.B.; Lieberman, M.D. Dissociable neural systems support retrieval of how and why action knowledge. Psychol. Sci. 2010, 21, 1593–1598. [Google Scholar] [CrossRef]
- Dodell-Feder, D.; Koster-Hale, J.; Bedny, M.; Saxe, R. fMRI item analysis in a theory of mind task. Neuroimage 2011, 55, 705–712. [Google Scholar] [CrossRef]
- Bruneau, E.G.; Jacoby, N.; Saxe, R. Empathic control through coordinated interaction of amygdala, theory of mind and extended pain matrix brain regions. Neuroimage 2015, 114, 105–119. [Google Scholar] [CrossRef] [Green Version]
- Bruneau, E.G.; Pluta, A.; Saxe, R. Distinct roles of the ‘shared pain’ and ‘theory of mind’ networks in processing others’ emotional suffering. Neuropsychologia 2012, 50, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, N.; Bruneau, E.; Koster-Hale, J.; Saxe, R. Localizing Pain Matrix and Theory of Mind networks with both verbal and non-verbal stimuli. Neuroimage 2016, 126, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanske, P.; Bockler, A.; Trautwein, F.M.; Singer, T. Dissecting the social brain: Introducing the EmpaToM to reveal distinct neural networks and brain-behavior relations for empathy and Theory of Mind. Neuroimage 2015, 122, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H. Development of brain networks for social functions: Confirmatory analyses in a large open source dataset. Dev. Cogn. Neurosci. 2019, 37, 100598. [Google Scholar] [CrossRef] [PubMed]
- Hasson, U.; Nir, Y.; Levy, I.; Fuhrmann, G.; Malach, R. Intersubject synchronization of cortical activity during natural vision. Science 2004, 303, 1634–1640. [Google Scholar] [CrossRef]
- Vanderwal, T.; Eilbott, J.; Castellanos, F.X. Movies in the magnet: Naturalistic paradigms in developmental functional neuroimaging. Dev. Cogn. Neurosci. 2019, 36, 100600. [Google Scholar] [CrossRef]
- Vanderwal, T.; Eilbott, J.; Finn, E.S.; Craddock, R.C.; Turnbull, A.; Castellanos, F.X. Individual differences in functional connectivity during naturalistic viewing conditions. Neuroimage 2017, 157, 521–530. [Google Scholar] [CrossRef]
- Redcay, E.; Moraczewski, D. Social cognition in context: A naturalistic imaging approach. Neuroimage 2020, 216, 116392. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Milham, M.; Vanderwal, T. Towards clinical applications of movie fMRI. Neuroimage 2020, 217, 116860. [Google Scholar] [CrossRef]
- Byrge, L.; Dubois, J.; Tyszka, J.M.; Adolphs, R.; Kennedy, D.P. Idiosyncratic brain activation patterns are associated with poor social comprehension in autism. J. Neurosci. 2015, 35, 5837–5850. [Google Scholar] [CrossRef]
- Finn, E.S.; Bandettini, P.A. Movie-watching outperforms rest for functional connectivity-based prediction of behavior. Neuroimage 2021, 235, 117963. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R.; Damasio, H.; Tranel, D.; Cooper, G.; Damasio, A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neurosci. 2000, 20, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Blonder, L.X.; Bowers, D.; Heilman, K.M. The role of the right hemisphere in emotional communication. Brain 1991, 114 Pt 3, 1115–1127. [Google Scholar] [CrossRef]
- Borod, J.C. Cerebral mechanisms underlying facial, prosodic, and lexical emotional expression: A review of neuropsychological studies and methodological issues. Neuropsychology 1993, 7, 445–463. [Google Scholar] [CrossRef]
- Borod, J.C.; Cicero, B.A.; Obler, L.K.; Welkowitz, J.; Erhan, H.M.; Santschi, C.; Grunwald, I.S.; Agosti, R.M.; Whalen, J.R. Right hemisphere emotional perception: Evidence across multiple channels. Neuropsychology 1998, 12, 446–458. [Google Scholar] [CrossRef]
- Ross, E.D.; Monnot, M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang. 2008, 104, 51–74. [Google Scholar] [CrossRef]
- Bowers, D.; Bauer, R.M.; Heilman, K.M. The nonverbal affect lexicon: Theoretical perspectives from neuropsychological studies of affect perception. Neuropsychology 1993, 7, 433–444. [Google Scholar] [CrossRef]
- Vallortigara, G. Right hemisphere advantage for social recognition in the chick. Neuropsychologia 1992, 30, 761–768. [Google Scholar] [CrossRef]
- Jonas, R.; Nguyen, S.; Hu, B.; Asarnow, R.F.; LoPresti, C.; Curtiss, S.; de Bode, S.; Yudovin, S.; Shields, W.D.; Vinters, H.V.; et al. Cerebral hemispherectomy: Hospital course, seizure, developmental, language, and motor outcomes. Neurology 2004, 62, 1712–1721. [Google Scholar] [CrossRef]
- Kim, J.S.; Park, E.K.; Shim, K.W.; Kim, D.S. Hemispherotomy and Functional Hemispherectomy: Indications and Outcomes. J. Epilepsy Res. 2018, 8, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Reher, K.; Sohn, P. Partly Cloudy [Motion Picture]. 2009. [Google Scholar]
- Wechsler, D. WAIS-III: Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence, 2nd ed.; The Psychological Corporation: San Antonio, TX, USA, 2011. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, D.; Clark, L.A.; Carey, G. Positive and negative affectivity and their relation to anxiety and depressive disorders. J. Abnorm. Psychol. 1988, 97, 346–353. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Luschene, R. Test Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Baron-Cohen, S.; Wheelwright, S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004, 34, 163–175. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Richler, J.; Bisarya, D.; Gurunathan, N.; Wheelwright, S. The systemizing quotient: An investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 361–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale (SRS): Manual; Western Psychological Services: Los Angeles, CA, USA, 2005. [Google Scholar]
- Mayer, J.; Salovey, P.; Caruso, D. Mayer-Salovey-Caruso Emotional Intelligence Test Manual; Multi-Health Systems: Toronto, CA, USA, 2002. [Google Scholar]
- Crawford, J.R.; Garthwaite, P.H.; Lawrie, C.J.; Henry, J.D.; MacDonald, M.A.; Sutherland, J.; Sinha, P. A convenient method of obtaining percentile norms and accompanying interval estimates for self-report mood scales (DASS, DASS-21, HADS, PANAS, and sAD). Br. J. Clin. Psychol. 2009, 48, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Wheelwright, S.; Baron-Cohen, S.; Goldenfeld, N.; Delaney, J.; Fine, D.; Smith, R.; Weil, L.; Wakabayashi, A. Predicting Autism Spectrum Quotient (AQ) from the Systemizing Quotient-Revised (SQ-R) and Empathy Quotient (EQ). Brain Res. 2006, 1079, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.; Saxe, R. Development of predictive responses in theory of mind brain regions. Dev. Sci. 2020, 23, e12863. [Google Scholar] [CrossRef] [Green Version]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 2019, 16, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L.; Brain Development Cooperative Group. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage 2011, 54, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, R.W.; Hyde, J.S. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997, 10, 171–178. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef] [Green Version]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, T.D.; Elliott, M.A.; Gerraty, R.T.; Ruparel, K.; Loughead, J.; Calkins, M.E.; Eickhoff, S.B.; Hakonarson, H.; Gur, R.C.; Gur, R.E.; et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 2013, 64, 240–256. [Google Scholar] [CrossRef] [Green Version]
- Lanczos, C. Evaluation of Noisy Data. J. Soc. Ind. Appl. Math. Ser. B Numer. Anal. 1964, 76–85. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [Green Version]
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.; Jenkinson, M.; Smith, S.M. Bayesian analysis of neuroimaging data in FSL. Neuroimage 2009, 45, S173–S186. [Google Scholar] [CrossRef]
- Kelly, R.E., Jr.; Alexopoulos, G.S.; Wang, Z.; Gunning, F.M.; Murphy, C.F.; Morimoto, S.S.; Kanellopoulos, D.; Jia, Z.; Lim, K.O.; Hoptman, M.J. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods 2010, 189, 233–245. [Google Scholar] [CrossRef] [Green Version]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Nichols, T.E.; Das, S.; Eickhoff, S.B.; Evans, A.C.; Glatard, T.; Hanke, M.; Kriegeskorte, N.; Milham, M.P.; Poldrack, R.A.; Poline, J.-B.; et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 2017, 20, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Sonkusare, S.; Breakspear, M.; Guo, C. Naturalistic Stimuli in Neuroscience: Critically Acclaimed. Trends Cogn. Sci. 2019, 23, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.A.; Hutcherson, C.A.; Adolphs, R.; Stanley, D.A. Deconstructing Theory-of-Mind Impairment in High-Functioning Adults with Autism. Curr. Biol. 2019, 29, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahern, G.L.; Schwartz, G.E. Differential lateralization for positive versus negative emotion. Neuropsychologia 1979, 17, 693–698. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.; Davidson, R.J. Differential contributions of the two cerebral hemispheres to the perception of happy and sad faces. Neuropsychologia 1981, 19, 609–613. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992, 20, 125–151. [Google Scholar] [CrossRef]
- Gainotti, G. Emotional behavior and hemispheric side of the lesion. Cortex 1972, 8, 41–55. [Google Scholar] [CrossRef]
- Gainotti, G. Emotions and the Right Hemisphere: Can New Data Clarify Old Models? Neuroscientist 2019, 25, 258–270. [Google Scholar] [CrossRef]
- Gainotti, G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia 2012, 50, 205–218. [Google Scholar] [CrossRef]
- Gainotti, G. The riddle of the right hemisphere’s contribution to the recovery of language. Eur. J. Disord. Commun. 1993, 28, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.R.; Tranel, D.; Damasio, H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav. Brain Res. 1990, 41, 81–94. [Google Scholar] [CrossRef]
- Frassle, S.; Krach, S.; Paulus, F.M.; Jansen, A. Handedness is related to neural mechanisms underlying hemispheric lateralization of face processing. Sci. Rep. 2016, 6, 27153. [Google Scholar] [CrossRef] [PubMed]

| Case | Hem | Etiol | Onset | Age HS | Sex | Hand | Age | FSIQ | VCI | POI |
|---|---|---|---|---|---|---|---|---|---|---|
| RHS1 | R | CD | birth | 3m | M | R | 22 | 80 | 91 | 72 |
| RHS2 | R | RS | 11y | 20y | M | R | 23 | 105 | 109 | 93 |
| RHS3 | R | PNS | 8y | 15y | F | R | 21 | 96 | 101 | 86 |
| LHS4 | L | PNS | 3y | 6y | F | L | 26 | 95 | 101 | 95 |
| CNT1 | na | na | na | na | M | R | 26 | 94 | 99 | 92 |
| CNT2 | na | na | na | na | F | R | 24 | 100 | 104 | 96 |
| CNT3 | na | na | na | na | F | L | 28 | 100 | 106 | 94 |
| CNT4 | na | na | na | na | M | R | 24 | 101 | 105 | 96 |
| Case | Temporal Mean FD (mm) | ||
|---|---|---|---|
| run1 | run2 | mean(run1,run2) | |
| RHS1 | 0.10 | n/a | n/a |
| RHS2 | 0.13 | 0.20 | 0.16 |
| RHS3 | 0.15 | 0.11 | 0.13 |
| LHS1 | 0.23 | 0.12 | 0.17 |
| CNT1 | 0.30 | n/a | n/a |
| CNT2 | 0.14 | n/a | n/a |
| CNT3 | 0.11 | n/a | n/a |
| CNT4 | 0.16 | n/a | n/a |
| Case | MSCEIT Exp | MSCEIT Rea | PANAS Pos | PANAS Neg | STAI State | STAI Trait | SRS-2 | EQ | SQ |
|---|---|---|---|---|---|---|---|---|---|
| RHS1 | 0.61 | −0.38 | 2.21 * | −0.75 | −1.00 | −0.50 | −0.50 | 1.64 * | 1.40 |
| RHS2 | −0.45 | 0.57 | −0.37 | −1.05 | −1.20 | −1.20 | −1.00 | 2.24 * | 0.30 |
| RHS3 | 0.28 | −0.16 | −1.59 * | −1.05 | −0.10 | 1.00 | 0.80 | −1.15 | −0.40 |
| LHS4 | −0.42 | −0.66 | −1.32 | −0.01 | 1.40 | 1.10 | 1.40 | −0.27 | 1.16 |
| CNT1 | na | na | na | na | na | 0 | 0.30 | −1.38 | −0.79 |
| CNT2 | 0.09 | −0.47 | na | na | na | 1.00 | 1.40 | 0.27 | 0.54 |
| CNT3 | 0.03 | 0.36 | na | na | na | 1.30 | 0.30 | 0.71 | 0.02 |
| CNT4 | na | na | na | na | na | 2.00* | 1.30 | −0.52 | −0.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliemann, D.; Adolphs, R.; Paul, L.K.; Tyszka, J.M.; Tranel, D. Reorganization of the Social Brain in Individuals with Only One Intact Cerebral Hemisphere. Brain Sci. 2021, 11, 965. https://doi.org/10.3390/brainsci11080965
Kliemann D, Adolphs R, Paul LK, Tyszka JM, Tranel D. Reorganization of the Social Brain in Individuals with Only One Intact Cerebral Hemisphere. Brain Sciences. 2021; 11(8):965. https://doi.org/10.3390/brainsci11080965
Chicago/Turabian StyleKliemann, Dorit, Ralph Adolphs, Lynn K. Paul, J. Michael Tyszka, and Daniel Tranel. 2021. "Reorganization of the Social Brain in Individuals with Only One Intact Cerebral Hemisphere" Brain Sciences 11, no. 8: 965. https://doi.org/10.3390/brainsci11080965





