Neuroanatomical Correlates of Binge-Eating Behavior: At the Roots of Unstoppable Eating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Self-Report Questionnaires
- EAT-26 [24]: It assesses the presence of an eating disorder, by providing a measure of the symptoms and concerns that are peculiar to eating disorders. Here, we focused on the behavioral questions investigating the presence of binge-eating episodes.
- BES [22]: It assesses the severity of binge-eating behavior relying upon both behavioral characteristics (e.g., amount of food consumed) and the emotional, cognitive responses (e.g., guilt/shame or preoccupation with food).
- Yale Food Addiction Scale (YFAS) [29]: A 25-items self-reported assessment adopted to identify individuals prone to exhibit traits of substance addiction (in this case, consumption of high fat/high sugar foods). Items used to spot food-addiction symptoms (e.g., loss of control, tolerance, withdrawal) are built on the criteria for substance dependence as described in the DSM IV-TR [30]. Usually, three or more symptoms—plus clinically significant impairment or distress—indicates the presence of “food addiction”.
- Barratt Impulsiveness Scale (BIS-11) [21]: It allows the investigation of three distinct forms of impulsivity: (i) attentional, (ii) motor, (iii) non-planning impulsivity.
2.3. Magnetic Resonance Imaging (MRI) Acquisition
2.4. MRI Analysis
2.5. Brain-Behavior Correlations
3. Results
3.1. Descriptive Statistics
3.2. Voxel-Based Morphometry (VBM)
3.2.1. Global Volumes: Between-Groups Comparison
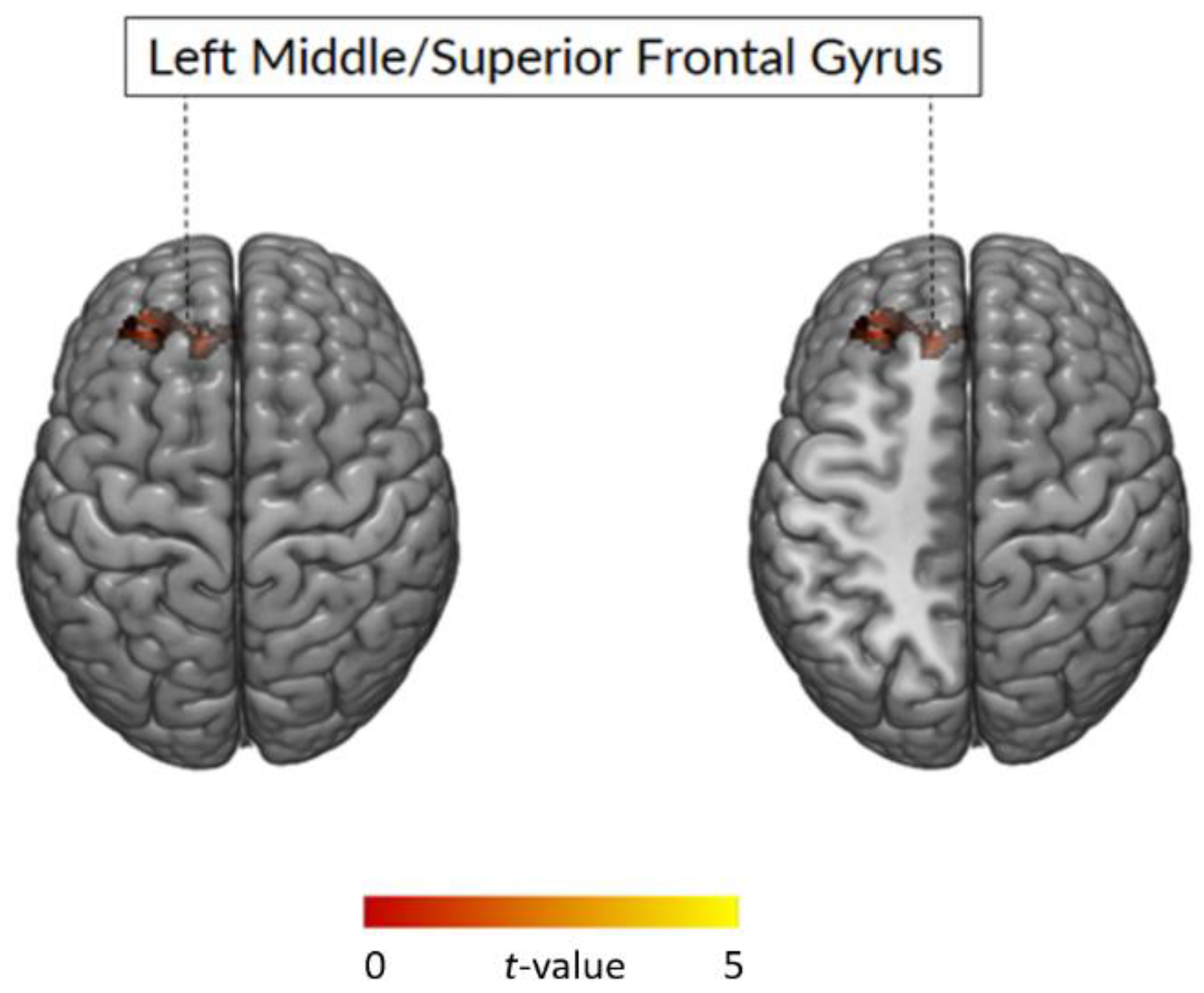
3.2.2. Whole Brain Analysis: Between-Group Comparison
3.2.3. Correlation between GMV and Impulsivity Traits
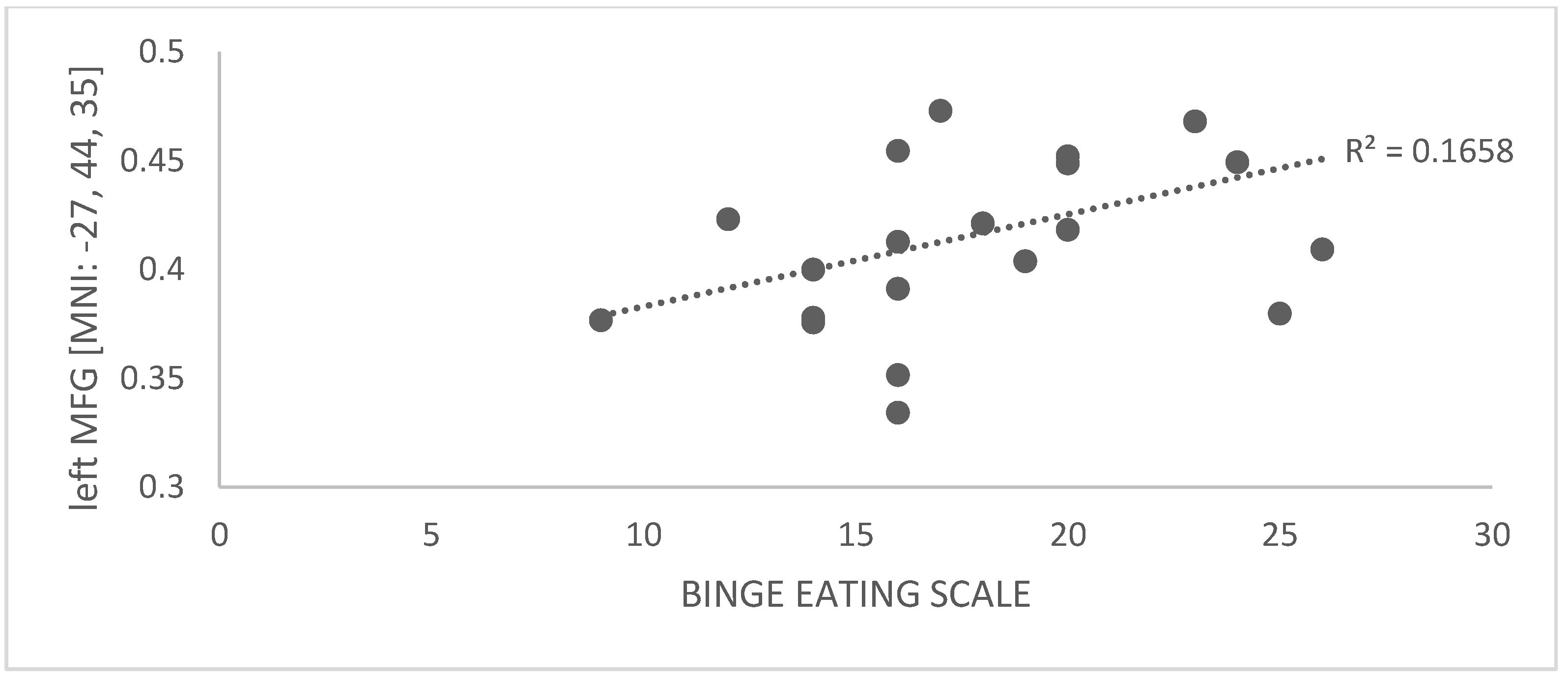
3.2.4. Correlation between GMV and Binge-Eating Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Extracted GMV in Left MFG [MNI: −27, 44, 35] | ||
|---|---|---|
| Spearman’s Rho | p-Value | |
| BIS-11 Total score | −0.048 | 0.835 |
| Subscale ATTENTION | 0.107 | 0.644 |
| Subscale MOTOR | −0.264 | 0.247 |
| Subscale NON-PLANNING | 0.047 | 0.839 |
| BES | 0.257 | 0.261 |
| Extracted GMV in Left MFG [MNI: −27, 44, 35] | ||
|---|---|---|
| Spearman’s Rho | p-Value | |
| BIS-11 Total score | 0.150 | 0.518 |
| Subscale ATTENTION | 0.289 | 0.204 |
| Subscale MOTOR | 0.084 | 0.719 |
| Subscale NON-PLANNING | 0.037 | 0.872 |
References
- Mayhew, A.J.; Pigeyre, M.; Couturier, J.; Meyre, D. An Evolutionary Genetic Perspective of Eating Disorders. Neuroendocrinology 2018, 106, 292–306. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Press Inc.: Washington, DC, USA, 1994. [Google Scholar]
- Davis, C. From passive overeating to “food addiction”: A spectrum of compulsion and severity. ISRN Obesity 2013, 2013, 435027. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.F.; Sabino, V.; Koob, G.F.; Cottone, P. Neuroscience of Compulsive Eating Behavior. Front. Neurosci. 2018, 11, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loxton, N.J. The Role of Reward Sensitivity and Impulsivity in Overeating and Food Addiction. Curr. Addict. Rep. 2018, 5, 212–222. [Google Scholar] [CrossRef]
- García-García, I.; Horstmann, A.; Jurado, M.A.; Garolera, M.; Chaudhry, S.J.; Margulies, D.S.; Villringer, A.; Neumann, J. Reward processing in obesity, substance addiction and non-substance addiction. Obes. Rev. 2014, 15, 853–869. [Google Scholar] [CrossRef]
- Beyer, F.; García-García, I.; Heinrich, M.; Schroeter, M.L.; Sacher, J.; Luck, T.; Riedel-Heller, S.G.; Stumvoll, M.; Villringer, A.; Witte, A.V. Neuroanatomical correlates of food addiction symptoms and body mass index in the general population. Hum. Brain Mapp 2019, 40, 2747–2758. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.M.; Hutson, P.H.; Herman, B.K.; Potenza, M.N. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev. 2016, 63, 223–238. [Google Scholar] [CrossRef] [Green Version]
- García-García, I.; Michaud, A.; Dadar, M.; Zeighami, Y.; Neseliler, S.; Collins, D.L.; Evans, A.C.; Dagher, A. Neuroanatomical differences in obesity: Meta-analytic findings and their validation in an independent dataset. Int. J. Obes. 2019, 43, 943–951. [Google Scholar] [CrossRef]
- Opel, N.; Redlich, R.; Kaehler, C.; Grotegerd, D.; Dohm, K.; Heindel, W.; Kugel, H.; Thalamuthu, A.; Koutsouleris, N.; Arolt, V.; et al. Prefrontal gray matter volume mediates genetic risks for obesity. Mol. Psychiatry 2017, 22, 703–710. [Google Scholar] [CrossRef]
- Vainik, U.; Baker, T.E.; Dadar, M.; Zeighami, Y.; Michaud, A.; Zhang, Y.; Dagher, A. Neurobehavioral correlates of obesity are largely heritable. PNAS 2018, 201718206. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.J.; Benedict, C.; Burgos, J.; Kempton, M.J.; Kullberg, J.; Nordenskjöld, R.; Kilander, L.; Nylander, R.; Larsson, E.M.; Johansson, L.; et al. Late-life obesity is associated with smaller global and regional gray matter volumes: A voxel-based morphometric study. Int. J. Obes. 2013, 37, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Schienle, A.; Unger, I.; Wabnegger, A. Comparison of women with high vs. low food addiction tendency: A pilot study with voxel-based morphometry. J. Eat Disord. 2020, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, W.; Dai, Z.; Dong, C. Eating behavior associated with gray matter volume alternations: A voxel based morphometry study. Appetite 2016, 96, 572–579. [Google Scholar] [CrossRef]
- Sedgmond, J.; Lawrence, N.S.; Verbruggen, F.; Morrison, S.; Chambers, C.D.; Adams, R.C. Prefrontal brain stimulation during food-related inhibition training: Effects on food craving, food consumption and inhibitory control. R Soc. Open Sci. 2019, 6, 181186. [Google Scholar] [CrossRef] [Green Version]
- Bartholdy, S.; O’Daly, O.G.; Campbell, I.C.; Banaschewski, T.; Barker, G.; Bokde, A.; Bromberg, U.; Büchel, C.; Quinlan, E.B.; Desrivières, S.; et al. Neural Correlates of Failed Inhibitory Control as an Early Marker of Disordered Eating in Adolescents. Biol. Psychiatry 2019, 85, 956–965. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Alonso, M.; Pascual-Leone, A. The right brain hypothesis for obesity. JAMA 2019, 297, 1819–1822. [Google Scholar] [CrossRef]
- Willette, A.A.; Kapogiannis, D. Does the brain shrink as the waist expands? Ageing Res. Rev. 2015, 20, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashburner, J.; Friston, K.J. Voxel-Based Morphometry—The Methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef] [Green Version]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Gormally, J.; Black, S.; Daston, S.; Rardin, D. The assessment of binge-eating severity among obese persons. Addict. Behav. 1982, 7, 47–55. [Google Scholar] [CrossRef]
- Gluck, M.E.; Viswanath, P.; Stinson, E.J. Obesity, appetite and the prefrontal cortex. Curr. Obes. Rep. 2017, 6, 380–388. [Google Scholar] [CrossRef]
- Garner, D.M.; Olmsted, M.P.; Bohr, Y.; Garfinkel, P.E. The eating attitudes test: Psychometric features and clinical correlates. Psychol. Med. 1982, 12, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Filbey, F.M.; Myers, U.S.; Dewitt, S. Reward circuit function in high BMI individuals with compulsive overeating: Similarities with addiction. NeuroImage 2012, 63, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Oliva, R.; Morys, F.; Horstmann, A.; Castiello, U.; Begliomini, C. The impulsive brain: Neural underpinnings of binge-eating behavior in normal-weight adults. Appetite 2019, 136, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Morys, F.; Horstmann, A.; Castiello, U.; Begliomini, C. Characterizing impulsivity and resting-state functional connectivity in normal-weight binge eaters. Int. J. Eat. Disord. 2020, 53, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Corbin, W.R.; Brownell, K.D. Preliminary validation of the Yale Food Addiction Scale. Appetite 2009, 52, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Press Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Carver, C.S.; White, T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 1994, 67, 319–333. [Google Scholar] [CrossRef]
- Gray, J.A. The Neuropsychology of Temperament. In Explorations in Temperament. Perspectives on Individual Differences; Strelau, J., Angleitner, A., Eds.; Springer: Boston, MA, USA, 1991; pp. 105–128. [Google Scholar]
- Kroll, D.S.; Feldman, D.E.; Biesecker, C.L.; McPherson, K.L.; Manza, P.; Joseph, P.V.; Volkow, N.D.; Wang, G.J. Neuroimaging of Sex/Gender Differences in Obesity: A Review of Structure, Function, and Neurotransmission. Nutrients 2020, 12, 1942. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.-L.; Valabregue, R.; Poline, J.-B. Region of Interest Analysis using an SPM Toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002; Volume 16. Available on CD-ROM in NeuroImage. [Google Scholar]
- Nederkoorn, C.; Van Eijs, Y.; Jansen, A. Restrained eaters act on impulse. Pers. Individ Diff. 2004, 37, 1651–1658. [Google Scholar] [CrossRef]
- Yeomans, M.R.; Brace, A. Cued to act on impulse: More impulsive choice and risky decision making by women susceptible to overeating after exposure to food stimuli. PLoS ONE 2015, 10, e0137626. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.L. Frontal-subcortical circuits and human behavior. Arch. Neurol. 1993, 50, 873–880. [Google Scholar] [CrossRef]
- Georgii, C.; Goldhofer, P.; Meule, A.; Richard, A.; Blechert, J. Food craving, food choice and consumption: The role of impulsivity and sham-controlled tDCS stimulation of the right dlPFC. Physiol. Behav. 2017, 177, 20–26. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar] [CrossRef]
- García-García, I.; Morys, F.; Michaud, A.; Dager, A. Food Addiction, Skating on Thin Ice: A Critical Overview of Neuroimaging Findings. Curr. Addict. Rep. 2020, 7, 20–29. [Google Scholar] [CrossRef]
- Schulte, E.M.; Yokum, S.; Jahn, A.; Gearhardt, A.N. Food cue reactivity in food addiction: A functional magnetic resonance imaging study. Physiol. Behav. 2019, 208, 112574. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.R. Functional networks for cognitive control in a stop signal task: Independent component analysis. Hum. Brain Mapp. 2012, 33, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Yokum, S.; Ng, J.; Stice, E. Attentional bias to food images associated with elevated weight and future weight gain: An fMRI study. Obesity 2011, 19, 1775–1783. [Google Scholar] [CrossRef]
- Davids, S.; Lauffer, H.; Thoms, K.; Jagdhuhn, M.; Hirschfeld, H.; Domin, M.; Lotze, M. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int. J. Obes 2010, 34, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Brodtmann, A.; Puce, A.; Darby, D.; Donnan, G. Regional fMRI brain activation does correlate with global brain volume. Brain Res. 2009, 1259, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Doallo, S.; Cadaveira, F.; Corral, M.; Mota, N.; López-Caneda, E.; Holguín, S.R. Larger Mid-Dorsolateral Prefrontal Gray Matter Volume in Young Binge Drinkers Revealed by Voxel-Based Morphometry. PLoS ONE 2014, 9, e96380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, S.S.; Sampaio, A.; Marques, P.; Gonçalves, Ó.F.; Crego, A. Gray Matter Abnormalities in the Inhibitory Circuitry of Young Binge Drinkers: A Voxel-Based Morphometry Study. Front. Psychol. 2017, 8, 1567. [Google Scholar] [CrossRef] [Green Version]
- Schulte, E.M.; Grilo, C.M.; Gearhardt, A.N. Shared and unique mechanisms underlying binge-eating disorder and addictive disorders. Clin. Psychol. Rev. 2016, 44, 125–139. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
| Characteristics | BE (n = 21) M ± SD | NON-BE (n = 21) M ± SD | Two-Samples t-Test | |
|---|---|---|---|---|
| AGE | 23.9 + 3.19 | 25.23 ± 3.08 | 2.05 | 0.191 |
| BMI (kg/m2) | 22.3 ± 2.1 | 21.29 ± 2.02 | 1.73 | 0.074 |
| BES | 17.7 ± 3.8 | 3.8 ± 2.6 | 17.1 | <0.001 * |
| YFAS | 3.05 ± 1.43 | 0.29 ± 0.56 | 8.23 | <0.001 * |
| BIS-11 | ||||
| Subscale ATTENTION | 17.05 ± 3.7 | 15 ± 3.3 | 1.8 | 0.075 |
| Subscale MOTOR | 20.73 ± 4.2 | 17.75 ± 3.3 | 2.5 | 0.015 * |
| Subscale NON-PLANNING | 26.32 ± 5.1 | 22.25 ± 4.1 | 2.8 | 0.007 * |
| TOTAL SCORE | 63.4 ± 8.8 | 56 ± 7.5 | 2.7 | 0.011 * |
| BIS/BAS | ||||
| BAS reward responsiveness | 7.3 ± 1.8 | 7.6 ± 2.1 | 0.43 | 0.075 |
| BAS Drive | 7.8 ± 1.7 | 9.3 ± 1.9 | 2.51 | 0.017 * |
| BAS Fun seeking | 8.7 ± 2.1 | 9.4 ± 2.4 | 1.03 | 0.13 |
| BIS | 13.3 ± 2.3 | 16.2 ± 3.6 | 2.88 | 0.007 * |
| Non-BE | BE | Two Sample t-Test | ||
|---|---|---|---|---|
| M ± SD | M ± SD | t | p | |
| (%) GM | 19.4 ± 7 | 19.6 ± 3.3 | 0.11 | 0.915 |
| (%) WM | 44.6 ± 1.8 | 44.1 ±3.4 | 0.62 | 0.537 |
| (%) CFS | 37 ± 1.3 | 36.3 ± 1.6 | 1.59 | 0.12 |
| TIV (mL) | 1422.4 ± 142.4 | 1483.2 ± 134.4 | 1.42 | 0.162 |
| Whole Brain Analysis | ||||||
|---|---|---|---|---|---|---|
| k | p (FWE) | t | z-Score | MNI | Side | Region |
| BE > non-BE | ||||||
| 604 | 0.002 | 4.92 | 4.29 | −27, 44, 35 | L | Middle Frontal Gyrus |
| 4.20 | 3.77 | −15, 41, 29 | L | Superior Frontal Gyrus | ||
| 3.89 | 3.54 | −35, 41, 41 | L | Middle Frontal Gyrus | ||
| Non-BE > BE | ||||||
| ns | ||||||
| Extracted GMV in Left MFG | ||
|---|---|---|
| Spearman’s Rho | p-Value | |
| BES | 0.463 | 0.035 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, R.; Budisavljević, S.; Castiello, U.; Begliomini, C. Neuroanatomical Correlates of Binge-Eating Behavior: At the Roots of Unstoppable Eating. Brain Sci. 2021, 11, 1162. https://doi.org/10.3390/brainsci11091162
Oliva R, Budisavljević S, Castiello U, Begliomini C. Neuroanatomical Correlates of Binge-Eating Behavior: At the Roots of Unstoppable Eating. Brain Sciences. 2021; 11(9):1162. https://doi.org/10.3390/brainsci11091162
Chicago/Turabian StyleOliva, Rossella, Sanja Budisavljević, Umberto Castiello, and Chiara Begliomini. 2021. "Neuroanatomical Correlates of Binge-Eating Behavior: At the Roots of Unstoppable Eating" Brain Sciences 11, no. 9: 1162. https://doi.org/10.3390/brainsci11091162
APA StyleOliva, R., Budisavljević, S., Castiello, U., & Begliomini, C. (2021). Neuroanatomical Correlates of Binge-Eating Behavior: At the Roots of Unstoppable Eating. Brain Sciences, 11(9), 1162. https://doi.org/10.3390/brainsci11091162







