Decision Making under Risk in Patients Suffering from Schizophrenia or Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Background Assessment
2.3. Assessment of Decision Making Abilities
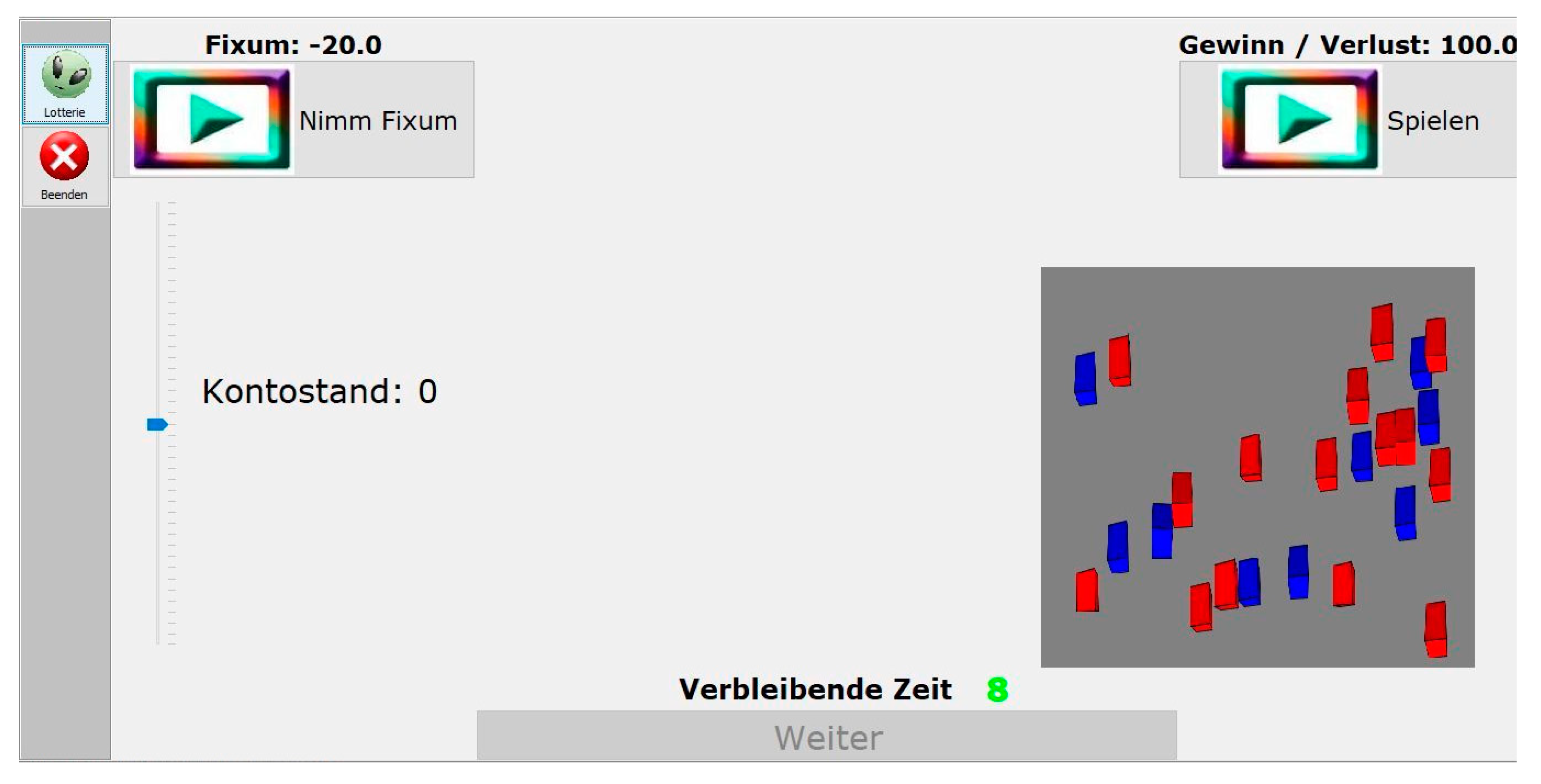
Probability-Associated Gambling Task (PAG)
- Arcsine-transformed frequency of gambles in each of the four winning probabilities in combination with a fixed sum of +20€;
- Arcsine-transformed frequency of gambles in each of the four winning probabilities in combination with a fixed sum of −20€;
- Ln-transformed total response time (RT) in ms;
- Total winning amount in €;
- Number of omission errors (trials where no alternative was chosen within the time limit of 10 s);
- Proportion of strategy changes from the fixed sum alternative to the gambling alternative when there is a positive change in the winning probability (from low to high);
- Proportion of strategy changes from the gambling alternative to the fixed sum alternative when there is a negative change in the winning probability (from high to low).
2.4. Statistical Analyses
3. Results
3.1. Sociodemographic and Clinical Data
3.2. Neuropsychological Background Scores
3.3. Decision Making Performance
3.4. Correlations between Clinical Variables and Neuropsychological Background Scores
3.5. Correlations between Clinical Variables and Decision Making Performance
3.6. Correlations between Neuropsychological Background Scores and Decision Making Performance
4. Discussion
4.1. Cognitive Performance
4.2. Decision Making under Risk
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manes, F.; Sahakian, B.; Clark, L.; Rogers, R.; Antoun, N.; Aitken, M.; Robbins, T. Decision-making processes following damage to the prefrontal cortex. Brain 2002, 125, 624–639. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.; Markowitsch, H.J. Aging and decision-making: A neurocognitive perspective. Gerontology 2010, 56, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gleichgerrcht, E.; Ibáñez, A.; Roca, M.; Torralva, T.; Manes, F. Decision-making cognition in neurodegenerative diseases. Nat. Rev. Neurol. 2010, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Labudda, K.; Markowitsch, H.J. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Netw. 2006, 19, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Bredemeier, K.; Warren, S.L.; Berenbaum, H.; Miller, G.A.; Heller, W. Executive function deficits associated with current and past major depressive symptoms. J. Affect. Disord. 2016, 204, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Beblo, T.; Lautenbacher, S. Neuropsychologie der Depression; Hogrefe Verlag: Gottingen, Germany, 2006. [Google Scholar]
- McDermott, L.M.; Ebmeier, K.P. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009, 119, 1–8. [Google Scholar] [CrossRef]
- Green, M.F.; Nuechterlein, K.H.; Gold, J.M.; Barch, D.M.; Cohen, J.; Essock, S.; Fenton, W.S.; Frese, F.; Goldberg, T.E.; Heaton, R.K.; et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry 2004, 56, 301–307. [Google Scholar] [CrossRef]
- Kurtz, M. Neurocognitive impairment across the lifespan in schizophrenia: An update. Schizophr. Res. 2005, 74, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, K.A.; Alexopoulos, G.S.; van Gorp, W.G. Executive dysfunction in geriatric depression. Am. J. Psychiatry 2002, 159, 1119–1126. [Google Scholar] [CrossRef]
- Mosiołek, A.; Gierus, J.; Koweszko, T.; Szulc, A. Cognitive impairment in schizophrenia across age groups: A case–control study. BMC Psychiatry 2016, 16, 37. [Google Scholar] [CrossRef] [Green Version]
- Cella, M.; Dymond, S.; Cooper, A. Impaired flexible decision-making in major depressive disorder. J. Affect. Disord. 2010, 124, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Deisenhammer, E.A.; Schmid, S.K.; Kemmler, G.; Moser, B.; Delazer, M. Decision making under risk and under ambiguity in depressed suicide attempters, depressed non-attempters and healthy controls. J. Affect. Disord. 2018, 226, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Szabó, Z.; Bódi, N.; Szász, A.; Janka, Z.; Kéri, S. Sensitivity to reward and punishment and the prefrontal cortex in major depression. J. Affect. Disord. 2006, 90, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Horvath, S.; Nemeth, V.L.; Janka, Z. The Iowa Gambling Task in depression—What have we learned about sub-optimal decision-making strategies? Front. Psychol. 2013, 4, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoski, M.J.; Lynch, T.R.; Rosenthal, M.Z.; Cheavens, J.S.; Chapman, A.L.; Krishnan, R.R. Decision-making and risk aversion among depressive adults. J. Behav. Ther. Exp. Psychiatry 2008, 39, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Bark, R.; Dieckmann, S.; Bogerts, B.; Northoff, G. Deficit in decision making in catatonic schizophrenia: An exploratory study. Psychiatry Res. 2005, 134, 131–141. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Dong, Y.; Ji, Y.; Tao, R.; Liang, Z.; Chen, J.; Wu, Y.; Wang, K. Similarities and differences in decision-making impairments between autism spectrum disorder and schizophrenia. Front. Behav. Neurosci. 2015, 9, 259. [Google Scholar] [CrossRef] [Green Version]
- Shurman, B.; Horan, W.P.; Nuechterlein, K.H. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr. Res. 2005, 72, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Sevy, S.; Burdick, K.E.; Visweswaraiah, H.; Abdelmessih, S.; Lukin, M.; Yechiam, E.; Bechara, A. Iowa Gambling Task in schizophrenia: A review and new data in patients with schizophrenia and co-occurring cannabis use disorders. Schizophr. Res. 2007, 92, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Kim, Y.-T.; Seo, E.; Park, O.; Jeong, S.-H.; Kim, S.H.; Lee, S.-J. Dissociation of Emotional decision-making from cognitive decision-making in chronic schizophrenia. Psychiatry Res. 2007, 152, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kester, H.M.; Sevy, S.; Yechiam, E.; Burdick, K.E.; Cervellione, K.L.; Kumra, S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr. Res. 2006, 85, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Bayard, S.; Capdevielle, D.; Del-Monte, J.; Mimoun, N.; Macgregor, A.; Boulenger, J.-P.; Gely-Nargeot, M.-C.; Raffard, S. A further evaluation of decision-making under risk and under ambiguity in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 249–257. [Google Scholar] [CrossRef]
- Schiebener, J.; Brand, M. Decision making under objective risk conditions—A review of cognitive and emotional correlates, strategies, feedback processing, and external influences. Neuropsychol. Rev. 2015, 25, 171–198. [Google Scholar] [CrossRef]
- Ernst, M.; Paulus, M.P. Neurobiology of decision making: A selective review from a neurocognitive and clinical perspective. Biol. Psychiatry 2005, 58, 597–604. [Google Scholar] [CrossRef]
- Trepel, C.; Fox, C.R.; Poldrack, R.A. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Cogn. Brain Res. 2005, 23, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.-R.; Jungermann, H.; Fischer, K. Die Psychologie der Entscheidung: Eine Einführung; Springer: Berlin, Germany, 2017. [Google Scholar] [CrossRef]
- Damasio, A.R. Descartes’ Irrtum. Fühlen, Denken Und Das Menschliche Gehirn; Ullstein Buchverlage: Berlin, Germany, 2004. [Google Scholar]
- Lee, R.S.C.; Hermens, D.F.; Porter, M.A.; Redoblado-Hodge, M.A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J. Affect. Disord. 2012, 140, 113–124. [Google Scholar] [CrossRef]
- Baune, B.T.; Fuhr, M.; Air, T.; Hering, C. Neuropsychological functioning in adolescents and young adults with major depressive disorder—A review. Psychiatry Res. 2014, 218, 261–271. [Google Scholar] [CrossRef]
- Beblo, T.; Sinnamon, G.; Baune, B.T. Specifying the neuropsychology of affective disorders: Clinical, demographic and neurobiological factors. Neuropsychol. Rev. 2011, 21, 337–359. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahern, E.; Semkovska, M. Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology 2017, 31, 52–72. [Google Scholar] [CrossRef]
- Bora, E.; Akdede, B.B.; Alptekin, K. Neurocognitive impairment in deficit and non-deficit schizophrenia: A meta-analysis. Psychol. Med. 2017, 47, 2401–2413. [Google Scholar] [CrossRef]
- Mesholam-Gately, R.I.; Giuliano, A.J.; Goff, K.P.; Faraone, S.V.; Seidman, L.J. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology 2009, 23, 315–336. [Google Scholar] [CrossRef] [Green Version]
- Fioravanti, M.; Bianchi, V.; Cinti, M.E. Cognitive deficits in schizophrenia: An updated metanalysis of the scientific evidence. BMC Psychiatry 2012, 12, 64. [Google Scholar] [CrossRef] [Green Version]
- Bechara, A.; Damasio, A.R.; Damasio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef]
- Adida, M.; Jollant, F.; Clark, L.; Besnier, N.; Guillaume, S.; Kaladjian, A.; Mazzola-Pomietto, P.; Jeanningros, R.; Goodwin, G.M.; Azorin, J.-M.; et al. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol. Psychiatry 2011, 70, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Larquet, M.; Coricelli, G.; Opolczynski, G.; Thibaut, F. Impaired decision making in schizophrenia and orbitofrontal cortex lesion patients. Schizophr. Res. 2010, 116, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.C.; Hack, S.M.; Gold, J.M.; Carpenter, W.T.; Fischer, B.A.; Prentice, K.P.; Waltz, J.A. Integrating frequency and magnitude information in decision-making in schizophrenia: An account of patient performance on the Iowa Gambling Task. J. Psychiatr. Res. 2015, 66, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Eshel, N.; Roiser, J.P. Reward and punishment processing in depression. Biol. Psychiatry 2010, 68, 118–124. [Google Scholar] [CrossRef]
- von Helversen, B.; Wilke, A.; Johnson, T.; Schmid, G.; Klapp, B. Performance benefits of depression: Sequential decision making in a healthy sample and a clinically depressed sample. J. Abnorm. Psychol. 2011, 120, 962–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, M.; Fujiwara, E.; Borsutzky, S.; Kalbe, E.; Kessler, J.; Markowitsch, H.J. Decision-making deficits of korsakoff patients in a new gambling task with explicit rules: Associations with executive functions. Neuropsychology 2005, 19, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.; Labudda, K.; Kalbe, E.; Hilker, R.; Emmans, D.; Fuchs, G.; Kessler, J.; Markowitsch, H.J. Decision-making impairments in patients with Parkinson’s disease. Behav. Neurol. 2004, 15, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Sinz, H.; Zamarian, L.; Benke, T.; Wenning, G.K.; Delazer, M. Impact of ambiguity and risk on decision making in mild Alzheimer’s disease. Neuropsychologia 2008, 46, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Delazer, M.; Sinz, H.; Zamarian, L.; Stockner, H.; Seppi, K.; Wenning, G.K.; Benke, T.; Poewe, W. Decision making under risk and under ambiguity in Parkinson’s disease. Neuropsychologia 2009, 47, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Kalbe, E.; Labudda, K.; Fujiwara, E.; Kessler, J.; Markowitsch, H.J. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005, 133, 91–99. [Google Scholar] [CrossRef]
- Zamarian, L.; Weiss, E.M.; Delazer, M. The impact of mild cognitive impairment on decision making in two gambling tasks. J. Gerontol. Ser. B 2011, 66, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Fujino, J.; Hirose, K.; Tei, S.; Kawada, R.; Tsurumi, K.; Matsukawa, N.; Miyata, J.; Sugihara, G.; Yoshihara, Y.; Ideno, T.; et al. Ambiguity aversion in schizophrenia: An FMRI study of decision-making under risk and ambiguity. Schizophr. Res. 2016, 178, 94–101. [Google Scholar] [CrossRef]
- Pedersen, A.; Göder, R.; Tomczyk, S.; Ohrmann, P. Risky decision-making under risk in schizophrenia: A deliberate choice? J. Behav. Ther. Exp. Psychiatry 2017, 56, 57–64. [Google Scholar] [CrossRef]
- Lehrl, S. Mehrfachwahl-Wortschatz-Intelligenztest, 5th ed.; PERIMED—Spitta Medizinische Verlagsgesellschaft: Balingen, Germany, 2005. [Google Scholar]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders; World Health Organization: Genève, Switzerland, 1993. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef]
- Niemann, H.; Sturm, W.; Thöne-Otto, A.I.T.; Willmes, K. CVLT California Verbal Learning Test. German Adaptation. Manual; Pearson Assessment: Frankfurt, Germany, 2008. [Google Scholar]
- Reitan, R.M. TMT, Trail Making Test. A & B; AR: Reitan Neuropsychology Laboratory: South Tucson, AZ, USA, 1992. [Google Scholar]
- Aschenbrenner, A.; Tucha, O.; Lange, K. RWT Regensburger Wortflüssigkeits-Test, Handanweisung; Hogrefe Verlag: Göttingen, Germany, 2000. [Google Scholar]
- Zamarian, L.; Sinz, H.; Bonatti, E.; Gamboz, N.; Delazer, M. Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology 2008, 22, 645–657. [Google Scholar] [CrossRef]
- Gooren, T.; Schlattmann, P.; Neu, P. A comparison of cognitive functioning in acute schizophrenia and depression. Acta Neuropsychiatr. 2013, 25, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Orzechowska, A.; Filip, M.; Gałecki, P. Influence of pharmacotherapy on cognitive functions in depression: A review of the literature. Med. Sci. Monit. 2015, 21, 3643–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, P.D.; Rabinowitz, J.; Eerdekens, M.; Davidson, M. Treatment of cognitive impairment in early psychosis: A comparison of risperidone and haloperidol in a large long-term trial. Am. J. Psychiatry 2005, 162, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Beninger, R.J.; Wasserman, J.; Zanibbi, K.; Charbonneau, D.; Mangels, J.; Beninger, B.V. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: A double dissociation. Schizophr. Res. 2003, 61, 281–292. [Google Scholar] [CrossRef]
- Ng, Q.X.; Loke, W.; Foo, N.X.; Tan, W.J.; Chan, H.W.; Lim, D.Y.; Yeo, W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2020, 34, 583–590. [Google Scholar] [CrossRef]
- Siris, S.G.; Adan, F.; Cohen, M.; Mandeli, J.; Aronson, A.; Casey, E. Postpsychotic depression and negative symptoms: An investigation of syndromal overlap. Am. J. Psychiatry 1988, 145, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Krynicki, C.R.; Upthegrove, R.; Deakin, J.F.W.; Barnes, T.R.E. The relationship between negative symptoms and depression in schizophrenia: A systematic review. Acta Psychiatr. Scand. 2018, 137, 380–390. [Google Scholar] [CrossRef]
- Bismark, A.W.; Thomas, M.L.; Tarasenko, M.; Shiluk, A.L.; Rackelmann, S.Y.; Young, J.W.; Light, G.A. Relationship between effortful motivation and neurocognition in schizophrenia. Schizophr. Res. 2018, 193, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Foussias, G.; Siddiqui, I.; Fervaha, G.; Mann, S.; McDonald, K.; Agid, O.; Zakzanis, K.K.; Remington, G. Motivated to do well: An examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophr. Res. 2015, 166, 276–282. [Google Scholar] [CrossRef]

| Participant Groups | |||
|---|---|---|---|
| S (n = 28) | D (n = 28) | C (n = 30) | |
| Age (M, SD) | 42.57 (12.26) | 42.79 (15.29) | 42.40 (15.28) |
| Sex | |||
| - Male | 15 | 12 | 11 |
| - Female | 13 | 16 | 19 |
| Education | |||
| - 1: Less than high school | 22 | 19 | 20 |
| - 2: High school degree | 4 | 9 | 7 |
| - 3: Some college | 2 | 0 | 3 |
| Depression severity (M, SD) [HAMD-21 score] | - | 18.11 (5.92) | - |
| Schizophrenia symptomatology (M, SD) [PANSS score] | |||
| - Positive symptoms | 17.96 (5.70) | - | - |
| - Negative symptoms | 23.11 (7.76) | - | - |
| - General psychopathology | 37.79 (9.73) | - | - |
| Participant Groups | Group Comparisons | |||||
|---|---|---|---|---|---|---|
| Neuropsychological Parameters | S (n = 28) | D (n = 28) | C (n = 30) | S vs. D (p-Value) | S vs. C (p-Value) | D vs. C (p-Value) |
| CVLT [correctly recalled items] (M, SD) | ||||||
| - learning (trials 1–5) | 35.71 (12.47) | 44.00 (12.00) | 54.53 (8.66) | 0.018 | 0.000 | 0.002 |
| - immediate memory (trial 1) | 4.39 (1.50) | 6.14 (2.17) | 7.53 (1.81) | 0.002 | 0.000 | 0.015 |
| - short-delay free recall (trial 6) | 7.04 (3.23) | 8.21 (3.75) | 11.87 (2.49) | 0.352 | 0.000 | 0.000 |
| - long-delay free recall (trial 7) | 6.43 (3.51) | 8.39 (4.31) | 11.17 (3.77) | 0.146 | 0.000 | 0.021 |
| - recognition corrected | 10.21 (3.63) | 11.25 (3.92) | 13.60 (1.69) | 0.452 | 0.000 | 0.018 |
| TMT [s, ln-transformed] (M, SD) | ||||||
| - psychomotor speed (Part A) | 51.89 (20.00) | 56.29 (41.89) | 34.37 (14.52) | 0.926 | 0.003 | 0.009 |
| - cognitive flexibility (Part B) | 149.43 (67.14) | 122.43 (67.77) | 65.57 (20.49) | 0.081 | 0.000 | 0.000 |
| RWT [number of produced words] (M, SD) | ||||||
| - Semantic-categorical verbal fluency (“Animals”/2 min) | 27.64 (8.82) | 30.21 (9.02) | 37.97 (8.23) | 0.512 | 0.000 | 0.003 |
| - Phonological verbal fluency (“S-words”/2 min) | 17.29 (5.66) | 23.00 (8.81) | 29.80 (7.45) | 0.014 | 0.000 | 0.002 |
| Participant Groups | Group Comparisons | |||||
|---|---|---|---|---|---|---|
| PAG Parameters (M, SD) | S (n = 28) | D (n = 28) | C (n = 30) | S vs. D (p-Value) | S vs. C (p-Value) | D vs. C (p-Value) |
| Response time [ms, ln-transformed] | 4064.15 (1477.69) | 3684.78 (1281.18) | 2451.21 (1026.67) | 0.646 | 0.000 | 0.001 |
| Total winning amount [€] | 530.00 (535.45) | 655.00 (494.01) | 805.33 (409.40) | 0.596 | 0.081 | 0.463 |
| Strategy Changes [proportions] | ||||||
| - From fixed sum to gambling at a positive change of winning probability | 0.57 (0.32) | 0.60 (0.30) | 0.75 (0.25) | 0.886 | 0.043 | 0.125 |
| - From gambling to fixed sum at a negative change of winning probability | 0.55 (0.31) | 0.61 (0.33) | 0.69 (0.25) | 0.747 | 0.175 | 0.541 |
| PAG Task Parameters | ||||||
|---|---|---|---|---|---|---|
| Frequency of Gambles with p = 0.125 | Total Response Times | Proportion of Strategy Changes | ||||
| Neuropsychological Parameters | r | p | r | p | r | p |
| CVLT [correctly recalled items] | ||||||
| - learning (trials 1–5) | −0.407 | 0.032 | −0.334 | 0.082 | 0.285 | 0.142 |
| - immediate memory (trial 1) | −0.018 | 0.929 | −0.210 | 0.283 | −0.027 | 0.893 |
| - short-delay free recall (trial 6) | −0.270 | 0.165 | −0.292 | 0.131 | 0.155 | 0.432 |
| - long-delay free recall (trial 7) | −0.102 | 0.604 | −0.301 | 0.119 | −0.090 | 0.650 |
| - recognition corrected | −0.166 | 0.398 | −0.305 | 0.115 | 0.007 | 0.972 |
| TMT [s] | ||||||
| - psychomotor speed (Part A) | 0.235 | 0.229 | 0.412 | 0.030 | −0.192 | 0.328 |
| - cognitive flexibility (Part B) | 0.255 | 0.190 | 0.448 | 0.017 | −0.341 | 0.076 |
| RWT [number of produced words] | ||||||
| - semantic-categorial verbal fluency (“Animals”/2 min) | −0.352 | 0.067 | −0.103 | 0.602 | 0.328 | 0.089 |
| - phonological verbal fluency (“S-words”/2 min) | −0.344 | 0.073 | −0.276 | 0.155 | 0.226 | 0.248 |
| PAG Task Parameters | ||||||
|---|---|---|---|---|---|---|
| Frequency of Gambles with p = 0.125 | Total Response Times | Proportion of Strategy Changes | ||||
| Neuropsychological Parameters | r | p | r | p | r | p |
| CVLT [correctly recalled items] | ||||||
| - learning (trials 1–5) | 0.030 | 0.880 | −0.159 | 0.418 | −0.223 | 0.255 |
| - immediate memory (trial 1) | −0.051 | 0.797 | −0.383 | 0.044 | −0.201 | 0.305 |
| - short-delay free recall (trial 6) | −0.095 | 0.632 | −0.154 | 0.434 | −0.129 | 0.512 |
| - long-delay free recall (trial 7) | −0.019 | 0.925 | −0.186 | 0.342 | −0.178 | 0.365 |
| - recognition corrected | −0.140 | 0.477 | −0.273 | 0.160 | −0.127 | 0.520 |
| TMT [s] | ||||||
| - psychomotor speed (Part A) | −0.034 | 0.865 | 0.510 | 0.006 | 0.220 | 0.261 |
| - cognitive flexibility (Part B) | 0.117 | 0.554 | 0.511 | 0.005 | −0.060 | 0.761 |
| RWT [number of produced words] | ||||||
| - semantic-categorial verbal fluency (“Animals”/2 min) | −0.229 | 0.241 | −0.044 | 0.825 | 0.172 | 0.383 |
| - phonological verbal fluency (“S-words”/2 min) | −0.338 | 0.078 | −0.468 | 0.012 | 0.303 | 0.117 |
| PAG Task Parameters | ||||||
|---|---|---|---|---|---|---|
| Frequency of Gambles with p = 0.125 | Total Response Times | Proportion of Strategy Changes | ||||
| Neuropsychological Parameters | r | p | r | p | r | p |
| CVLT [correctly recalled items] | ||||||
| - learning (trials 1–5) | 0.110 | 0.564 | −0.009 | 0.963 | −0.196 | 0.299 |
| - immediate memory (trial 1) | −0.033 | 0.861 | −0.011 | 0.953 | −0.032 | 0.866 |
| - short-delay free recall (trial 6) | 0.249 | 0.185 | 0.271 | 0.147 | −0.162 | 0.394 |
| - long-delay free recall (trial 7) | 0.271 | 0.147 | 0.367 | 0.046 | −0.191 | 0.311 |
| - recognition corrected | 0.115 | 0.546 | 0.131 | 0.490 | −0.148 | 0.436 |
| TMT [s] | ||||||
| - psychomotor speed (Part A) | −0.448 | 0.013 | −0.083 | 0.662 | 0.465 | 0.010 |
| - cognitive flexibility (Part B) | −0.069 | 0.719 | 0.136 | 0.472 | 0.026 | 0.890 |
| RWT [number of produced words] | ||||||
| - semantic-categorial verbal fluency (“Animals”/2 min) | 0.149 | 0.431 | 0.195 | 0.301 | −0.060 | 0.755 |
| - phonological verbal fluency (“S-words”/2 min) | −0.012 | 0.949 | 0.007 | 0.970 | 0.038 | 0.843 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benke, T.; Marksteiner, J.; Ruepp, B.; Weiss, E.M.; Zamarian, L. Decision Making under Risk in Patients Suffering from Schizophrenia or Depression. Brain Sci. 2021, 11, 1178. https://doi.org/10.3390/brainsci11091178
Benke T, Marksteiner J, Ruepp B, Weiss EM, Zamarian L. Decision Making under Risk in Patients Suffering from Schizophrenia or Depression. Brain Sciences. 2021; 11(9):1178. https://doi.org/10.3390/brainsci11091178
Chicago/Turabian StyleBenke, Theresa, Josef Marksteiner, Beatrix Ruepp, Elisabeth M. Weiss, and Laura Zamarian. 2021. "Decision Making under Risk in Patients Suffering from Schizophrenia or Depression" Brain Sciences 11, no. 9: 1178. https://doi.org/10.3390/brainsci11091178
APA StyleBenke, T., Marksteiner, J., Ruepp, B., Weiss, E. M., & Zamarian, L. (2021). Decision Making under Risk in Patients Suffering from Schizophrenia or Depression. Brain Sciences, 11(9), 1178. https://doi.org/10.3390/brainsci11091178







