Differential Effects of Lateral and Medial Entorhinal Cortex Lesions on Trace, Delay and Contextual Fear Memories
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intracranial Surgery
2.3. Apparatus
2.4. Fear Conditioning
2.5. Fear Testing
2.6. Open Field
2.7. Histology
3. Results
3.1. Histological Verification
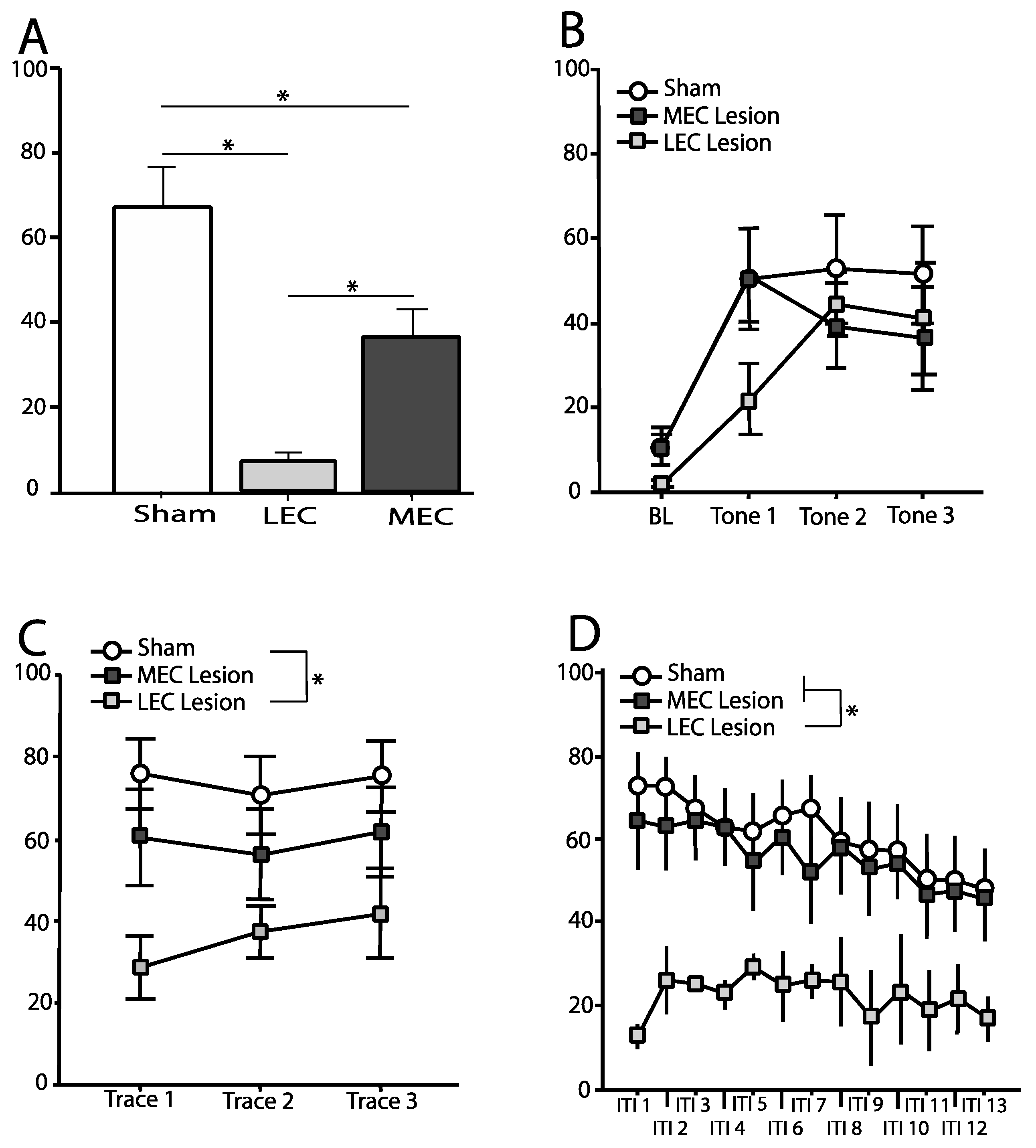
3.2. Trace Fear Conditioned Animals—Context Test
3.3. Trace Fear Conditioned Animals—Tone Test
3.3.1. Baseline
3.3.2. Tone
3.3.3. Trace Interval
3.3.4. Intertrial Interval
3.4. Delay Fear Conditioned Animals—Context Test
3.5. Delay Fear Conditioned Animals—Tone Test
3.5.1. Baseline
3.5.2. Tone
3.5.3. Trace Interval Equivalent
3.5.4. Intertrial Interval
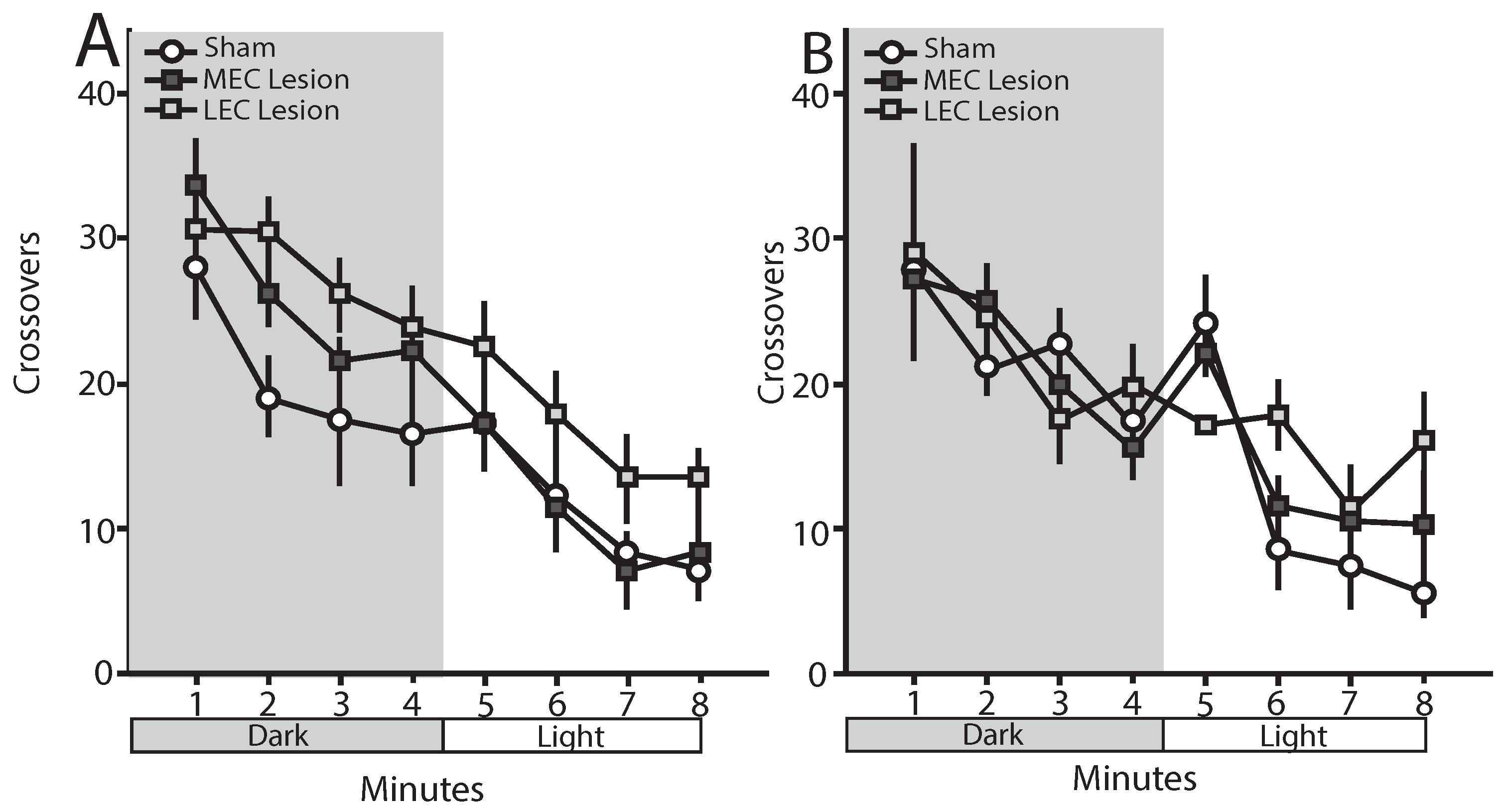
3.6. Trace Fear Conditioned Animals—Open Field
3.7. Delay Fear Conditioned Animals—Open Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burwell, R.D.; Amaral, D.G. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices. J. Comp. Neurol. 1998, 398, 179–205. [Google Scholar] [CrossRef]
- Witter, M.P.; Wouterlood, F.G.; Naber, P.A.; Van Haeften, T. Anatomical organization of the parahippocampal-hippocampal network. Ann. N. Y. Acad. Sci. 2000, 911, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, E.L.; Rao, G.; Lee, I.; Knierim, J.J. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 2005, 308, 1792–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, K.M.; Agster, K.L.; Furtak, S.C.; Burwell, R.D. Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus 2007, 17, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Takehara-Nishiuchi, K. Entorhinal cortex and consolidated memory. Neurosci. Res. 2014, 84C, 27–33. [Google Scholar] [CrossRef]
- Morrissey, M.D.; Takehara-Nishiuchi, K. Diversity of mnemonic function within the entorhinal cortex: A meta-analysis of rodent behavioral studies. Neurobiol. Learn. Mem. 2014, 115, 95–107. [Google Scholar] [CrossRef]
- Maren, S.; Fanselow, M.S. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol. Learn. Mem. 1997, 67, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Good, M.; Honey, R.C. Dissociable effects of selective lesions to hippocampal subsystems on exploratory behavior, contextual learning, and spatial learning. Behav. Neurosci. 1997, 111, 487–493. [Google Scholar] [CrossRef]
- Phillips, G.; LeDoux, J.E. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J. Neurosci. 1995, 15, 5308–5315. [Google Scholar] [CrossRef] [Green Version]
- Esclassan, F.; Coutureau, E.; Di Scala, G.; Marchand, A.R. A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. J. Neurosci. 2009, 29, 8087–8093. [Google Scholar] [CrossRef] [Green Version]
- Suter, E.E.; Weiss, C.; Disterhoft, J.F. Perirhinal and postrhinal, but not lateral entorhinal, cortices are essential for acquisition of trace eyeblink conditioning. Learn. Mem. 2013, 20, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, M.; Ferry, B.; Marchand, A.R.; Herbeaux, K.; Seillier, A.; Barbelivien, A. Entorhinal cortex lesions disrupt fear conditioning to background context but spare fear conditioning to a tone in the rat. Hippocampus 2006, 16, 114–124. [Google Scholar] [CrossRef]
- Suh, J.; Rivest, A.J.; Nakashiba, T.; Tominaga, T.; Tonegawa, S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science 2011, 334, 1415–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, T.; Pignatelli, M.; Suh, J.; Kohara, K.; Yoshiki, A.; Abe, K.; Tonegawa, S. Island cells control temporal association memory. Science 2014, 343, 896–901. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T. Driving and regulating temporal association learning coordinated by entorhinal-hippocampal network. Neurosci. Res. 2017, 121, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ryou, J.W.; Cho, S.Y.; Kim, H.T. Lesions of the entorhinal cortex impair acquisition of hippocampal-dependent trace conditioning. Neurobiol. Learn. Mem. 2001, 75, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanninen, S.E.; Yu, X.; Giritharan, T.; Tran, L.; Bakir, R.; Volle, J.; Morrissey, M.D.; Takehara-Nishiuchi, K. Cholinergic, but not NMDA, receptors in the lateral entorhinal cortex mediate acquisition in trace eyeblink conditioning. Hippocampus 2015, 25, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.D.; Maal-Bared, G.; Brady, S.; Takehara-Nishiuchi, K. Functional dissociation within the entorhinal cortex for memory retrieval of an association between temporally discontiguous stimuli. J. Neurosci. 2012, 32, 5356–5361. [Google Scholar] [CrossRef] [PubMed]
- Tanninen, S.E.; Morrissey, M.D.; Takehara-Nishiuchi, K. Unilateral lateral entorhinal inactivation impairs memory expression in trace eyeblink conditioning. PLoS ONE 2013, 8, e84543. [Google Scholar] [CrossRef]
- Maren, S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: Cautions and caveats. Eur. J. Neurosci. 2008, 28, 1661–1666. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.J.; Oommen, S.S.; Morrison, G.E.; Fanselow, M.S. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus 2002, 12, 495–504. [Google Scholar] [CrossRef]
- Quinn, J.J.; Wied, H.M.; Ma, Q.D.; Tinsley, M.R.; Fanselow, M.S. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus 2008, 18, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.C.; Blanchard, R.J.; Lee, E.M.C.; Fukunaga, K.K. Movement arrest and the hippocampus. Physiol. Psychol. 1977, 5, 331–335. [Google Scholar] [CrossRef]
- Maren, S.; Aharonov, G.; Fanselow, M.S. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditiong in rats. Behav. Brain Res. 1997, 88, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Godsil, B.P.; Stefanacci, L.; Fanselow, M.S. Bright light suppresses hyperactivity induced by excitotoxic dorsal hippocampus lesions in the rat. Behav. Neurosci. 2005, 119, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Hunsaker, M.R.; Chen, V.; Tran, G.T.; Kesner, R.P. The medial and lateral entorhinal cortex both contribute to contextual and item recognition memory: A test of the binding ofitems and context model. Hippocampus 2013, 23, 380–391. [Google Scholar] [CrossRef]
- Anagnostaras, S.G.; Maren, S.; Fanselow, M.S. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J. Neurosci. 1999, 19, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Kochli, D.E.; Thompson, E.C.; Fricke, E.A.; Postle, A.F.; Quinn, J.J. The amygdala is critical for trace, delay, and contextual fear conditioning. Learn. Mem. 2015, 22, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.W.; Han, J.S.; Kim, J.J. Selective neurotoxin lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J. Neurosci. 2004, 24, 7654–7662. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Pierson, J.L.; Pullins, S.E.; Quinn, J.J. Dorsal hippocampus infusions of CNQX into the dentate gyrus disrupt expression of trace fear conditioning. Hippocampus 2015, 25, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Holland, P.C.; Bouton, M.E. Hippocampus and context in classical conditioning. Curr. Opin. Neurobiol. 1999, 9, 195–202. [Google Scholar] [CrossRef]
- Pilkiw, M.; Insel, N.; Cui, Y.; Finney, C.; Morrissey, M.D.; Takehara-Nishiuchi, K. Phasic and tonic neuron ensemble codes for stimulus-environment conjunctions in the lateral entorhinal cortex. eLife 2017, 6, e28611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.I.; Langston, R.F.; Schlesiger, M.I.; Wagner, M.; Watanabe, S.; Ainge, J.A. Lateral entorhinal cortex is critical for novel object-context recognition. Hippocampus 2013, 23, 352–366. [Google Scholar] [CrossRef] [Green Version]
- Van Cauter, T.; Camon, J.; Alvernhe, A.; Elduayen, C.; Sargolini, F.; Save, E. Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb. Cortex 2013, 23, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuruvilla, M.V.; Ainge, J.A. Lateral entorhinal cortex lesions impair local spatial frameworks. Front. Syst. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, S.; Nakamura, N.H.; Maingret, N.; Mahnke, L.; Yoshida, M.; Sauvage, M.M. Regional specific evidence for memory-load dependent activity in the dorsal subiculum and the lateral entorhinal cortex. Front. Syst. Neurosci. 2017, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Bucci, D.J.; Phillips, R.G.; Burwell, R.D. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behav. Neurosci. 2000, 114, 882–894. [Google Scholar] [CrossRef]
- Bucci, D.J.; Saddoris, M.P.; Burwell, R.D. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behav. Neurosci. 2002, 116, 479–488. [Google Scholar] [CrossRef]
- Burwell, R.D.; Bucci, D.J.; Sanborn, M.R.; Jutras, M.J. Perirhinal and postrhinal contributions to remote memory for context. J. Neurosci. 2004, 24, 11023–11028. [Google Scholar] [CrossRef]
- Rosen, J.B.; Hitchcock, J.M.; Miserendino, M.J.; Falls, W.A.; Campeau, S.; Davis, M. Lesions of the perirhinal cortex but not of the frontal, medial prefrontal, visual, or insular cortex block fear-potentiated startle using a visual conditioned stimulus. J. Neurosci. 1992, 12, 4624–4633. [Google Scholar] [CrossRef] [Green Version]
- Campeau, S.; Davis, M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J. Neurosci. 1995, 15 Pt 2, 2312–2327. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.J.; Brown, T.H. Muscarinic receptors in perirhinal cortex control trace conditioning. J. Neurosci. 2009, 29, 4346–4350. [Google Scholar] [CrossRef] [PubMed]
- McNish, K.A.; Gewirtz, J.C.; Davis, M. Evidence of contextual fear after lesions of the hippocampus: A disruption of freezing but not fear-potentiated startle. J. Neurosci. 1997, 17, 9353–9360. [Google Scholar] [CrossRef] [PubMed]

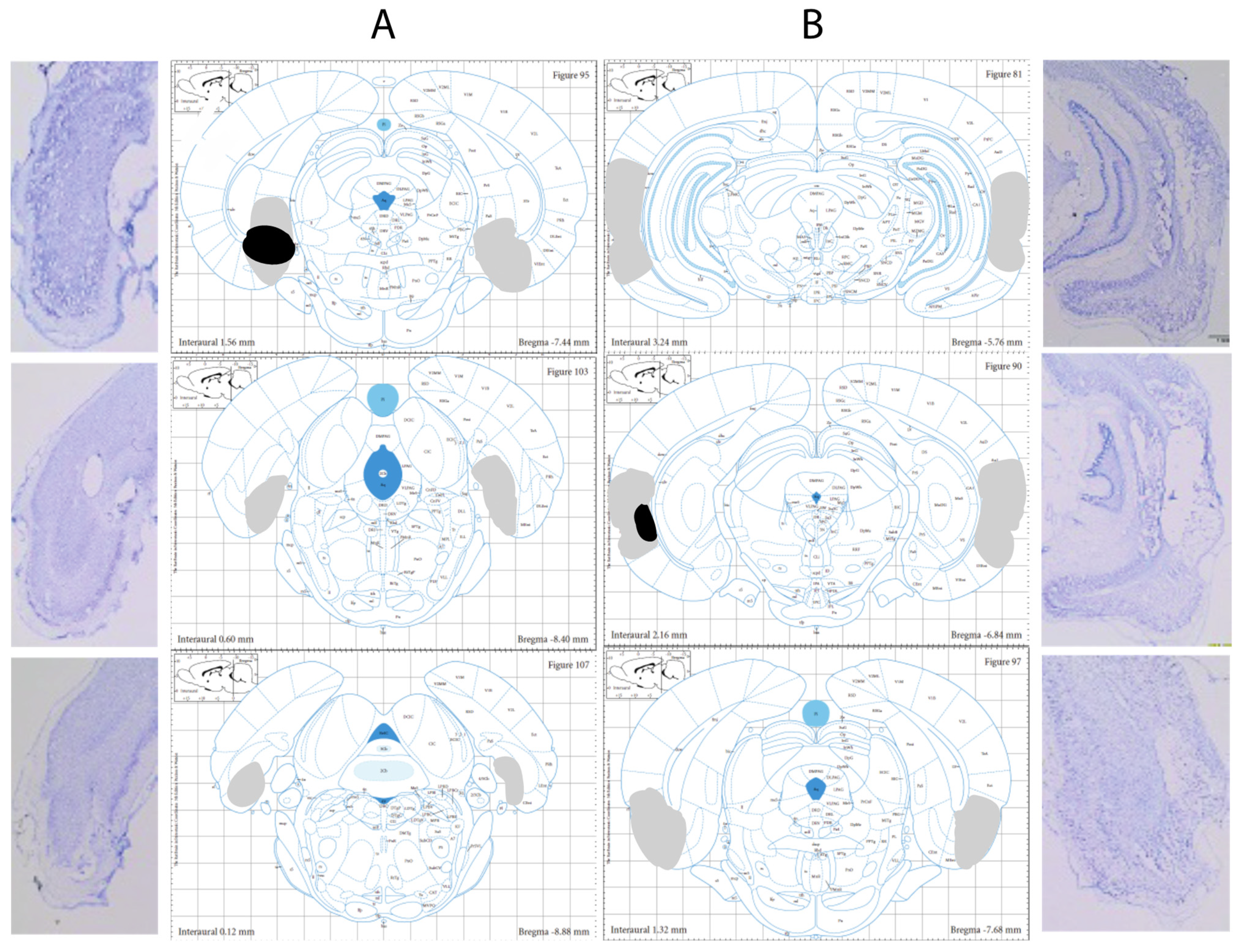

| LEC | Site 1 | Site 2 | Site 3 | Site 4 |
| AP | −4.8 | −4.8 | −6.8 | −6.8 |
| ML | ±6.8 | ±6.8 | ±6.5 | ±6.5 |
| DV | −7.8 | −8.8 | −7.5 | −8.5 |
| MEC | Site 1 | Site 2 | Site 3 | |
| AP | −8.3 | −8.8 | −8.8 | |
| ML | ±4.8 | ±6.2 | ±6.2 | |
| DV | −7 | −7 | −8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
East, B.S., Jr.; Brady, L.R.; Quinn, J.J. Differential Effects of Lateral and Medial Entorhinal Cortex Lesions on Trace, Delay and Contextual Fear Memories. Brain Sci. 2022, 12, 34. https://doi.org/10.3390/brainsci12010034
East BS Jr., Brady LR, Quinn JJ. Differential Effects of Lateral and Medial Entorhinal Cortex Lesions on Trace, Delay and Contextual Fear Memories. Brain Sciences. 2022; 12(1):34. https://doi.org/10.3390/brainsci12010034
Chicago/Turabian StyleEast, Brett S., Jr., Lauren R. Brady, and Jennifer J. Quinn. 2022. "Differential Effects of Lateral and Medial Entorhinal Cortex Lesions on Trace, Delay and Contextual Fear Memories" Brain Sciences 12, no. 1: 34. https://doi.org/10.3390/brainsci12010034





