MRI-Based Radiomic Machine-Learning Model May Accurately Distinguish between Subjects with Internet Gaming Disorder and Healthy Controls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. MRI Data Pre-Processing and Feature Extraction
2.4. Feature Selection and Assessment of the Relevance of the Selected Features
2.5. Construction of the RF Classifier
2.6. Statistical Analysis
3. Results
3.1. Demographic and Volumetric Comparison
3.2. Classification Performance and Significantly Relevant Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Song, K.R.; Potenza, M.N.; Fang, X.Y.; Gong, G.L.; Zhang, J.T. Resting-state connectome-based support-vector-machine predictive modeling of internet gaming disorder. Addict. Biol. 2020, 26, e12969. [Google Scholar] [CrossRef]
- Francesmonneris, A.; Pincus, H.; First, M. Diagnostic and Statistical Manual of Mental Disorders: DSM-V. Am. Psychiatr. Assoc. 2013, 101, 89–96. [Google Scholar]
- Beard, K.W.; Wolf, E.M. Modification in the Proposed Diagnostic Criteria for Internet Addiction. Cyber. Psychol. Behav. 2001, 4, 377–383. [Google Scholar] [CrossRef]
- Weinstein, A.; Livny, A.; Weizman, A. New developments in brain research of internet and gaming disorder. Neurosci. Biobehav. Rev. 2017, 75, 314–330. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, T.; Cai, C.; Bi, Y.; Li, Y.; Yu, D.; Zhang, M.; Yuan, K. Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imaging Behav. 2015, 10, 719–729. [Google Scholar] [CrossRef]
- Jeong, B.S.; Han, D.H.; Kim, S.M.; Lee, S.W.; Renshaw, P.F. White matter connectivity and Internet gaming disorder. Addict. Biol. 2015, 21, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Volkow, G.F.K.N. Neurocircuitry of Addiction. Neuropsychopharmacology 2009, 35, 217. [Google Scholar]
- Evren, C.; Evren, B.; Dalbudak, E.; Topcu, M.; Kutlu, N. Relationships of Internet addiction and Internet gaming disorder symptom severities with probable attention deficit/hyperactivity disorder, aggression and negative affect among university students. Atten. Deficit Hyperact. Disord. 2019, 11, 413–421. [Google Scholar] [CrossRef]
- West, G.L.; Konishi, K.; Diarra, M.; Benady-Chorney, J.; Drisdelle, B.L.; Dahmani, L.; Sodums, D.J.; Lepore, F.; Jolicoeur, P.; Bohbot, V.D. Impact of video games on plasticity of the hippocampus. Mol. Psychiatry 2017, 23, 1566–1574. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Luo, L.; Qiu, L.; Kang, Y.; Liu, B.; Yu, D.; Lu, X.; Yuan, K. The topological organization of white matter network in internet gaming disorder individuals. Brain Imaging Behav. 2016, 11, 1769–1778. [Google Scholar] [CrossRef]
- Du, X.; Liu, L.; Yang, Y.; Qi, X.; Gao, P.; Zhang, Y.; Zhu, J.; Du, G.; Dai, S.; Li, X.; et al. Diffusion tensor imaging of the structural integrity of white matter correlates with impulsivity in adolescents with internet gaming disorder. Brain Behav. 2017, 7, e00753. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jin, C.; Yuan, K.; Shakir, T.M.; Mao, C.; Niu, X.; Niu, C.; Guo, L.; Zhang, M. The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front. Behav. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chen, Y.; Huang, Q.; Lui, S.; Huang, X.; Shi, Y.; Xu, X.; Sweeney, J.A.; Gong, Q. Psychoradiologic Utility of MR Imaging for Diagnosis of Attention Deficit Hyperactivity Disorder: A Radiomics Analysis. Radiology 2018, 287, 620–630. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.-H.; Yen, C.-F.; Yen, C.-N.; Yen, J.-Y.; Chen, C.-C.; Chen, S.-H. Screening for Internet Addiction: An Empirical Study on Cut-off Points for the Chen Internet Addiction Scale. Kaohsiung J. Med. Sci. 2005, 21, 545–551. [Google Scholar] [CrossRef] [Green Version]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Klein, A.; Tourville, J. 101 Labeled Brain Images and a Consistent Human Cortical Labeling Protocol. Front. Behav. Neurosci. 2012, 6, 171. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Kursa, M.B. Robustness of Random Forest-based gene selection methods. BMC Bioinform. 2014, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, M.; Morris, R.G.; Huckstep, B.; Jones, D.K.; Williams, S.; Markus, H.S. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Cheng, P.; Dong, T.; Bi, Y.; Xing, L.; Yu, D.; Zhao, L.; Dong, M.; von Deneen, K.M.; Liu, Y.; et al. Cortical Thickness Abnormalities in Late Adolescence with Online Gaming Addiction. PLoS ONE 2013, 8, e53055. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Han, D.H.; Lee, Y.S.; Kim, J.E.; Renshaw, P.F. Changes in brain activity in response to problem solving during the abstinence from online game play. J. Behav. Addict. 2012, 1, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Han, D.H.; Kim, S.M.; Lee, Y.S.; Renshaw, P.F. The effect of family therapy on the changes in the severity of on-line game play and brain activity in adolescents with on-line game addiction. Psychiatry Res. Neuroimaging 2012, 202, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Ying, H.; Seetohul, R.M.; Xuemei, W.; Ya, Z.; Qian, L.; Guoqing, X.; Ye, S. Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents). Behav. Brain Res. 2012, 233, 563–576. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, S.M.; Bae, S.; Han, D.H. Impulsive Internet Game Play Is Associated with Increased Functional Connectivity Between the Default Mode and Salience Networks in Depressed Patients with Short Allele of Serotonin Transporter Gene. Front. Psychiatry 2018, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wu, X.; Wang, Y.; Sun, Y.; Ding, W.; Cao, M.; Du, Y.; Lin, F.; Zhou, Y. Alterations of Resting-State Static and Dynamic Functional Connectivity of the Dorsolateral Prefrontal Cortex in Subjects with Internet Gaming Disorder. Front. Hum. Neurosci. 2018, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Mushiake, H.; Inase, M.; Tanji, J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J. Neurophysiol. 1991, 66, 705–718. [Google Scholar] [CrossRef]
- Thoenissen, D.; Zilles, K.; Toni, I. Differential Involvement of Parietal and Precentral Regions in Movement Preparation and Motor Intention. J. Neurosci. 2002, 22, 9024–9034. [Google Scholar] [CrossRef] [Green Version]
- Kelley, T.A.; Serences, J.T.; Giesbrecht, B.; Yantis, S. Cortical Mechanisms for Shifting and Holding Visuospatial Attention. Cereb. Cortex 2007, 18, 114–125. [Google Scholar] [CrossRef]
- Du, X.; Yang, Y.; Gao, P.; Qi, X.; Du, G.; Zhang, Y.; Li, X.; Zhang, Q. Compensatory increase of functional connectivity density in adolescents with internet gaming disorder. Brain Imaging Behav. 2016, 11, 1901–1909. [Google Scholar] [CrossRef]
- Crockford, D.N.; Goodyear, B.; Edwards, J.; Quickfall, J.; El-Guebaly, N. Cue-Induced Brain Activity in Pathological Gamblers. Biol. Psychiatry 2005, 58, 787–795. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Yuan, K.; Hu, Y.; Zheng, H.; Du, X.; Dong, G. Cortical thickness and volume abnormalities in Internet gaming disorder: Evidence from comparison of recreational Internet game users. Eur. J. Neurosci. 2018, 48, 1654–1666. [Google Scholar] [CrossRef]
- Pezawas, L.; Fischer, G.; Podreka, I.; Schindler, S.; Brücke, T.; Jagsch, R.; Thurnher, M.; Kasper, S. Opioid addiction changes cerebral blood flow symmetry. Neuropsychobiology 2002, 45, 67–73. [Google Scholar] [CrossRef]
- Levitt, J.J.; Kubicki, M.; Nestor, P.G.; Ersner-Hershfield, H.; Westin, C.-F.; Alvarado, J.L.; Kikinis, R.; Jolesz, F.A.; McCarley, R.W.; Shenton, M.E. A diffusion tensor imaging study of the anterior limb of the internal capsule in schizophrenia. Psychiatry Res. Neuroimaging 2010, 184, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Zhou, Y.; Du, Y.; Qin, L.; Zhao, Z.; Xu, J.; Lei, H. Abnormal White Matter Integrity in Adolescents with Internet Addiction Disorder: A Tract-Based Spatial Statistics Study. PLoS ONE 2012, 7, e30253. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Qin, W.; Wang, G.; Zeng, F.; Zhao, L.; Yang, X.; Liu, P.; Liu, J.; Sun, J.; von Deneen, K.M.; et al. Microstructure Abnormalities in Adolescents with Internet Addiction Disorder. PLoS ONE 2011, 6, e20708. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoshida, T.; Kubicki, M.; Bouix, S.; Westin, C.-F.; Kindlmann, G.; Niznikiewicz, M.; Cohen, A.; McCarley, R.; Shenton, M.E. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: A Diffusion Tensor Imaging study. Schizophr. Res. 2009, 108, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Tromp, D.P.; Fox, A.S.; Oler, J.A.; Alexander, A.L.; Kalin, N.H. The Relationship Between the Uncinate Fasciculus and Anxious Temperament Is Evolutionarily Conserved and Sexually Dimorphic. Biol. Psychiatry 2019, 86, 890–898. [Google Scholar] [CrossRef]
- Borkowski, K.; Krzyżak, A.T. Analysis and correction of errors in DTI-based tractography due to diffusion gradient inhomogeneity. J. Magn. Reson. 2018, 296, 5–11. [Google Scholar] [CrossRef] [PubMed]

| IGD | HC | Statistic | Degree of Freedom | p Value | |
|---|---|---|---|---|---|
| Age | 21.39 ± 3.06 (15–28) | 20.34 ± 3.98 (13–28) | 1.639 | 126 | 0.104 |
| Gender | 0.417 χ2 | 1 | 0.518 | ||
| Male | 47 | 58 | |||
| Female | 12 | 11 | |||
| Total gray matter volume (mm3) | 709,119.83 ± 59,534.46 | 751,018.21 ± 58,611.32 | −0.563 | 126 | 0.574 |
| Total white matter volume (mm3) | 465,054.49 ± 51,862.65 | 470,600.22 ± 47,006.67 | 0.634 | 126 | 0.527 |
| Subcortical region volume (mm3) | 63,882.71 ± 5110.42 | 64,764.36 ± 4332.33 | −1.056 | 126 | 0.293 |
| Total brain volume (mm3) | 1,555,295.64 ± 152,316.31 | 15,4491.19 ± 151,241.11 | 0.03 | 126 | 0.976 |
| IGD | HC | Statistic | Degree of Freedom | p Value | |
|---|---|---|---|---|---|
| CIAS | 78.27 ± 10.31 | 44.38 ± 11.34 | 17.57 | 126 | <0.0001 * |
| BIS-11 | 63.02 ± 7.72 | 53.81 ± 7.42 | 6.87 | 126 | <0.0001 * |
| SAS | 50.51 ± 8.19 | 42.65 ± 6.39 | 6.09 | 126 | <0.0001 * |
| SDS | 51.97 ± 7.09 | 45.74 ± 8.92 | 4.32 | 126 | <0.0001 * |
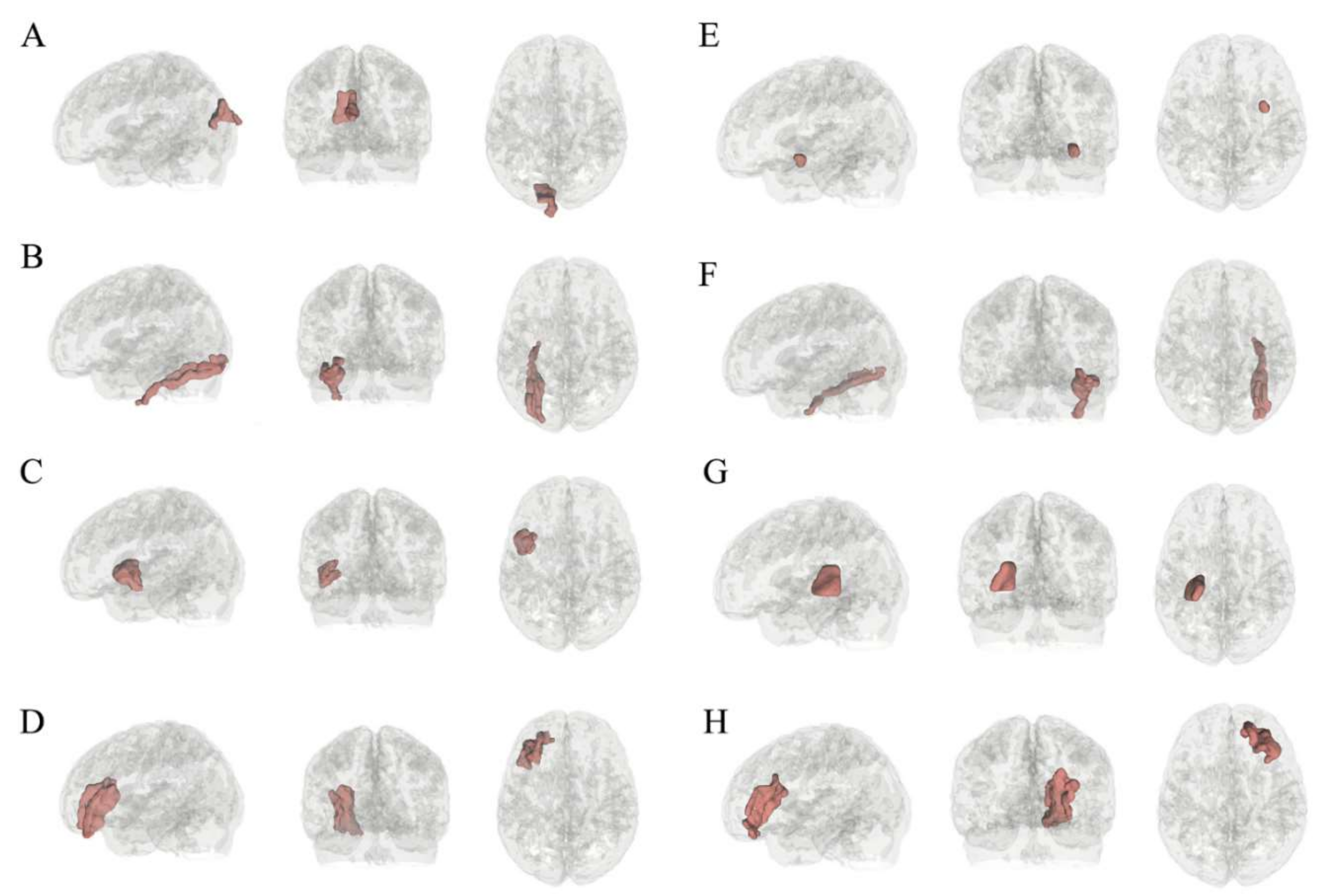
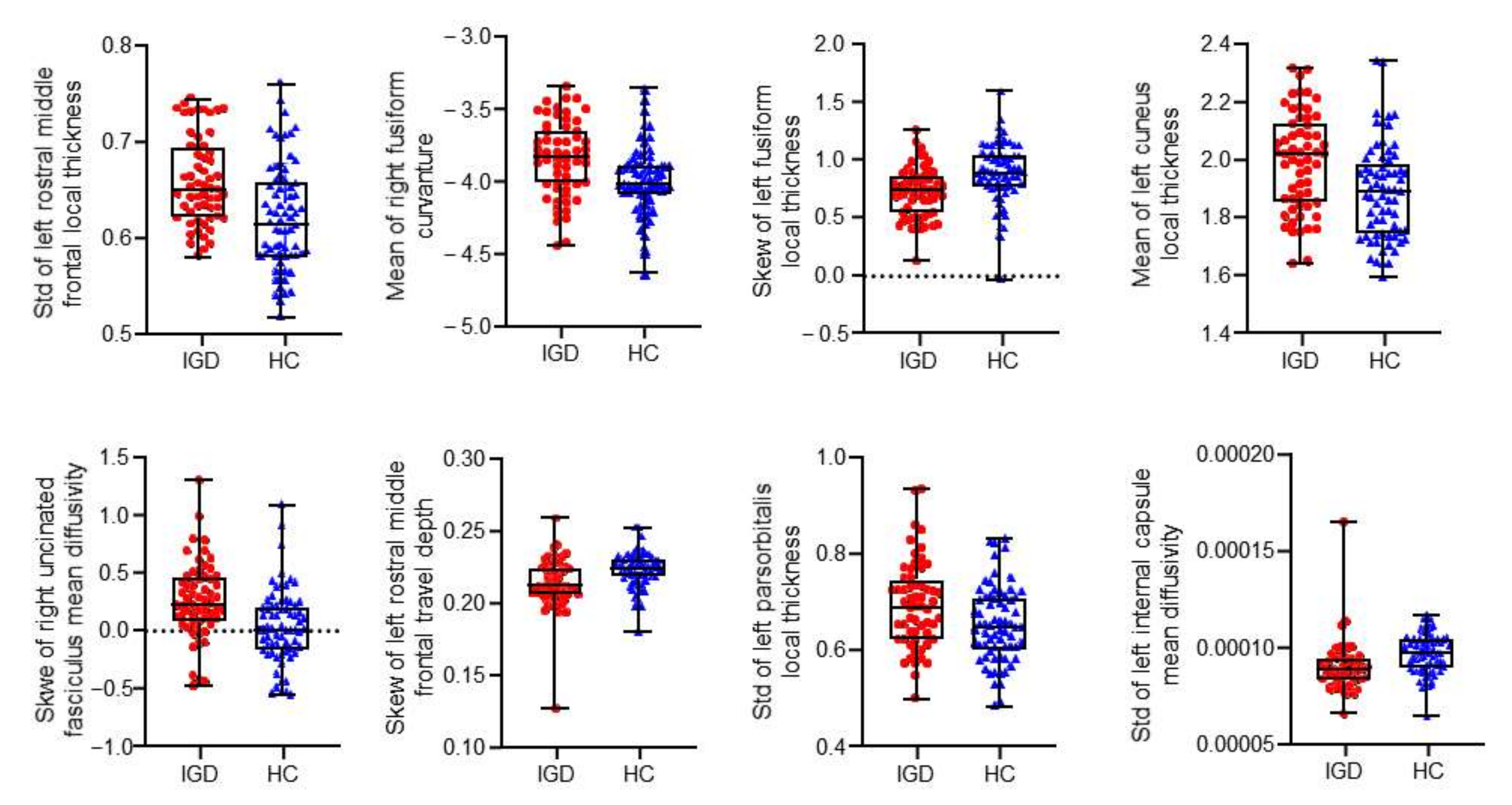
| Selection Frequency (%) | Hemisphere | Label | Feature Type | Statistic | IGD * | HC * |
|---|---|---|---|---|---|---|
| 99.6 | Left | Rostral middle frontal | Local thickness | Standard deviation | 0.66 ± 0.05 | 0.62 ± 0.06 |
| 96.8 | Left | Internal capsule | Mean diffusivity | Standard deviation | 0.000089 ± 0.000013 | 0.000095 ± 0.000010 |
| 84.0 | Right | Fusiform | Mean curvature | Mean | −3.83 ± 0.26 | −4.00 ± 0.23 |
| 83.8 | Left | Fusiform | Local thickness | Skewness | 0.70 ± 0.22 | 0.86 ± 0.26 |
| 83.2 | Left | Cuneus | Local thickness | Mean | 1.99 ± 0.17 | 1.88 ± 0.16 |
| 77.8 | Right | Uncinate fasciculus | Mean diffusivity | Skewness | 0.25 ± 0.33 | 0.03 ± 0.32 |
| 74.4 | Left | Rostral middle frontal | Travel depth | Skewness | 0.21 ± 0.02 | 0.22 ± 0.01 |
| 72.6 | Left | Parsorbitalis | Local thickness | Standard deviation | 0.52 ± 0.05 | 0.49 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Wei, L.; Sun, Y.; Hu, Y.; Wang, Y.; Ding, W.; Wang, Z.; Jiang, W.; Wang, H.; Zhou, Y. MRI-Based Radiomic Machine-Learning Model May Accurately Distinguish between Subjects with Internet Gaming Disorder and Healthy Controls. Brain Sci. 2022, 12, 44. https://doi.org/10.3390/brainsci12010044
Han X, Wei L, Sun Y, Hu Y, Wang Y, Ding W, Wang Z, Jiang W, Wang H, Zhou Y. MRI-Based Radiomic Machine-Learning Model May Accurately Distinguish between Subjects with Internet Gaming Disorder and Healthy Controls. Brain Sciences. 2022; 12(1):44. https://doi.org/10.3390/brainsci12010044
Chicago/Turabian StyleHan, Xu, Lei Wei, Yawen Sun, Ying Hu, Yao Wang, Weina Ding, Zhe Wang, Wenqing Jiang, He Wang, and Yan Zhou. 2022. "MRI-Based Radiomic Machine-Learning Model May Accurately Distinguish between Subjects with Internet Gaming Disorder and Healthy Controls" Brain Sciences 12, no. 1: 44. https://doi.org/10.3390/brainsci12010044
APA StyleHan, X., Wei, L., Sun, Y., Hu, Y., Wang, Y., Ding, W., Wang, Z., Jiang, W., Wang, H., & Zhou, Y. (2022). MRI-Based Radiomic Machine-Learning Model May Accurately Distinguish between Subjects with Internet Gaming Disorder and Healthy Controls. Brain Sciences, 12(1), 44. https://doi.org/10.3390/brainsci12010044







