Mapping Actuarial Criteria for Parkinson’s Disease-Mild Cognitive Impairment onto Data-Driven Cognitive Phenotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Neuropsychological Measures
2.4. PD-MCI Classification
2.5. Cluster Analyses
2.6. Other Measures
2.7. Statistics
3. Results
3.1. Sample Characteristics
3.2. Prevalence of PD-MCI and Subtypes
3.3. Cluster-Derived Cognitive Phenotypes
3.4. Relationship between Cognitive Phenotypes and PD-MCI Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Comparison of Deep Brain Stimulation and General Clinic Patients’ Cognitive Performance
| Measure | DBS | General Clinic | Significance |
|---|---|---|---|
| (n = 338) | (n = 156) | ||
| Mean (SD) | Means (SD) | p-value | |
| Dementia Rating Scale-2, raw total | 137.05 (4.28) | 136.86 (4.92) | 0.69 |
| Cognitive Composites (z-scores) # | |||
| Executive Function | −0.55 (0.88) | −0.64 (0.93) | 0.32 |
| Verbal Delayed Memory | −0.37 (1.00) | −0.36 (1.04) | 0.95 |
| Language | 0.001 (0.94) | −0.09 (1.00) | 0.35 |
| Visuospatial Abilities | 0.01 (0.78) | 0.02 (0.79) | 0.85 |
| Attention/Working Memory | 0.20 (0.77) | 0.11 (0.76) | 0.22 |
Appendix B. Relationship between Mood and Cognitive Domains
| Measure | Executive Function | Verbal Delayed Memory | Language | Visuospatial | Attention/ Working Memory |
|---|---|---|---|---|---|
| BDI-II | −0.10 * | −0.13 * | −0.09 * | −0.10 * | −0.16 * |
| STAI | |||||
| State | −0.18 * | −0.10 * | −0.18 * | −0.10 * | −0.08 |
| Trait | −0.18 * | −0.15 * | −0.17 * | −0.14 * | −0.15 * |
| Apathy Scale | −0.14 * | −0.10 | −0.09 | −0.13 * | −0.09 |
Appendix C. Exploratory Analyses of Language Metrics
| Metric | Cognitively Average | Low EF | Prominently Impaired EF/Memory |
|---|---|---|---|
| Boston Naming Test | 1.05 (0.96) | 0.18 (1.01) | −0.67 (0.96) |
| Semantic Fluency (Animals) | 0.52 (0.90) | −0.35 (0.96) | −1.37 (1.04) |
References
- Baiano, C.; Barone, P.; Trojano, L.; Santangelo, G. Prevalence and Clinical Aspects of Mild Cognitive Impairment in Parkinson’s Disease: A Meta-Analysis. Mov. Disord. 2020, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.J.; Adena, M.A.; Halliday, G.M.; Morris, J.G.L. The Sydney Multicenter Study of Parkinson’s Disease: The Inevitability of Dementia at 20 Years. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Pigott, K.; Rick, J.; Hurtig, H.; Chen-Plotkin, A.; Duda, J.; Morley, J.; Chahine, L.; Dahodwala, N.; Fleisher, J.; Akhtar, R.; et al. Long-Term Outcomes of Parkinson’s Disease Patients with Normal Cognition (P5.260). Neurology 2014, 82, P5.260. [Google Scholar]
- Edmonds, E.C.; Smirnov, D.S.; Thomas, K.R.; Graves, L.V.; Bangen, K.J.; Delano-Wood, L.; Galasko, D.R.; Salmon, D.P.; Bondi, M.W. Data-Driven vs. Consensus Diagnosis of MCI: Enhanced Sensitivity for Detection of Clinical, Biomarker, and Neuropathologic Outcomes. Neurology 2021, 97, e1288–e1299. [Google Scholar] [CrossRef]
- Edmonds, E.C.; McDonald, C.R.; Marshall, A.; Thomas, K.R.; Eppig, J.; Weigand, A.J.; Delano-Wood, L.; Galasko, D.R.; Salmon, D.P.; Bondi, M.W. Early vs. Late MCI: Improved MCI Staging Using a Neuropsychological Approach. Alzheimers Dement. J. Alzheimers Assoc. 2019, 15, 699–708. [Google Scholar] [CrossRef]
- Brennan, L.; Devlin, K.M.; Xie, S.X.; Mechanic-Hamilton, D.; Tran, B.; Hurtig, H.H.; Chen-Plotkin, A.; Chahine, L.M.; Morley, J.F.; Duda, J.E.; et al. Neuropsychological Subgroups in Non-Demented Parkinson’s Disease: A Latent Class Analysis. J. Park. Dis. 2017, 7, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourzinal, D.; Yang, J.H.J.; Byrne, G.J.; O’Sullivan, J.D.; Mitchell, L.; McMahon, K.L.; Copland, D.A.; Dissanayaka, N.N. Identifying Subtypes of Mild Cognitive Impairment in Parkinson’s Disease Using Cluster Analysis. J. Neurol. 2020, 267, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Holden, S.; Bernard, B.; Ouyang, B.; Goetz, C.G.; Stebbins, G.T. Defining Optimal Cutoff Scores for Cognitive Impairment Using Movement Disorder Society Task Force Criteria for Mild Cognitive Impairment in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1972–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, G.W.; Abbott, R.D.; Petrovitch, H.; Tanner, C.M.; White, L.R. Pre-Motor Features of Parkinson’s Disease: The Honolulu-Asia Aging Study Experience. Parkinsonism Relat. Disord. 2012, 18 (Suppl. S1), S199–S202. [Google Scholar] [CrossRef]
- Calabresi, P.; Castrioto, A.; Di Filippo, M.; Picconi, B. New Experimental and Clinical Links between the Hippocampus and the Dopaminergic System in Parkinson’s Disease. Lancet Neurol. 2013, 12, 811–821. [Google Scholar] [CrossRef]
- Weintraub, D.; Moberg, P.J.; Culbertson, W.C.; Duda, J.E.; Stern, M.B. Evidence for Impaired Encoding and Retrieval Memory Profiles in Parkinson Disease. Cogn. Behav. Neurol. Off. J. Soc. Behav. Cogn. Neurol. 2004, 17, 195–200. [Google Scholar]
- Mahieux, F.; Fénelon, G.; Flahault, A.; Manifacier, M.J.; Michelet, D.; Boller, F. Neuropsychological Prediction of Dementia in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1998, 64, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Sollinger, A.B.; Goldstein, F.C.; Lah, J.J.; Levey, A.I.; Factor, S.A. Mild Cognitive Impairment in Parkinson’s Disease: Subtypes and Motor Characteristics. Parkinsonism Relat. Disord. 2010, 16, 177–180. [Google Scholar] [CrossRef] [Green Version]
- Hobson, P.; Meara, J. Risk and Incidence of Dementia in a Cohort of Older Subjects with Parkinson’s Disease in the United Kingdom. Mov. Disord. 2004, 19, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Liepelt-Scarfone, I.; Gräber, S.; Fruhmann Berger, M.; Feseker, A.; Baysal, G.; Csoti, I.; Godau, J.; Gaenslen, A.; Huber, H.; Srulijes, K.; et al. Cognitive Profiles in Parkinson’s Disease and Their Relation to Dementia: A Data-Driven Approach. Int. J. Alzheimers Dis. 2012, 2012, e910757. [Google Scholar] [CrossRef] [Green Version]
- McKinlay, A.; Grace, R.C.; Dalrymple-Alford, J.C.; Roger, D. Cognitive Characteristics Associated with Mild Cognitive Impairment in Parkinson’s Disease. Dement. Geriatr. Cogn. Disord. 2009, 28, 121–129. [Google Scholar] [CrossRef]
- Souza, C.P.; Oliveira, G.N.; Foss, M.P.; Tumas, V. Cluster Analysis of Cognitive Performance in a Sample of Patients with Parkinson’s Disease. Dement. Neuropsychol. 2016, 10, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Recio, L.; Martín-Plasencia, P.; Ruiz, M.; Serrano, J.M. Differences in Cognitive Performance in Nondemented Parkinson’s Disease: A Latent Profile Analysis of Cognitive Subtypes. J. Clin. Exp. Neuropsychol. 2018, 40, 777–789. [Google Scholar] [CrossRef]
- Dujardin, K.; Leentjens, A.F.G.; Langlois, C.; Moonen, A.J.H.; Duits, A.A.; Carette, A.-S.; Duhamel, A. The Spectrum of Cognitive Disorders in Parkinson’s Disease: A Data-Driven Approach. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 183–189. [Google Scholar] [CrossRef]
- van Balkom, T.D.; Vriend, C.; Berendse, H.W.; Foncke, E.M.J.; van der Werf, Y.D.; van den Heuvel, O.A.; Klein, M. Profiling Cognitive and Neuropsychiatric Heterogeneity in Parkinson’s Disease. Parkinsonism Relat. Disord. 2016, 28, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.J.; Banan, G.; Amin, M.; Tanner, J.J.; Hizel, L.; Nguyen, P.; Brumback, B.; Rodriguez, K.; McFarland, N.; Bowers, D.; et al. Statistically Defined Parkinson’s Disease Executive and Memory Cognitive Phenotypes: Demographic, Behavioral, and Structural Neuroimaging Comparisons. J. Park. Dis. 2021, 11, 283–297. [Google Scholar] [CrossRef]
- Jak, A.J.; Bondi, M.W.; Delano-Wood, L.; Wierenga, C.; Corey-Bloom, J.; Salmon, D.P.; Delis, D.C. Quantification of Five Neuropsychological Approaches to Defining Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2009, 17, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic Criteria for Mild Cognitive Impairment in Parkinson’s Disease: Movement Disorder Society Task Force Guidelines. Mov. Disord. Off. J. Mov. Disord. Soc. 2012, 27, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogland, J.; Boel, J.A.; de Bie, R.M.A.; Geskus, R.B.; Schmand, B.A.; Dalrymple-Alford, J.C.; Marras, C.; Adler, C.H.; Goldman, J.G.; Tröster, A.I.; et al. Mild Cognitive Impairment as a Risk Factor for Parkinson’s Disease Dementia. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 1056–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, J.G.; Vernaleo, B.A.; Camicioli, R.; Dahodwala, N.; Dobkin, R.D.; Ellis, T.; Galvin, J.E.; Marras, C.; Edwards, J.; Fields, J.; et al. Cognitive Impairment in Parkinson’s Disease: A Report from a Multidisciplinary Symposium on Unmet Needs and Future Directions to Maintain Cognitive Health. NPJ Park. Dis. 2018, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Liepelt-Scarfone, I.; Graeber, S.; Feseker, A.; Baysal, G.; Godau, J.; Gaenslen, A.; Maetzler, W.; Berg, D. Influence of Different Cut-off Values on the Diagnosis of Mild Cognitive Impairment in Parkinson’s Disease. Park. Dis. 2011, 2011, 540843. [Google Scholar] [CrossRef] [Green Version]
- Wood, K.-L.; Myall, D.J.; Livingston, L.; Melzer, T.R.; Pitcher, T.L.; MacAskill, M.R.; Geurtsen, G.J.; Anderson, T.J.; Dalrymple-Alford, J.C. Different PD-MCI Criteria and Risk of Dementia in Parkinson’s Disease: 4-Year Longitudinal Study. NPJ Park. Dis. 2016, 2, 15027. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Müller, M.L.T.M.; Kotagal, V.; Koeppe, R.A.; Kilbourn, M.R.; Gilman, S.; Albin, R.L.; Frey, K.A. Heterogeneity of Cholinergic Denervation in Parkinson’s Disease without Dementia. J. Cereb. Blood Flow Metab. 2012, 32, 1609–1617. [Google Scholar] [CrossRef]
- Dubois, B.; Pillon, B. Cognitive Deficits in Parkinson’s Disease. J. Neurol. 1996, 244, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Leverenz, J.B.; Schneider, J.S.; Adler, C.H. The Neurobiological Basis of Cognitive Impairment in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 634–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive Impairment in Parkinson’s Disease: The Dual Syndrome Hypothesis. Neurodegener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Muslimović, D.; Schmand, B.; Speelman, J.D.; Haan, R.J.D. Course of Cognitive Decline in Parkinson’s Disease: A Meta-Analysis. J. Int. Neuropsychol. Soc. 2007, 13, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Hobson, P.; Meara, J. Mild Cognitive Impairment in Parkinson’s Disease and Its Progression onto Dementia: A 16-Year Outcome Evaluation of the Denbighshire Cohort. Int. J. Geriatr. Psychiatry 2015, 30, 1048–1055. [Google Scholar] [CrossRef]
- Janvin, C.C.; Larsen, J.P.; Aarsland, D.; Hugdahl, K. Subtypes of Mild Cognitive Impairment in Parkinson’s Disease: Progression to Dementia. Mov. Disord. 2006, 21, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Evans, J.R.; Goris, A.; Foltynie, T.; Ban, M.; Robbins, T.W.; Brayne, C.; Kolachana, B.S.; Weinberger, D.R.; Sawcer, S.J.; et al. The Distinct Cognitive Syndromes of Parkinson’s Disease: 5 Year Follow-up of the CamPaIGN Cohort. Brain J. Neurol. 2009, 132, 2958–2969. [Google Scholar] [CrossRef] [Green Version]
- Jurica, P.J.; Leitten, C.L.; Mattis, S. Dementia Rating Scale-2: Professional Manual; Psychological Assessment Resources: Lutz, FL, USA, 2001. [Google Scholar]
- Lucas, J.A.; Ivnik, R.J.; Smith, G.E.; Bohac, D.L.; Tangalos, E.G.; Kokmen, E.; Graff-Radford, N.R.; Petersen, R.C. Normative Data for the Mattis Dementia Rating Scale. J. Clin. Exp. Neuropsychol. 1998, 20, 536–547. [Google Scholar] [CrossRef]
- Kirsh-Darrow, L. Apathy, Neurocognitive Functioning, and Parkinson’s Disease; University of Florida: Gainesville, FL, USA, 2010. [Google Scholar]
- Jones, J.D.; Hass, C.; Mangal, P.; Lafo, J.; Okun, M.S.; Bowers, D. The Cognition and Emotional Well-Being Indices of the Parkinson’s Disease Questionnaire-39: What Do They Really Measure? Parkinsonism Relat. Disord. 2014, 20, 1236–1241. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.D.; Tanner, J.J.; Okun, M.; Price, C.C.; Bowers, D. Are Parkinson’s Patients More Vulnerable to the Effects of Cardiovascular Risk: A Neuroimaging and Neuropsychological Study. J. Int. Neuropsychol. Soc. JINS 2017, 23, 322–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaton, R.K.; Miller, S.; Taylor, M.J.; Grant, I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults; Psychological Assessment Resources: Lutz, FL, USA, 2004. [Google Scholar]
- Cholerton, B.A.; Zabetian, C.P.; Wan, J.Y.; Montine, T.J.; Quinn, J.F.; Mata, I.F.; Chung, K.A.; Peterson, A.; Espay, A.J.; Revilla, F.J.; et al. Evaluation of Mild Cognitive Impairment Subtypes in Parkinson’s Disease. Mov. Disord. 2014, 29, 756–764. [Google Scholar] [CrossRef]
- Goldman, J.G.; Holden, S.; Ouyang, B.; Bernard, B.; Goetz, C.G.; Stebbins, G.T. Diagnosing PD-MCI by MDS Task Force Criteria: How Many and Which Neuropsychological Tests? Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 402–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golden, C.J. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses; Stoelting Company: Wood Dale, IL, USA, 1978. [Google Scholar]
- Army Individual Test Battery. Manual of Directions and Scoring; War Department, Adjunct General’s Office: Washington, DC, USA, 1944. [Google Scholar]
- Spreen, O.; Benton, A.L. Neurosensory Center Comprehensive Examination for Aphasia; University of Victoria, Neuropsychology Laboratory: Victoria, BC, Canada, 1977. [Google Scholar]
- Brandt, J. The Hopkins Verbal Learning Test: Development of a New Memory Test with Six Equivalent Forms. Clin. Neuropsychol. 1991, 5, 125–142. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Memory Scale; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Kaplan, E.F.; Goodglass, H.; Weintraub, S. The Boston Naming Test, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Goodglass, H.; Kaplan, E. The Assessment of Aphasia and Related Disorders; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Benton, A.L.; Varney, N.R.; Hamsher, K.D. Visuospatial Judgment. A Clinical Test. Arch. Neurol. 1978, 35, 364–367. [Google Scholar] [CrossRef]
- Benton, A.L.; Sivan, A.B.; Hamsher, K.D.; Varney, N.R.; Spreen, O. Contributions to Neuropsychological Assessment: A Clinical Manual, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1994; ISBN 978-0-19-509179-3. [Google Scholar]
- Wechsler, D. WAIS-III Administration and Scoring Manual; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild Cognitive Impairment: Clinical Characterization and Outcome. Arch. Neurol. 1999, 56, 303. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. APA PsycTests 1996. [Google Scholar] [CrossRef]
- Starkstein, S.E.; Mayberg, H.S.; Preziosi, T.J.; Andrezejewski, P.; Leiguarda, R.; Robinson, R.G. Reliability, Validity, and Clinical Correlates of Apathy in Parkinson’s Disease. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 134–139. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Fahn, S.; Elton, R.L. UPDRS Development Committee the Unified Parkinson’s Disease Rating Scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Calne, D.B., Goldstein, M., Eds.; Macmillan Healthcare Information: Florham Park, NJ, USA, 1987; pp. 153–163, 293–304. [Google Scholar]
- Zhao, Y.J.; Wee, H.L.; Chan, Y.-H.; Seah, S.H.; Au, W.L.; Lau, P.N.; Pica, E.C.; Li, S.C.; Luo, N.; Tan, L.C.S. Progression of Parkinson’s Disease as Evaluated by Hoehn and Yahr Stage Transition Times. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 710–716. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp.: Armonk, NY, USA, 2019. [Google Scholar]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Marras, C.; Armstrong, M.J.; Meaney, C.A.; Fox, S.; Rothberg, B.; Reginold, W.; Tang-Wai, D.F.; Gill, D.; Eslinger, P.J.; Zadikoff, C.; et al. Measuring Mild Cognitive Impairment in Patients with Parkinson’s Disease. Mov. Disord. 2013, 28, 626–633. [Google Scholar] [CrossRef]
- Loftus, A.M.; Bucks, R.S.; Thomas, M.; Kane, R.; Timms, C.; Barker, R.A.; Gasson, N. Retrospective Assessment of Movement Disorder Society Criteria for Mild Cognitive Impairment in Parkinson’s Disease. J. Int. Neuropsychol. Soc. 2015, 21, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Domellöf, M.E.; Ekman, U.; Forsgren, L.; Elgh, E. Cognitive Function in the Early Phase of Parkinson’s Disease, a Five-Year Follow-Up. Acta Neurol. Scand. 2015, 132, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.; Larsen, J.P.; Emre, M.; Wentzel-Larsen, T.; Aarsland, D. Changes in Motor Subtype and Risk for Incident Dementia in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 1123–1130. [Google Scholar] [CrossRef]
- Braak, H.; Rüb, U.; Del Tredici, K. Cognitive Decline Correlates with Neuropathological Stage in Parkinson’s Disease. J. Neurol. Sci. 2006, 248, 255–258. [Google Scholar] [CrossRef]
- Goldman, J.G.; Weis, H.; Stebbins, G.; Bernard, B.; Goetz, C.G. Clinical Differences among Mild Cognitive Impairment Subtypes in Parkinson’s Disease. Mov. Disord. 2012, 27, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Perry, R.; Wentzel-Larsen, T.; Lolk, A.; Kragh-Sørensen, P. The Rate of Cognitive Decline in Parkinson Disease. Arch. Neurol. 2004, 61, 1906–1911. [Google Scholar] [CrossRef]
- Locascio, J.J.; Corkin, S.; Growdon, J.H. Relation between Clinical Characteristics of Parkinson’s Disease and Cognitive Decline. J. Clin. Exp. Neuropsychol. 2003, 25, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.-C.; Chan, S.-H. Education and Cognitive Decline in Parkinson’s Disease: A Study of 102 Patients. Acta Neurol. Scand. 2001, 103, 243–247. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; Livingston, L.; MacAskill, M.R.; Graham, C.; Melzer, T.R.; Porter, R.J.; Watts, R.; Anderson, T.J. Characterizing Mild Cognitive Impairment in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 629–636. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.L.; Fisher, N.; Bradford, S.; Camicioli, R. Parkinson’s Disease Mild Cognitive Impairment Classifications and Neurobehavioral Symptoms. Int. Psychogeriatr. 2018, 30, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.L.; Salow, M.J.; Angelini, M.C.; McGlinchey, R.E. The Anticholinergic Risk Scale and Anticholinergic Adverse Effects in Older Persons. Arch. Intern. Med. 2008, 168, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, B.J.; Gasson, N.; Loftus, A.M. Prevalence and Subtypes of Mild Cognitive Impairment in Parkinson’s Disease. Sci. Rep. 2016, 6, 33929. [Google Scholar] [CrossRef]
- Nicoletti, A.; Luca, A.; Baschi, R.; Cicero, C.E.; Mostile, G.; Davì, M.; Pilati, L.; Restivo, V.; Zappia, M.; Monastero, R. Incidence of Mild Cognitive Impairment and Dementia in Parkinson’s Disease: The Parkinson’s Disease Cognitive Impairment Study. Front. Aging Neurosci. 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santangelo, G.; Vitale, C.; Picillo, M.; Moccia, M.; Cuoco, S.; Longo, K.; Pezzella, D.; di Grazia, A.; Erro, R.; Pellecchia, M.T.; et al. Mild Cognitive Impairment in Newly Diagnosed Parkinson’s Disease: A Longitudinal Prospective Study. Parkinsonism Relat. Disord. 2015, 21, 1219–1226. [Google Scholar] [CrossRef]
- Copeland, J.N.; Lieberman, A.; Oravivattanakul, S.; Tröster, A.I. Accuracy of Patient and Care Partner Identification of Cognitive Impairments in Parkinson’s Disease—Mild Cognitive Impairment. Mov. Disord. 2016, 31, 693–698. [Google Scholar] [CrossRef]
- Purri, R.; Brennan, L.; Rick, J.; Xie, S.X.; Deck, B.L.; Chahine, L.M.; Dahodwala, N.; Chen-Plotkin, A.; Duda, J.E.; Morley, J.F.; et al. Subjective Cognitive Complaint in Parkinson Disease Patients with Normal Cognition: Canary in the Coal Mine? Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1618–1625. [Google Scholar] [CrossRef]
- Bondi, M.W.; Edmonds, E.C.; Jak, A.J.; Clark, L.R.; Delano-Wood, L.; McDonald, C.R.; Nation, D.A.; Libon, D.J.; Au, R.; Galasko, D.; et al. Neuropsychological Criteria for Mild Cognitive Impairment Improves Diagnostic Precision, Biomarker Associations, and Progression Rates. J. Alzheimers Dis. JAD 2014, 42, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, L.R.; Delano-Wood, L.; Libon, D.J.; McDonald, C.R.; Nation, D.A.; Bangen, K.J.; Jak, A.J.; Au, R.; Salmon, D.P.; Bondi, M.W. Are Empirically-Derived Subtypes of Mild Cognitive Impairment Consistent with Conventional Subtypes? J. Int. Neuropsychol. Soc. JINS 2013, 19, 635–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jak, A.J.; Preis, S.R.; Beiser, A.S.; Seshadri, S.; Wolf, P.A.; Bondi, M.W.; Au, R. Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. J. Int. Neuropsychol. Soc. 2016, 22, 937–943. [Google Scholar] [CrossRef] [Green Version]
| Cognitive Domain | Tests | Raw Score Used |
|---|---|---|
| Executive Functioning | Stroop Test (Interference trial) TMT Part B Letter Fluency (FAS) | Total Number of Correct Items Completion Time Total Number of Words (all 3 trials) |
| Verbal Delayed Memory | HVLT-R WMS-III Logical Memory | Delayed Total Recall Delayed Total Recall |
| Language | BNT Semantic Fluency (Animals) * | Total Correct Spontaneous Responses Total Number of Words |
| Visuospatial Skills | Benton JOLO Benton FRT | Total Items Correct Total Items Correct |
| Attention/Working Memory | WAIS-III Digit Span Forward WAIS-III Digit Span Backward | Total Number of Points Total Number of Points |
| Measure | Overall Sample (n = 494) |
|---|---|
| Variable | Mean/% (SD) |
| Age | 64.73 (9.04) |
| Education (years) | 15.01 (2.79) |
| % Male | 72% |
| % White, non-Hispanic | 94% |
| Years since diagnosis | 7.84 (4.94) |
| Years since symptom onset | 9.61 (5.26) |
| PD motor subtype | |
| Tremor predominant | 77% |
| Akinetic-rigid | 22% |
| PIGD | 1% |
| UPDRS III, on medication | 25.28 (9.80) |
| Hoehn and Yahr (H-Y) Scale ^ | |
| 0 | 0.30% |
| 1 | 1% |
| 1.5 | 1% |
| 2 | 58% |
| 2.5 | 21% |
| 3 | 15% |
| 4 | 3% |
| BDI-II, raw total | 10.10 (6.86) |
| STAI: State anxiety, percentile | 61.33 (29.87) |
| STAI: Trait anxiety, percentile | 58.38 (30.73) |
| Apathy scale, raw total | 11.22 (6.31) |
| Dementia Rating Scale-2, raw total | 136.99 (4.49) |
| Cognitive composites (z-scores) # | |
| Executive function | −0.58 (0.90) |
| Verbal delayed memory | −0.36 (1.01) |
| Language | −0.03 (0.96) |
| Visuospatial abilities | 0.01 (0.78) |
| Attention/working memory | 0.18 (0.77) |
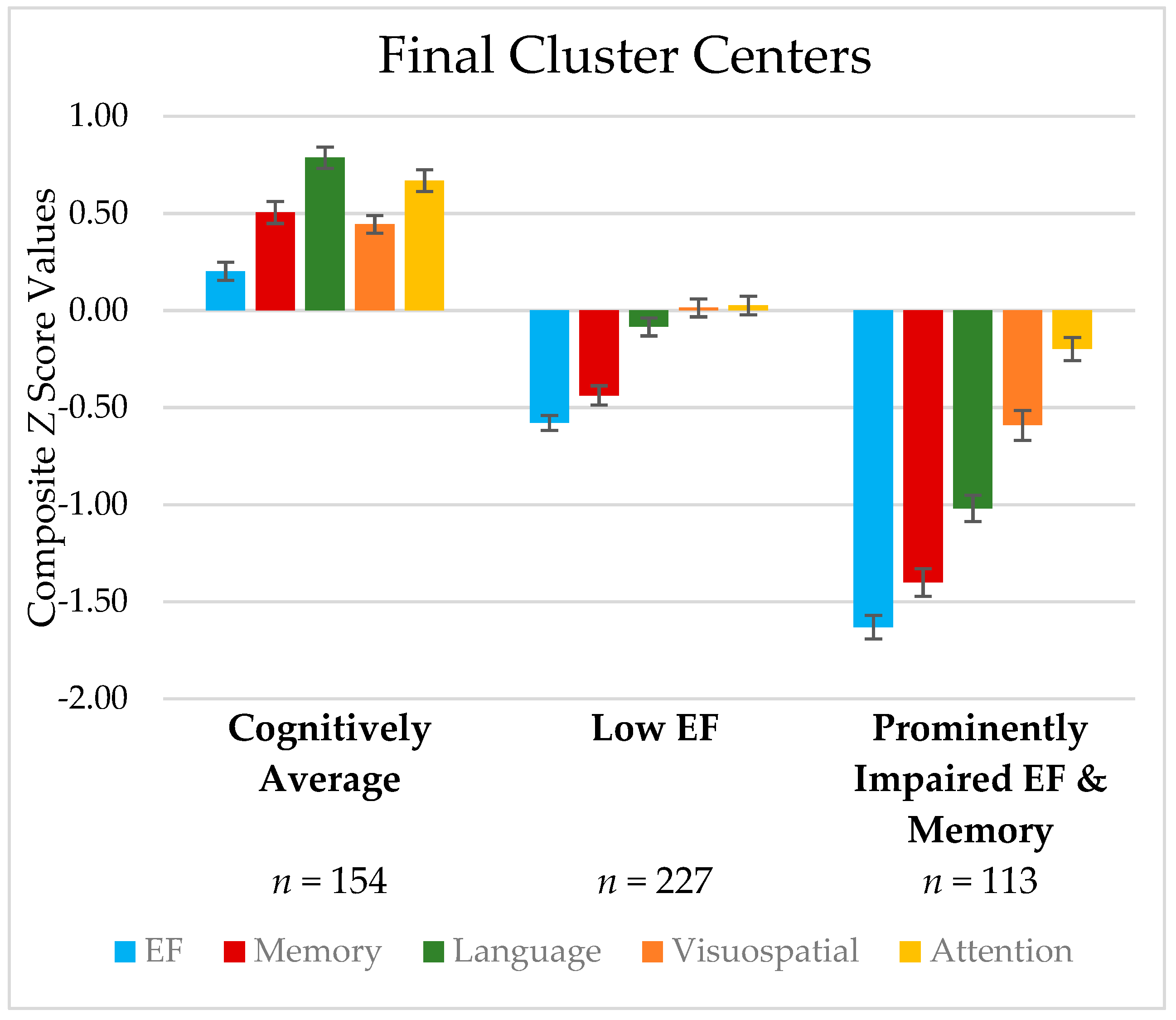
| Characteristic Measure | Cluster 1 Cognitively Average | Cluster 2 Low EF | Cluster 3 Prominently Impaired EF/Memory | Omnibus Kruskal– Wallis H-Test | Effect Size | Post hoc Differences (Bonferroni Corrected) |
|---|---|---|---|---|---|---|
| N = 154 | N = 227 | N = 113 | ||||
| Mean (SD)/ | Mean (SD)/ | Mean (SD)/ | p-value | Eta squared /Cramer’s V # | ||
| % | % | % | ||||
| Age (years) | 64.51 (8.75) | 65.31 (9.12) | 63.85 (9.15) | 0.38 | 0.004 | -- |
| Education (years) | 15.35 (2.55) | 14.91 (2.91) | 14.73 (2.83) | 0.13 | 0.01 | -- |
| Sex (% Male) | 68% | 73% | 75% | 0.33 ^ | 0.07 # | -- |
| % Caucasian, Non | 99% | 93% | 91% | 0.01 ^ | 0.15 # | 1 < [2 = 3] * |
| Hispanic | ||||||
| % H-Y Stages 2–3 | 95% | 93% | 95% | 0.57 ^ | 0.06 # | - |
| % Tremor Subtype | 73% | 76% | 82% | 0.16 ^ | 0.08 # | -- |
| Years Since Symptom | 9.45 (5.14) | 9.51 (5.20) | 10.03 (5.57) | 0.58 | 0.002 | -- |
| Onset | ||||||
| Years Since Diagnosis | 7.70 (5.06) | 7.58 (4.73) | 8.56 (5.15) | 0.23 | 0.01 | -- |
| UPDRS Part III | 22.78 (8.85) | 25.18 (9.38) | 28.91 (10.81) | <0.001 | 0.05 | [1 = 2] < 3 ** |
| BDI-II | 9.15 (7.04) | 9.81 (6.06) | 12.09 (7.79) | 0.001 | 0.03 | 1 < 3 ** |
| Apathy Scale | 10.47 (6.33) | 10.89 (6.25) | 13.01 (6.14) | 0.01 | 0.02 | [1 = 2] < 3 ** |
| STAI: State Pct. | 53.20 (31.34) | 63.26 (28.51) | 68.73 (28.09) | <0.001 | 0.04 | 1 < [2 = 3] ** |
| STAI: Trait Pct. | 50.11 (31.29) | 59.21 (30.30) | 68.33 (27.73) | <0.001 | 0.04 | 1 < 2 < 3 ** |
| DRS-2 | 139.54 (3.10) | 136.92 (4.22) | 133.65 (4.42) | <0.001 | 0.23 | 1 > 2 > 3 * |
| Cognitive Domain | ||||||
| Z-Score Composites | ||||||
| Executive Function | 0.20 (0.59) | −0.58 (0.58) | −1.63 (0.65) | <0.001 | 0.56 | 1 > 2 > 3 * |
| Memory | 0.51 (0.70) | −0.44 (0.75) | −1.40 (0.75) | <0.001 | 0.47 | 1 > 2 > 3 * |
| Language | 0.79 (0.68) | −0.08 (0.70) | −1.02 (0.72) | <0.001 | 0.48 | 1 > 2 > 3 * |
| Visuospatial Skills | 0.44 (0.56) | 0.01 (0.70) | −0.59 (0.82) | <0.001 | 0.21 | 1 > 2 > 3 * |
| Attention/WM | 0.67 (0.70) | 0.03 (0.72) | −0.20 (0.63) | <0.001 | 0.20 | 1 > 2 > 3 ** |
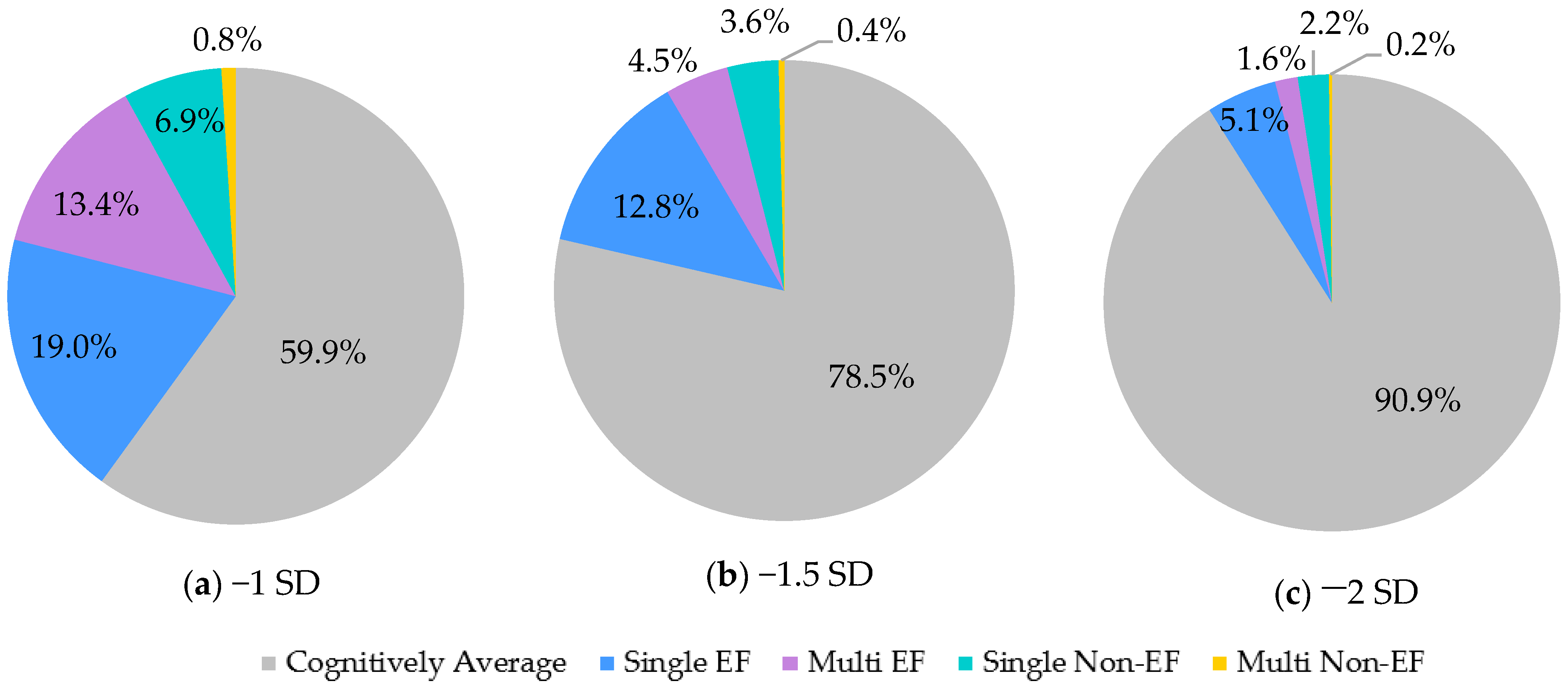
| Impairment Cutoff | Cognitively Average % (n) | Low EF % (n) | Prominently Impaired EF/Memory % (n) | Pearson Chi Square | p-Value | Cramer’s V |
|---|---|---|---|---|---|---|
| −1 SD | 1.01% (2) | 46.46% (92) | 52.53% (194) | 223.47 | <0.001 | 0.67 |
| −1.5 SD | 0.95% (1) | 23.81% (25) | 75.24% (79) | 213.13 | <0.001 | 0.66 |
| −2 SD | 0% (0) | 4.44% (2) | 95.56% (43) | 148.33 | <0.001 | 0.55 |
| Impairment Cutoff | Sensitivity | Specificity | C Stat. | Sample | 0.25 | Base Rate 0.45 | 0.65 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PPV NPV | PPV NPV | PPV NPV | PPV NPV | ||||||||
| −1 SD | 0.53 | 0.97 | 0.86 | 0.92 | 0.76 | 0.85 | 0.86 | 0.94 | 0.72 | 0.97 | 0.53 |
| −1.5 SD | 0.75 | 0.91 | 0.88 | 0.70 | 0.93 | 0.74 | 0.92 | 0.87 | 0.82 | 0.94 | 0.66 |
| −2 SD | 0.00 | 1.00 | 0.91 | -- | 0.91 | -- | 0.75 | -- | 0.55 | -- | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenney, L.E.; Ratajska, A.M.; Lopez, F.V.; Price, C.C.; Armstrong, M.J.; Bowers, D. Mapping Actuarial Criteria for Parkinson’s Disease-Mild Cognitive Impairment onto Data-Driven Cognitive Phenotypes. Brain Sci. 2022, 12, 54. https://doi.org/10.3390/brainsci12010054
Kenney LE, Ratajska AM, Lopez FV, Price CC, Armstrong MJ, Bowers D. Mapping Actuarial Criteria for Parkinson’s Disease-Mild Cognitive Impairment onto Data-Driven Cognitive Phenotypes. Brain Sciences. 2022; 12(1):54. https://doi.org/10.3390/brainsci12010054
Chicago/Turabian StyleKenney, Lauren E., Adrianna M. Ratajska, Francesca V. Lopez, Catherine C. Price, Melissa J. Armstrong, and Dawn Bowers. 2022. "Mapping Actuarial Criteria for Parkinson’s Disease-Mild Cognitive Impairment onto Data-Driven Cognitive Phenotypes" Brain Sciences 12, no. 1: 54. https://doi.org/10.3390/brainsci12010054
APA StyleKenney, L. E., Ratajska, A. M., Lopez, F. V., Price, C. C., Armstrong, M. J., & Bowers, D. (2022). Mapping Actuarial Criteria for Parkinson’s Disease-Mild Cognitive Impairment onto Data-Driven Cognitive Phenotypes. Brain Sciences, 12(1), 54. https://doi.org/10.3390/brainsci12010054






