Multiple Case Studies in German Children with Dyslexia: Characterization of Phonological, Auditory, Visual, and Cerebellar Processing on the Group and Individual Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Sample Criteria
2.2. Behavioral Test Battery
2.2.1. Assessment of Phonological Performance
2.2.2. Assessment of Auditory Performance
2.2.3. Assessment of Visual Performance
2.2.4. Assessment of Cerebellar Performance
2.3. Statistical Analysis
3. Results
3.1. Sample Criteria and Attention Performance
3.2. Group-Level Analyses
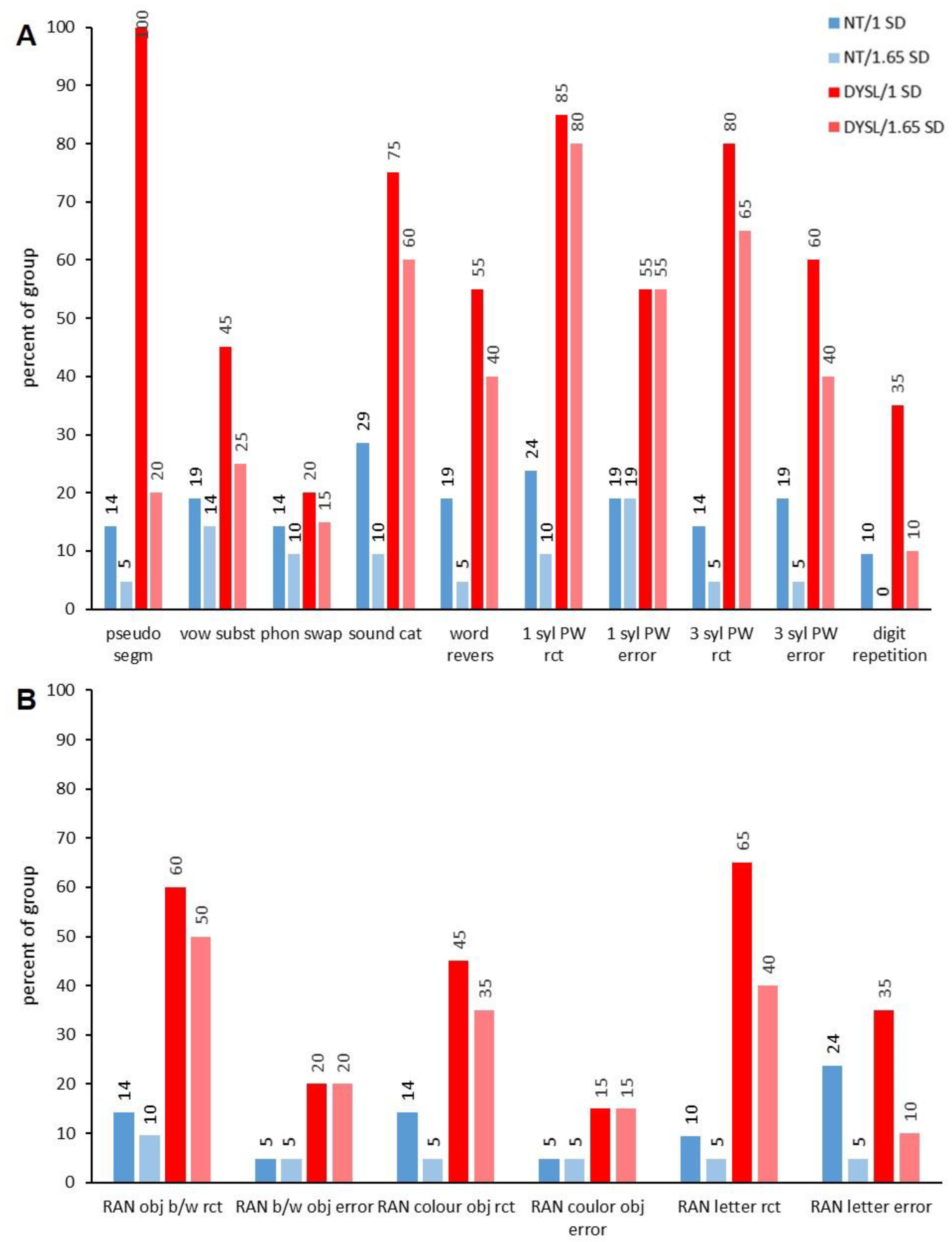
3.2.1. Analysis of Phonological Performance
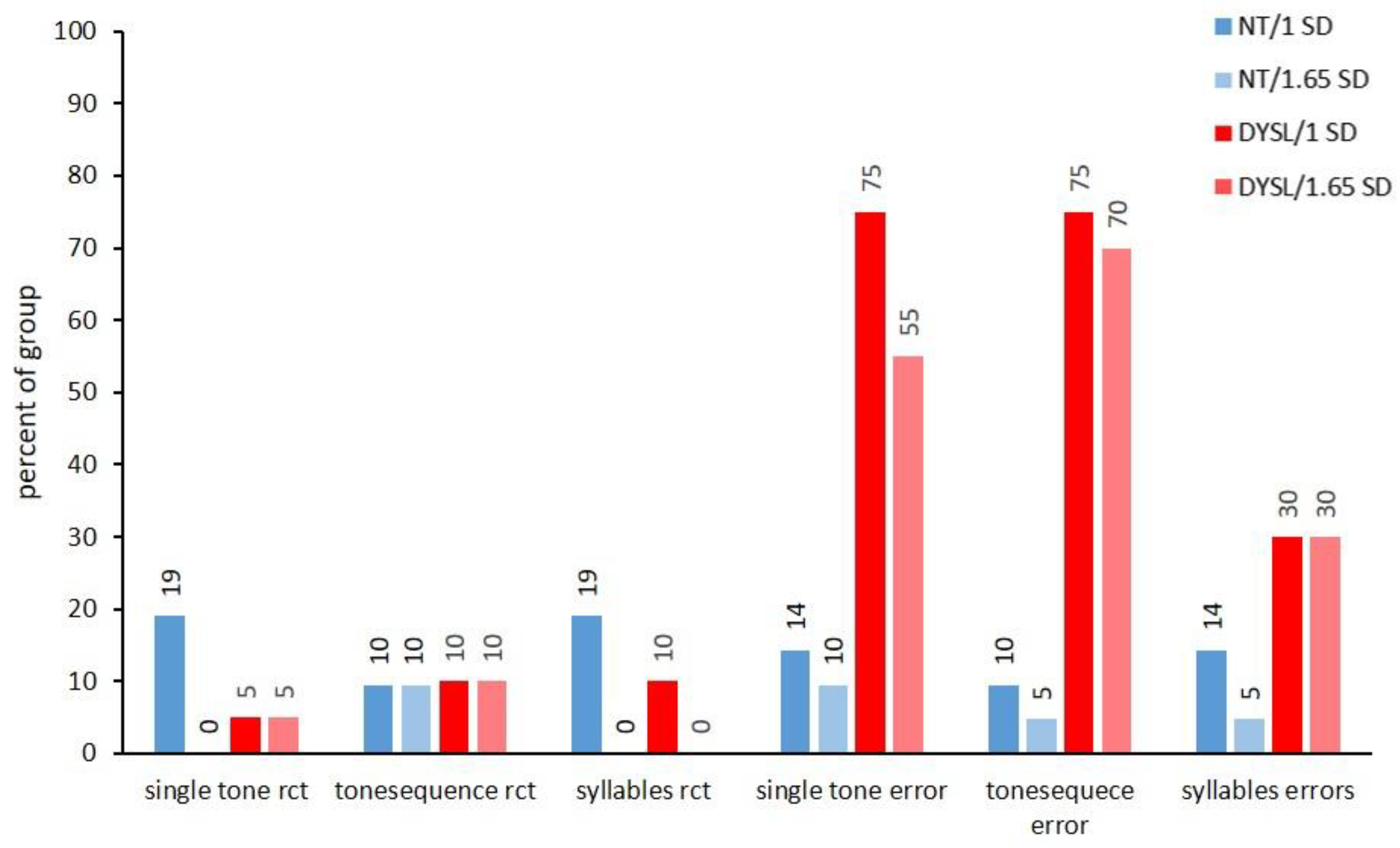
3.2.2. Analysis of Auditory Performance
3.2.3. Analysis of Visual Performance
3.2.4. Analysis of Cerebellar Performance
3.3. Analyses of Deviant Performance in the Four Domains
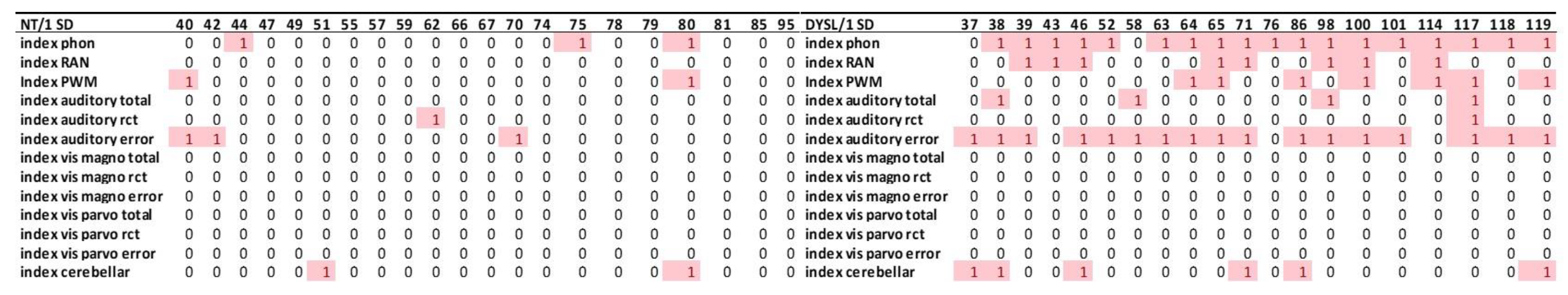
3.3.1. Index Level
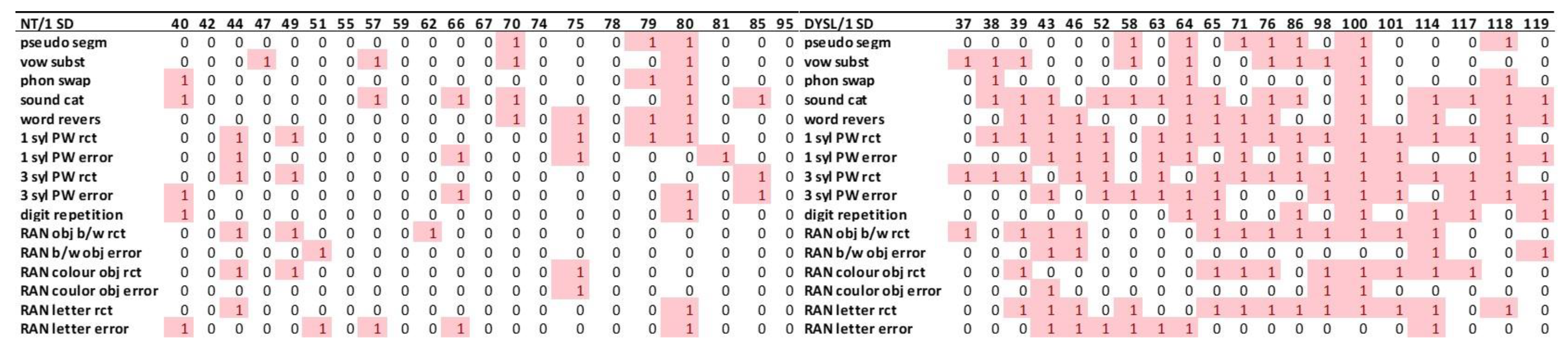
3.3.2. Subtest Level
3.3.3. Deviant Performance in the Individual Level Presented as Heatmaps
3.4. Predicting Reading Performance
4. Discussion
4.1. Which Performance Domain Showed the Most Impairments?
4.2. Are There Typical Individual Performance Profiles?
4.3. Which Domains Were Predictors for Reading Performance?
4.4. Methodological Issues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dilling, H.; Mombour, W.; Schmidt, M.H.; Schulte-Markwort, E. Weltgesundheitsorganisation: Internationale Klassifikation psychischer Störungen (ICD-10 Kapitel V (F), Diagnostische Kriterien für Forschung und Praxis); Verlag Hans Huber: Bern, Switzerland, 2000. [Google Scholar]
- Yang, L.; Li, C.; Li, X.; Zhai, M.; An, Q.; Zhang, Y.; Zhao, J.; Weng, X. Prevalence of Developmental Dyslexia in Primary School Children: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 240. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, S.E.; Morris, R.; Shaywitz, B.A. The education of dyslexic children from childhood to young adulthood. Annu. Rev. Psychol. 2008, 59, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Strehlow, U.; Haffner, J.; Haffner, J. Definitionsmöglichkeiten und sich daraus ergebende Häufigkeit der umschriebenen Lese- bzw. Rechtschreibstörung-theoretische Überlegungen und empirische Befunde an einer repräsentativen Stichprobe junger Erwachsener (Possible definitions and subsequent incidence of circumscribed reading and spelling disorders--theoretical considerations and empirical findings in a representative sample of young adults). Z. Für Kinder-Und Jugendpsychiatrie Und Psychother. 2002, 30, 113–126. [Google Scholar] [CrossRef]
- Snowling, M.J.; Hulme, C.; Nation, K. Defining and understanding dyslexia: Past, present and future. Oxf. Rev. Educ. 2020, 46, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Stanovich, K.E.; Siegel, L.S. Phenotypic performance profile of children with reading disabilities: A regression-based test of the phonological-core variable-difference model. J. Educ. Psychol. 1994, 86, 24–53. [Google Scholar] [CrossRef]
- Wyschkon, A.; Schulz, F.; Gallit, F.S.; Poltz, N.; Kohn, J.; Moraske, S.; Bondü, R.; Von Aster, M.; Esser, G. 5-Jahres-Verlauf der LRS: Stabilität, Geschlechtseffekte, Schriftsprachniveau und Schulerfolg (5-year course of dyslexia—Persistence, sex effects, performance in reading and spelling, and school-related success). Z. Für Kinder-Und Jugendpsychiatrie Und Psychother. 2018, 46, 107–122. [Google Scholar] [CrossRef]
- Esser, G.; Wyschkon, A.; Schmidt, M.H. Was wird aus Achtjährigen mit einer Lese- und Rechtschreibstörung. Zeitschrit Für Klin. Psychol. Und Psychother. 2002, 31, 235–242. [Google Scholar] [CrossRef]
- Chyl, K.; Fraga-González, G.; Brem, S.; Jednoróg, K. Brain dynamics of (a)typical reading development-a review of longitudinal studies. NPJ Sci. Learn 2021, 6, 4. [Google Scholar] [CrossRef]
- Erbeli, F.; Rice, M.; Paracchini, S. Insights into Dyslexia Genetics Research from the Last Two Decades. Brain Sci. 2021, 12, 27. [Google Scholar] [CrossRef]
- Kearns, D.M.; Hancock, R.; Hoeft, F.; Pugh, K.R.; Frost, S.J. The Neurobiology of Dyslexia. Teach. Except. Child. 2019, 51, 175–188. [Google Scholar] [CrossRef]
- Gialluisi, A.; Andlauer, T.F.M.; Mirza-Schreiber, N.; Moll, K.; Becker, J.; Hoffmann, P.; Ludwig, K.U.; Czamara, D.; Pourcain, B.S.; Brandler, W.; et al. Genome-wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Transl. Psychiatry 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, U.; Neef, N.E.; Kraft, I.; Schaadt, G.; Dörr, L.; Brauer, J.; Czepezauer, I.; Müller, B.; Wilcke, A.; Kirsten, H.; et al. The emergence of dyslexia in the developing brain. NeuroImage 2020, 211, 116633. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; White, S.; Frith, U. Weighing the evidence between competing theories of dyslexia. Dev. Sci. 2006, 9, 265–269. [Google Scholar] [CrossRef]
- Ali, S.A.; Fadzil, N.A.; Reza, F.; Mustafar, F.; Begum, T. A Mini Review: Visual and Auditory Perception in Dyslexia. Iium Med. J. Malays. 2021, 20, 121–130. [Google Scholar] [CrossRef]
- Goswami, U. Sensory theories of developmental dyslexia: Three challenges for research. Nat. Rev. Neurosci. 2015, 16, 43–54. [Google Scholar] [CrossRef]
- Ozernov-Palchik, O.; Gaab, N. Tackling the ‘dyslexia paradox’: Reading brain and behavior for early markers of developmental dyslexia. Wiley Interdiscip. Rev. Cogn. Sci. 2016, 7, 156–176. [Google Scholar] [CrossRef]
- Pennington, B.F. From single to multiple deficit models of developmental disorders. Cognition 2006, 101, 385–413. [Google Scholar] [CrossRef]
- Stein, J.F. What is Developmental Dyslexia? Brain Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Share, D.L. Common Misconceptions about the Phonological Deficit Theory of Dyslexia. Brain Sci. 2021, 11, 1510. [Google Scholar] [CrossRef]
- Habib, M. The Neurological Basis of Developmental Dyslexia and Related Disorders: A Reappraisal of the Temporal Hypothesis, Twenty Years on. Brain Sci. 2021, 11, 708. [Google Scholar] [CrossRef]
- Catts, H.W.; Petscher, Y. A Cumulative Risk and Resilience Model of Dyslexia. J. Learn. Disabil. 2022, 55, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; Rosen, S.; Dakin, S.C.; Day, B.L.; Castellote, J.M.; White, S.; Frith, U. Theories of developmental dyslexia: Insights from a multiple case study of dyslexic adults. Brain 2003, 126, 841–865. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F. Developmental dyslexia: Specific phonological deficit or general sensorimotor dysfunction? Curr. Opin. Neurobiol. 2003, 13, 212–218. [Google Scholar] [CrossRef]
- Wagner, R.K.; Torgesen, J.K. The Nature of Phonological Processing and its Causal Role in the Acquisition of Reading-Skills. Psychol. Bull. 1987, 101, 192–212. [Google Scholar] [CrossRef]
- Snowling, M.J. Phonemic Deficits in Developmental Dyslexia. Psychol. Res. 1981, 43, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Georgiewa, P.; Grunling, C.; Ligges, M.; Filz, C.; Moller, U.; Blanz, B. Age dependent changes in phonological reading in dyslexia. Zeitschrit Für Klin. Psychol. Und Psychother. 2004, 33, 281–289. [Google Scholar] [CrossRef]
- Snowling, M.J. Dyslexia as a Phonological Deficit: Evidence and Implications. Child Adolesc. Ment. Health. 1998, 3, 4–11. [Google Scholar] [CrossRef]
- Werth, R. Is Developmental Dyslexia Due to a Visual and Not a Phonological Impairment? Brain Sci. 2021, 11, 1313. [Google Scholar] [CrossRef]
- Gori, S.; Seitz, A.R.; Ronconi, L.; Franceschini, S.; Facoetti, A. Multiple Causal Links Between Magnocellular-Dorsal Pathway Deficit and Developmental Dyslexia. Cerebral Cortex 2016, 26, 4356–4369. [Google Scholar] [CrossRef]
- Ashburn, S.M.; Flowers, D.L.; Napoliello, E.M.; Eden, G.F. Cerebellar function in children with and without dyslexia during single word processing. Hum. Brain Mapp. 2020, 41, 120–138. [Google Scholar] [CrossRef] [Green Version]
- van Ermingen-Marbach, M.; Pape-Neumann, J.; Grande, M.; Grabowska, A.; Heim, S. Distinct neural signatures of cognitive subtypes of dyslexia: Effects of lexicality during phonological processing. Acta Neurobiol. Exp. 2013, 73, 404–416. [Google Scholar]
- Heim, S.; Freeman, R.B.; Eulitz, C.; Elbert, T. Auditory temporal processing deficit in dyslexia is associated with enhanced sensitivity in the visual modality. Neuroreport 2001, 12, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.F. The magnocellular theory of developmental dyslexia. Dyslexia 2001, 7, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Gavril, L.; Roșan, A.; Szamosközi, Ș. The role of visual-spatial attention in reading development: A meta-analysis. Cogn. Neuropsychol. 2021, 38, 387–407. [Google Scholar] [CrossRef]
- Talcott, J.B.; Hansen, P.C.; Willis-Owen, C.; McKinnell, I.W.; Richardson, A.J.; Stein, J.F. Visual magnocellular impairment in adult developmental dyslexics. Neuro-ophthalmology 1998, 20, 187–201. [Google Scholar] [CrossRef]
- Eden, G.F.; VanMeter, J.W.; Rumsey, J.M.; Zeffiro, T.A. The visual deficit theory of developmental Dyslexia. NeuroImage 1996, 4, S108–S117. [Google Scholar] [CrossRef]
- Skottun, B.C. The need to differentiate the magnocellular system from the dorsal stream in connection with dyslexia. Brain Cogn. 2015, 95, 62–66. [Google Scholar] [CrossRef]
- Livingstone, M.; Hubel, D. Segregation of Form, Color, Movement, and Depth: Anatomy, Physiology, and Perception. Science 1988, 240, 740–749. [Google Scholar] [CrossRef]
- Tallal, P. Auditory temporal perception, phonics, and reading disabilites in children. Brain Lang. 1980, 9, 182–198. [Google Scholar] [CrossRef]
- Stein, J.F.; Talcott, J. Impaired neuronal timing in developmental dyslexia—the magnocellular hypothesis. Dyslexia 1999, 5, 59–77. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Automaticity: A new framework for dyslexia research? Cognition 1990, 35, 159–182. [Google Scholar] [CrossRef]
- Ramus, F. A neurological model of dyslexia and other domain-specific developmental disorders with an associated sensorimotor syndrome. In The Dyslexic Brain: New Pathways in Neuroscience Discovery: A Neurological Model of Dyslexia and Other Domain-Specific Developmental Disorders With an Associated Sensorimotor Syndrome; Rosen, G.D., Ed.; Routledge: London, UK, 2006. [Google Scholar]
- Dyslexia, R.F. Talk of two theories. Nature 2001, 412, 393–395. [Google Scholar]
- Frith, U. Paradoxes in the definition of dyslexia. Dyslexia 1999, 5, 192–214. [Google Scholar] [CrossRef]
- Jednoróg, K.; Gawron, N.; Marchewka, A.; Heim, S.; Grabowska, A. Cognitive subtypes of dyslexia are characterized by distinct patterns of grey matter volume. Brain Struct. Funct 2014, 219, 1697–1707. [Google Scholar] [CrossRef]
- Heim, S.; Tschierse, J.; Amunts, K.; Wilms, M.; Vossel, S.; Willmes, K.; Grabowska, A.; Huber, W. Cognitive subtypes of dyslexia. Acta Neurobiol. Exp. 2008, 68, 73–82. [Google Scholar]
- Dębska, A.; Łuniewska, M.; Zubek, J.; Chyl, K.; Dynak, A.; Dzięgiel-Fivet, G.; Plewko, J.; Jednoróg, K.; Grabowska, A. The cognitive basis of dyslexia in school-aged children: A multiple case study in a transparent orthography. Dev. Sci. 2022, 25, e13173. [Google Scholar] [CrossRef]
- Danelli, L.; Berlingeri, M.; Bottini, G.; Borghese, N.A.; Lucchese, M.; Sberna, M.; Price, C.J.; Paulesu, E. How many deficits in the same dyslexic brains? A behavioural and fMRI assessment of comorbidity in adult dyslexics. Cortex 2017, 97, 125–142. [Google Scholar] [CrossRef]
- Carroll, J.M.; Solity, J.; Shapiro, L.R. Predicting dyslexia using prereading skills: The role of sensorimotor and cognitive abilities. J. Child Psychol. Psychiatry 2016, 57, 750–758. [Google Scholar] [CrossRef]
- White, S.; Milne, E.; Rosen, S.; Hansen, P.; Swettenham, J.; Frith, U.; Ramus, F. The role of sensorimotor impairments in dyslexia: A multiple case study of dyslexic children. Dev. Sci. 2006, 9, 237–255. [Google Scholar] [CrossRef]
- Wimmer, H.; Schurz, M. Dyslexia in regular orthographies: Manifestation and causation. Dyslexia 2010, 16, 283–299. [Google Scholar] [CrossRef]
- Zimmermann, P.; Gondan, M.; Fimm, B. Testbatterie zur Aufmerksamkeitsprüfung für Kinder—KiTAP.; Psychologische Testsysteme: Herzogenrath, Germany, 2006. [Google Scholar]
- Steingrüber, H.-J. Hand-Dominanz-Test. 3., Überarbeitete und Neu Normierte Auflage; Hogrefe: Göttingen, Germany, 2010. [Google Scholar]
- Tewes, U.; Rossmann, P.; Schallberger, U. HAWIK-III Hamburg-Wechsler-Intelligenztest fuer Kinder, 3rd ed.; Hans Huber: Bern, Switzerland, 2002. [Google Scholar]
- Grissemann, H. Zürcher Lesetest (ZLT); Hans Huber: Göttingen, Germany, 2000. [Google Scholar]
- Rathenow, P.; Laupenmühle, D.; Vöge, J. Westermann Rechtschreibtest 6+ (WRT6+), 2nd ed.; Georg Westermann: Braunschweig, Germany, 1981. [Google Scholar]
- Grund, M.; Haug, G.; Naumann, C.L. Diagnostischer Rechtschreibtest für 4. Klassen (DRT 4), 2nd ed.; Beltz Test GmbH: Göttingen, Germany, 2004. [Google Scholar]
- Grund, M.; Haug, G.; Naumann, C.L. Diagnostischer Rechtschreibtest für 5. Klassen (DRT 5); Beltz Test GmbH: Göttingen, Germany, 2004. [Google Scholar]
- Leibert, D.; Roth, E.; Schneider, W. Test zur Bestimmung der phonologischen Bewusstheit bei älteren Schulkindern (PTB).
- Stock, C.; Marx, P.; Schneider, W. BAKO 1-4—Basiskompetenzen für Lese-Rechtschreibleistungen. In Ein Test zur Erfassung der Phonologischen Bewusstheit vom Ersten Bis Vierten Grundschuljahr; Beltz: Göttingen, Germany, 2003. [Google Scholar]
- Skowronek, H.; Marx, H. Die Bielefelder Längsschnittstudie zur Früherkennung von Risiken der Lese-Rechtschreibschwäche: Theoretischer Hintergrund und erste Befunde. Heilpädagogische Forschung XV. 1989; 38–49. [Google Scholar]
- Jansen, H.; Mannhaupt, G.; Marx, H. BISC—Bielefelder Screening zur Früherkennung von Lese-Rechtschreibschwierigkeiten. 1999. [Google Scholar]
- Ahissar, M.; Protopapas, A.; Reid, M.; Merzenich, M.M. Auditory processing parallels reading abilities in adults. Proc. Natl. Acad. Sci. USA 2000, 97, 6832–6837. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Körne, G.; Deimel, W.; Bartling, J.; Remschmidt, H. Speech perception deficit in dyslexic adults as measured by mismatch negativity (MMN). Int. J. Psychophysiol. 2001, 40, 77–87. [Google Scholar] [CrossRef]
- Wilmer, J.B.; Richardson, A.J.; Chen, Y.; Stein, J.F. Two visual motion processing deficits in developmental dyslexia associated with different reading skills deficits. J. Cogn. Neurosci. 2004, 16, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Körne, G.; Bartling, J.; Deimel, W.; Remschmidt, H. Visual evoked potentials elicited by coherently moving dots in dyslexic children. Neurosci. Lett. 2004, 357, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Körne, G.; Bartling, J.; Deimel, W.; Remschmidt, H. Motion-onset VEPs in dyslexia. Evid. Vis. Percept. Deficit. Neuroreport 2004, 15, 1075–1078. [Google Scholar] [CrossRef]
- Rusiak, P.; Lachmann, T.; Jaskowski, P.; Van Leeuwen, C. Mental rotation of letters and shapes in developmental dyslexia. Perception 2007, 36, 617–631. [Google Scholar] [CrossRef]
- Korkman, M. NEPSY. A Developmental Neuro-Psychological Assessment; The Psychological Corporation: San Antonio, TX, USA, 1998. [Google Scholar]
- Hemmerich, W. StatistikGuru: Rechner zur Adjustierung des α-Niveaus. 2016. Available online: https://statistikguru.de/rechner/adjustierung-des-alphaniveaus.html (accessed on 14 August 2021).
- Ziegler, J.C.; Goswami, U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychol. Bull. 2005, 131, 3–29. [Google Scholar] [CrossRef]
- Melby-Lervåg, M.; Lyster, S.-A.H.; Hulme, C. Phonological skills and their role in learning to read: A meta-analytic review. Psychol. Bull. 2012, 138, 322–352. [Google Scholar] [CrossRef]
- Kujala, T.; Myllyviita, K.; Tervaniemi, M.; Alho, K.; Kallio, J.; Naatanen, R. Basic auditory dysfunction in dyslexia as demonstrated by brain activity measurements. Psychophysiology 2000, 37, 262–266. [Google Scholar] [CrossRef]
- Farmer, M.E.; Klein, R.M. The evidence for a temporal processing deficit linked to dyslexia: A review. Psychon. Bull. Rev. 1995, 2, 460–493. [Google Scholar] [CrossRef] [PubMed]
- Studdert-Kennedy, M.; Mody, M. Auditory temporal perception deficits in the reading-impaired: A critical review of the evidence. Psychon. Bull. Rev. 1995, 2, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Stein, J.F. Cerebellar Function in Developmental Dyslexia. Cerebellum 2013, 12, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Bowers, P.G. The double-deficit hypothesis for the developmental dyslexias. J. Educ. Psychol. 1999, 91, 415–438. [Google Scholar] [CrossRef]
- Raberger, T.; Wimmer, H. On the automaticity/cerebellar deficit hypothesis of dyslexia: Balancing and continuous rapid naming in dyslexic and ADHD children. Neuropsychologia 2003, 41, 1493–1497. [Google Scholar] [CrossRef]






| Deficit Theory | References | Presumed Neurobiological Background | Explanation for Dyslexia-Specific Performance Problems | Behavioral Deficits in Readers with Dyslexia |
|---|---|---|---|---|
| Phonological deficit | [28] Snowling, M. J. (1998) | left-hemisphere perisylvian areas of the reading network | deficient acquisition of grapheme–phoneme conversion, reading, and spelling deficits | poor phonological performance (phonological awareness skills, phonological working memory, access to semantic memory) |
| Magnocellular deficit—visual | [37] Eden et al. (1996) | visual magnocellular system | unstable eye movements hinder word perception, unstable visual perception impairs transformation of visual letter to speech sound—phonological deficits are a secondary consequence | worse performance of fast-moving, low-contrast visual stimuli, unstable visual perception, unstable eye control |
| Magnocellular deficit—auditory | [40] Tallal, P. (1980) [41] Stein and Talcott (1999) | auditory (magnocellular) system | deficient frequency discrimination hinders disassembly of the auditory speech—phonological deficits are a secondary consequence | deficient ability to process fast-changing acoustic stimuli |
| Cerebellar deficit | [42] Nicolson and Fawcett (1990) | cerebellum | impairment in automation of learned skills—phonological deficits are the result of impairment in the automation of grapheme–phoneme mapping | motoric clumsiness, worse performance in tasks requiring the automation of motor processes |
| NT Mean (SD) | DYSL Mean (SD) | Statistics | p-Value | |
|---|---|---|---|---|
| n | 21 | 20 | ||
| Age | 11.48 (0.75) | 11.38 (1.08) | t (33.65) = 0.33 | p = 1.000 |
| Nonverbal IQ | 115.86 (12.18) | 114.40 (9.81) | t (37.98) = 0.42 | p = 1.000 |
| Spelling performance | 107.62 (11.41) | 71.85 (12.10) | t (38.55) = 9.73 | p < 0.01 |
| Reading performance | 106.49 (5.00) | 78.71 (7.45) | t (33.03) = 13.95 | p < 0.01 |
| Discrepancy IQ and spelling | 0.55 (0.80) | 2.84 (1.04) | t (35.62) = -7.85 | p < 0.01 |
| Discrepancy IQ and reading | 0.63 (0.78) | 2.38 (0.64) | t (38.24) = -7.90 | p < 0.01 |
| Test score handedness | 47.00 (19.17) | 43.72 (14.15) | t (36.76) = 0.62 | p = 1.000 |
| NT | DYSL | Statistics | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| n | 21 | 20 | ||
| Distractability rct | 536.48 (64.37) | 530.30 (74.94) | t (37.50) = 0.28 | p = 1.000 |
| Sustained attention rct | 688.24 (160.51) | 665.75 (93.70) | t (32.49) = 0.55 | p = 1.000 |
| Gonogo rct | 475.67 (77.34) | 457.80 (53.96) | t (35.82) = 0.86 | p = 1.000 |
| Distractability cor | 39.19 (1.60) | 39.40 (0.82) | t (30.15) = −0.53 | p = 1.000 |
| Distractability com | 4.14 (3.81) | 7.05 (7.31) | t (28.31) = −1.59 | p = 1.000 |
| Distractability om | 0.81 (1.60) | 0.60 (0.82) | t (30.15) = 0.53 | p = 1.000 |
| Sustained attention cor | 47.71 (2.67) | 46.75 (3.46) | t (35.71) = 1.00 | p = 1.000 |
| Sustained attention com | 2.24 (3.42) | 4.90 (6.51) | t (28.42) = −1.63 | p = 1.000 |
| Sustained attention om | 2.29 (2.67) | 3.25 (3.46) | t (35.71) = −1.00 | p = 1.000 |
| GoNogo cor | 19.95 (0.22) | 19.80 (0.52) | t (25.16) = 1.21 | p = 1.000 |
| GoNogo om | 0.95 (0.92) | 1.60 (1.60) | t (30.00) = −1.58 | p = 1.000 |
| GoNogo com | 0.05 (0.22) | 0.20 (0.52) | t (25.16) = −1.21 | p = 1.000 |
| NT | DYSL | Statistics | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| n | 21 | 20 | ||
| Phonological Awareness | ||||
| Pseudo Segm | 7.57 (1.86) | 6.23 (2.79) | t (30.91) = 1.79 | p = 1.000 |
| Vow Subst | 8.57 (1.36) | 7.01 (2.16) | t (29.82) = 2.79 | p = 0.085 |
| Phon Swap | 7.71 (1.95) | 6.26 (2.15) | t (36.57) = 2.28 | p = 0.914 |
| Sound Cat | 9.10 (1.04) | 6.90 (2.13) | t (25.62) = 4.20 | p <0.001 |
| Word Revers | 6.76 (2.45) | 3.53 (2.93) | t (35.24) = 3.85 | p < 0.001 |
| 1 syl PW rct | 11.10 (3.53) | 22.75 (8.66) | t (24.91) = −5.59 | p < 0.001 |
| 1 syl PW error | 1.00 (1.14) | 2.65 (1.87) | t (31.11) = −3.39 | p < 0.017 |
| 3 syl PW rct | 31.14 (11.21) | 55.20 (19.82) | t (29.73) = −4.75 | p < 0.001 |
| 3 syl PW error | 2.81 (2.82) | 8.60 (11.58) | t (21.15) = −2.18 | p = 0.510 |
| Phonological recoding in working memory | ||||
| digit repetition | 12.60 (3.63) | 9.20 (2.09) | t (30.37) = 3.72 | p < 0.001 |
| Phonological recoding with access to the semantic lexicon | ||||
| RAN obj b/w rct | 22.33 (3.12) | 28.80 (7.87) | t (24.59) = −3.43 | p < 0.017 |
| RAN b/w obj error | 0.05 (0.22) | 0.25 (0.55) | t (24.60) = −1.53 | p = 1.000 |
| RAN colour obj rct | 26.33 (5.56) | 33.60 (11.62) | t (26.98) = −2.53 | p = 0.187 |
| RAN colour obj error | 0.05 (0.22) | 0.25 (0.72) | t (22.34) = −1.21 | p = 1.000 |
| RAN letter rct | 25.67 (5.28) | 34.50 (13.72) | t (24.27) = −2.70 | p = 0.119 |
| RAN letter error | 0.62 (0.97) | 1.30 (1.42) | t (33.48) = −1.78 | p = 1.000 |
| NT | DYSL | Statistics | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| n | 21 | 20 | ||
| Single tone rct | 570.79 (72.38) | 481.09 (97.02) | t (35.11) = 3.34 | p < 0.01 |
| Tone sequence rct | 1043.84 (330.72) | 1050.56 (393.38) | t (37.18) = −0.06 | p = 1.000 |
| Syllable rct | 607.54 (83.11) | 538.63 (130.97) | t (31.91) = 2.00 | p = 0.270 |
| Single tone error | 6.67 (5.97) | 16.35 (7.82) | t (35.55) = −4.44 | p < 0.001 |
| Tone sequence error | 11.95 (15.23) | 46.60 (25.27) | t (30.90) = −5.29 | p < 0.001 |
| Syllable errors | 3.76 (4.76) | 9.20 (7.14) | t (32.90) = −2.85 | p < 0.001 |
| NT | Dysl | Statistics | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| n | 21 | 20 | ||
| Magnocellular System | ||||
| CM rct | 742.78 (135.60) | 709.09 (99.37) | t (36.50) = 0.92 | p = 1.000 |
| CM error | 19.76 (28.53) | 24.249 (24.28) | t (37.87) = −0.55 | p = 1.000 |
| MG rct | 717.49 (125.29) | 721.99 (77.87) | t (33.68) = −0.14 | p = 1.000 |
| MG error | 23.24 (2.79) | 24.659 (4.64) | t (30.88) = −1.17 | p = 1.000 |
| Parvocellular System | ||||
| CC rct | 571.51 (55.29) | 577.19 (54.95) | t (37.65) = −0.33 | p = 1.000 |
| CC error | 0.00 (0.00) | 0.09 (0.23) | t (18.00) = −1.20 | p = 1.000 |
| PS rct | 739.29 (536.87) | 668.99 (240.96) | t (28.34) = 0.55 | p = 1.000 |
| PS error | 2.00 (2.12) | 5.39 (3.83) | t (27.46) = −3.46 | p < 0.006 |
| StG rct | 622.29 (126.91) | 622.29 (95.46) | t (37.06) = 0.00 | p = 1.000 |
| StG error | 0.86 (3.05) | 0.59 (1.47) | t (29.08) = 0.41 | p = 1.000 |
| Other Visual Control Tasks | ||||
| Ro rct | 1464.30 (491.18) | 1404.9 (409.74) | t (38.35) = 0.42 | p = 1.000 |
| Ro error | 11.71 (5.17) | 13.709 (4.16) | t (37.96) = −1.36 | p = 1.000 |
| NT | DYSL | Statistics | p-Value | |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| n | 21 | 20 | ||
| Tapping | 45.57 (8.15) | 51.75 (10.24) | t (36.28) = −2.13 | p = 0.064 |
| Statue | 29.24 (1.61) | 28.55 (1.85) | t (37.68) = 1.27 | p = 0.406 |
| Regression Coefficient B | Standard Error | t | p | 95.0% Confidence Interval for B | ||
|---|---|---|---|---|---|---|
| Upper | Lower | |||||
| Index phon | −8.285 | 2.513 | −3.298 | <0.001 | −13.210 | −3.361 |
| Index PWM | −0.748 | 2.262 | −0.331 | 0.741 | −5.181 | 3.684 |
| Index RAN | −2.026 | 1.466 | −1.382 | 0.167 | −4.900 | 0.848 |
| Index auditory rct | 0.348 | 2.012 | 0.173 | 0.863 | −3.596 | 4.293 |
| Index auditory error | −3.669 | 1.556 | −2.357 | 0.018 | −6.719 | −0.619 |
| Index vis magno total | −2.521 | 2.846 | −0.886 | 0.376 | −8.100 | 3.058 |
| Index vis parvo total | −0.857 | 2.500 | −0.343 | 0.732 | −5.756 | 4.042 |
| Index cerebellar | 2.735 | 1.825 | 1.498 | 0.134 | −0.843 | 6.312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ligges, C.; Lehmann, T. Multiple Case Studies in German Children with Dyslexia: Characterization of Phonological, Auditory, Visual, and Cerebellar Processing on the Group and Individual Levels. Brain Sci. 2022, 12, 1292. https://doi.org/10.3390/brainsci12101292
Ligges C, Lehmann T. Multiple Case Studies in German Children with Dyslexia: Characterization of Phonological, Auditory, Visual, and Cerebellar Processing on the Group and Individual Levels. Brain Sciences. 2022; 12(10):1292. https://doi.org/10.3390/brainsci12101292
Chicago/Turabian StyleLigges, Carolin, and Thomas Lehmann. 2022. "Multiple Case Studies in German Children with Dyslexia: Characterization of Phonological, Auditory, Visual, and Cerebellar Processing on the Group and Individual Levels" Brain Sciences 12, no. 10: 1292. https://doi.org/10.3390/brainsci12101292





