Transcutaneous Vagus Nerve Stimulation Could Improve the Effective Rate on the Quality of Sleep in the Treatment of Primary Insomnia: A Randomized Control Trial
Abstract
:1. Introduction
2. Methods and Materials
2.1. Study Design
2.2. Patients
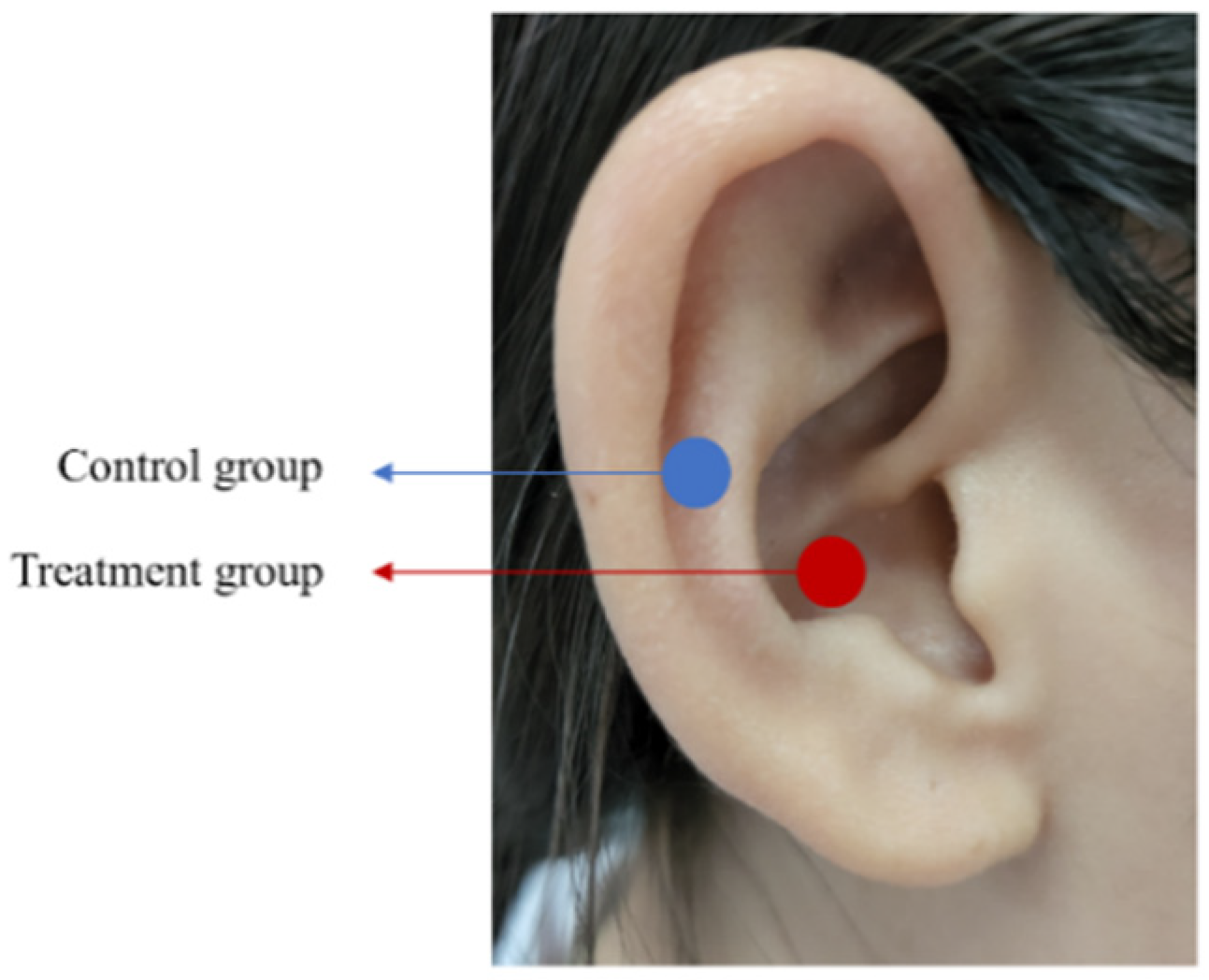
2.3. Interventions
2.4. Outcomes
2.5. Estimation of Sample Size
2.6. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, A.B.; Dzierzewski, J.M.; Griffin, S.C.; Lind, M.J.; Dick, D.; Rybarczyk, B.D. Insomnia Disorder and Behaviorally Induced Insufficient Sleep Syndrome: Prevalence and Relationship to Depression in College Students. Behav. Sleep Med. 2020, 18, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Luo, X.-N.; Li, H.-Y.; Ke, X.-Y.; Dai, Q.; Zhang, C.-J.; Ng, C.H.; Ungvari, G.S.; Xiang, Y.-T.; Ning, Y.-P. Prevalence of insomnia symptoms and their associated factors in patients treated in outpatient clinics of four general hospitals in Guangzhou, China. BMC Psychiatry 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.E.; Al-Jahdali, H.; Fatani, A.; Al-Rouqi, K.; Al-Jahdali, F.; Al-Harbi, A.; Baharoon, S.; Ali, Y.Z.; Khan, M.; Rumayyan, A. The effects of age and gender on the prevalence of insomnia in a sample of the Saudi population. Ethn. Health 2017, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-H.; Kim, B.-S.; Kim, S.-K.; Chang, S.-M.; Lee, D.-W.; Cho, M.-J.; Bae, J.-N. Prevalence of insomnia and associated factors in a community sample of elderly individuals in South Korea. Int. Psychogeriatr. 2013, 25, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Medina-Chávez, J.H.; Fuentes-Alexandro, S.A.; Gil-Palafox, I.B.; Adame-Galván, L.; Solís-Lam, F.; Sánchez-Herrera, L.Y.; Sánchez-Narváez, F. Clinical practice guideline. Diagnosis and treatment of insomnia in the elderly. Rev. Med. Inst. Mex. Seguro Soc. 2014, 52, 108–119. [Google Scholar]
- Winkelman, J.W. CLINICAL PRACTICE. Insomnia Disorder. N. Engl. J. Med. 2015, 373, 1437–1444. [Google Scholar] [CrossRef]
- Ge, L.; Guyatt, G.; Tian, J.; Pan, B.; Chang, Y.; Chen, Y.; Li, H.; Zhang, J.; Li, Y.; Ling, J.; et al. Insomnia and risk of mortality from all-cause, cardiovascular disease, and cancer: Systematic review and meta-analysis of prospective cohort studies. Sleep Med. Rev. 2019, 48, 101215. [Google Scholar] [CrossRef]
- Sen, A.; Opdahl, S.; Strand, L.B.; Vatten, L.J.; Laugsand, L.E.; Janszky, I. Insomnia and the Risk of Breast Cancer: The HUNT Study. Psychosom. Med. 2017, 79, 461–468. [Google Scholar] [CrossRef]
- Sateia, M.J.; Buysse, D.J.; Krystal, A.D.; Neubauer, D.N.; Heald, J.L. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 307–349. [Google Scholar] [CrossRef]
- He, W.; Li, M.; Zuo, L.; Wang, M.; Jiang, L.; Shan, H.; Han, X.; Yang, K.; Han, X. Acupuncture for treatment of insomnia: An overview of systematic reviews. Complement. Ther. Med. 2019, 42, 407–416. [Google Scholar] [CrossRef]
- Haynes, J.; Talbert, M.; Fox, S.; Close, E. Cognitive Behavioral Therapy in the Treatment of Insomnia. South Med. J. 2018, 111, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Choi, J.-W.; Lee, J.; Shin, A.; Oh, S.M.; Jung, S.J.; Lee, Y.J. Trends in prescriptions for sedative-hypnotics among Korean adults: A nationwide prescription database study for 2011–2015. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S. Benzodiazepines. Curr. Top. Behav. Neurosci. 2017, 34, 141–159. [Google Scholar] [PubMed]
- Fine, L. Pharmacologic Approach to Insomnia. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 255–264. [Google Scholar] [CrossRef]
- Yuan, H.; Silberstein, S.D. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache 2016, 56, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ramani, R. Vagus nerve stimulation therapy for seizures. J. Neurosurg. Anesthesiol. 2008, 20, 29–35. [Google Scholar] [CrossRef]
- Aaronson, S.T.; Conway, C.R. Vagus Nerve Stimulation: Changing the Paradigm for Chronic Severe Depression? Psychiatr. Clin. N. Am. 2018, 41, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Guo, X.; Luo, M.; Li, S.; Liu, A.; Zhao, Y.; Zhao, B.; Wang, D.; Li, Z.; Zheng, X.; et al. Effect of Transcutaneous Vagus Nerve Stimulation at Auricular Concha for Insomnia: A Randomized Clinical Trial. Evid.-Based Complement. Altern. Med. 2020, 2020, 6049891. [Google Scholar]
- Beckelhymer, L.M.; Fink, D.S.; Litts, J.K. Behavioral Management of Laryngeal Complaints Caused by Vagal Nerve Stimulation for Medically Refractory Epilepsy. J. Voice 2019, 35, 651–654. [Google Scholar] [CrossRef]
- Selner, A.N.; Rosinski, C.L.; Chiu, R.G.; Rosenberg, D.; Chaker, A.N.; Drammeh, H.; Esfahani, D.R.; Mehta, A.I. Vagal Nerve Stimulation for Epilepsy in Adults: A Database Risk Analysis and Review of the Literature. World Neurosurg. 2019, 121, e947–e953. [Google Scholar] [CrossRef]
- Salvadé, A.; Ryvlin, P.; Rossetti, A.O. Impact of vagus nerve stimulation on sleep-related breathing disorders in adults with epilepsy. Epilepsy Behav. 2018, 79, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Bauer, S. Lessons learned from transcutaneous vagus nerve stimulation (tVNS). Epilepsy Res. 2019, 153, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.; Day, D.; Leung, H.; Laud, P.; Ali, A.; Lindert, R.; Majid, A. Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans; a systematic review. Brain Stimul. 2018, 11, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Baier, H.; Baumgartner, C.; Bohlmann, K.; Fauser, S.; Graf, W.; Hillenbrand, B.; Hirsch, M.; Last, C.; Lerche, H.; et al. Transcutaneous Vagus Nerve Stimulation (tVNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial (cMPsE02). Brain Stimul. 2016, 9, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Mtui, E.; Gruener, G.; Dockery, P. Fitzgerald’s Clinical Neuroanatomy and Neuroscience E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Bretherton, B.; Atkinson, L.; Murray, A.; Clancy, J.; Deuchars, S.; Deuchars, J. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: Potential benefits of daily stimulation. Aging 2019, 11, 4836–4857. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.V.H.F.D.; Francisco, A.N.Z.D., Jr.; Stebel, S.L. The role of vagus nerve stimulation in refractory epilepsy. Arq. Neuro-Psiquiatr. 2017, 75, 657–666. [Google Scholar] [CrossRef]
- Fang, J.; Rong, P.; Hong, Y.; Fan, Y.; Liu, J.; Wang, H.; Zhang, G.; Chen, X.; Shi, S.; Wang, L.; et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 2016, 79, 266–273. [Google Scholar] [CrossRef]
- De Lartigue, G. Role of the vagus nerve in the development and treatment of diet-induced obesity. J. Physiol. 2016, 594, 5791–5815. [Google Scholar] [CrossRef]
- Luo, M.; Qu, X.; Li, S.; Zhao, J.; Zhao, Y.; Jiao, Y.; Rong, P. Transcutaneous vagus nerve stimulation for primary insomnia and affective disorder: A report of 35 cases. Zhongguo Zhen Jiu 2017, 37, 269–273. [Google Scholar]
- Galli, R.; Bonanni, E.; Pizzanelli, C.; Maestri, M.; Lutzemberger, L.; Giorgi, F.S.; Iudice, A.; Murri, L. Daytime vigilance and quality of life in epileptic patients treated with vagus nerve stimulation. Epilepsy Behav. 2003, 4, 185–191. [Google Scholar] [CrossRef]
- Shekleton, J.A.; Flynn-Evans, E.E.; Miller, B.; Epstein, L.J.; Kirsch, D.; Brogna, L.A.; Burke, L.M.; Bremer, E.; Murray, J.M.; Gehrman, P.; et al. Neurobehavioral performance impairment in insomnia: Relationships with self-reported sleep and daytime functioning. Sleep 2014, 37, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, S.; Majoie, M.; van der Heijden, M.; Rijkers, K.; Leenen, L.; Aldenkamp, A. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clin. Neurol. Neurosurg. 2012, 114, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Zhao, Y.; Wang, H.; Vangel, M.; Sun, S.; et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect. Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E. Hamilton Rating Scale for Anxiety (HAM-A). Occup. Med. 2015, 65, 601. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Rohan, K.J.; Rough, J.N.; Evans, M.; Ho, S.-Y.; Meyerhoff, J.; Roberts, L.M.; Vacek, P.M. A protocol for the Hamilton Rating Scale for Depression: Item scoring rules, Rater training, and outcome accuracy with data on its application in a clinical trial. J. Affect. Disord. 2016, 200, 111–118. [Google Scholar] [CrossRef] [Green Version]


| Treatment Group (n = 15) | Control Group (n = 15) | p | |
|---|---|---|---|
| Gender | |||
| Female | 13 | 11 | 0.651 |
| Age (mean ± SD) | 45.9 ± 15.4 | 47.1 ± 13.2 | 0.687 |
| Course of disease (months) | 52.5 ± 38.6 | 59.1 ± 43.5 | 0.253 |
| Family history | 1 | 3 | 0.598 |
| Baseline PSQI (median, IQR) | 14 (9–18) | 12 (8–16) | 0.791 |
| Baseline HAMD (mean ± SD) | 13.4 ± 3.5 | 15.6 ± 6.2 | 0.458 |
| Baseline HAMA (mean ± SD) | 15.9 ± 4.7 | 12.1 ± 5.3 | 0.443 |
| Baseline | Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|---|
| Treatment group | |||||
| PSQI (median, IQR) | 14 (9–18) | 11 (7–13) | 8 (5–11) | 8 (4–10.5) | 6 (3–9.5) |
| Z | 1 (referent) | −2.045 | −3.303 | −3.416 | −3.353 |
| P | 0.041 | 0.001 | 0.001 | 0.001 | |
| Control group | |||||
| PSQI (median, IQR) | 12 (8–16) | 11 (6–13) | 9 (5–12) | 9 (5–12.5) | 7 (4–11) |
| Z | 1 (referent) | −0.709 | −1.866 | −2.166 | −2.269 |
| P | 0.478 | 0.062 | 0.030 | 0.023 |
| Treatment Group (n = 15) | Control Group (n = 15) | p | |
|---|---|---|---|
| PSQI (median) | |||
| Baseline | 14 (9–18) | 12 (8–16) | 0.791 |
| Week 1 | 11 (7–13) | 11 (6–13) | 0.982 |
| Week 2 | 8 (5–11) | 9 (5–12) | 0.652 |
| Week 3 | 8 (4–10.5) | 9 (5–12.5) | 0.534 |
| Week 4 | 6 (3–9.5) | 7 (4–11) | 0.422 |
| Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|
| Response rate | ||||
| Treatment group | 9 (9/15.60%) | 13 (13/15.87%) | 14 (14/15.93%) | 14 (14/15.93%) |
| Control group | 5 (5/15.33%) | 10 (10/15.67%) | 11 (11/15.73%) | 11 (11/15.73%) |
| p value | 0.143 | 0.390 | 0.330 | 0.330 |
| Effective rate | ||||
| Treatment group | 2 (2/15.13%) | 3 (3/15.20%) | 5 (5/15.33%) | 11 (11/15.73%) |
| Control group | 0% | 1 (1/15.7%) | 2 (2/15.13%) | 4 (4/15.27%) |
| p value | NA | 0.589 | 0.390 | 0.027 |
| Treatment Group (n = 15) | Control Group (n = 15) | p | |
|---|---|---|---|
| HAMD | |||
| Final (mean ± SD) | 8.2 ± 4.4 | 7.5 ± 3.0 | |
| Change | −5.3 | −6.5 | 0.324 |
| HAMA | |||
| Final (mean ± SD) | 6.5 ± 5.0 | 6.1 ± 4.0 | |
| Change | −3.1 | −3.2 | 0.809 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Song, L.; Wang, X.; Li, N.; Zhan, S.; Rong, P.; Wang, Y.; Liu, A. Transcutaneous Vagus Nerve Stimulation Could Improve the Effective Rate on the Quality of Sleep in the Treatment of Primary Insomnia: A Randomized Control Trial. Brain Sci. 2022, 12, 1296. https://doi.org/10.3390/brainsci12101296
Wu Y, Song L, Wang X, Li N, Zhan S, Rong P, Wang Y, Liu A. Transcutaneous Vagus Nerve Stimulation Could Improve the Effective Rate on the Quality of Sleep in the Treatment of Primary Insomnia: A Randomized Control Trial. Brain Sciences. 2022; 12(10):1296. https://doi.org/10.3390/brainsci12101296
Chicago/Turabian StyleWu, Yating, Lu Song, Xian Wang, Ning Li, Shuqin Zhan, Peijing Rong, Yuping Wang, and Aihua Liu. 2022. "Transcutaneous Vagus Nerve Stimulation Could Improve the Effective Rate on the Quality of Sleep in the Treatment of Primary Insomnia: A Randomized Control Trial" Brain Sciences 12, no. 10: 1296. https://doi.org/10.3390/brainsci12101296






