Effects of Parental Internalizing and Externalizing Behavior Problems on Children’s Limbic Brain Structures—An MRI Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kessler, R.C.; McLaughlin, K.A.; Green, J.G.; Gruber, M.J.; Sampson, N.A.; Zaslavsky, A.M.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.O.; Alonso, J.; Angermeyer, M.; et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry 2010, 197, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Everett, Y.; Martin, C.G.; Zalewski, M. A Systematic Review Focusing on Psychotherapeutic Interventions that Impact Parental Psychopathology, Child Psychopathology and Parenting Behavior. Clin. Child Fam. Psychol. Rev. 2021, 24, 579–598. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.Z.; Oppenheimer, C.W.; Ladouceur, C.D.; Butterfield, R.D.; Silk, J.S. A review of associations between parental emotion socialization behaviors and the neural substrates of emotional reactivity and regulation in youth. Dev. Psychol. 2020, 56, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, S.; Bray, S.; McGirr, A.; Lee, K.; Kopala-Sibley, D.C. A Narrative Review of Methodological Considerations in Magnetic Resonance Imaging of Offspring Brain Development and the Influence of Parenting. Front. Hum. Neurosci. 2021, 15, 694845. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry 2016, 57, 241–266. [Google Scholar] [CrossRef]
- Caspi, A.; Moffitt, T. All for One and One for All: Mental Disorders in One Dimension. Am. J. Psychiatry 2018, 175, 831–844. [Google Scholar] [CrossRef]
- Pagliaccio, D.; Alqueza, K.L.; Marsh, R.; Auerbach, R.P. Brain Volume Abnormalities in Youth at High Risk for Depression: Adolescent Brain and Cognitive Development Study. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 1178–1188. [Google Scholar] [CrossRef]
- Meyer, A.; Weinberg, A.; Klein, D.N.; Hajcak, G. The development of the error-related negativity (ERN) and its relationship with anxiety: Evidence from 8 to 13 year-olds. Dev. Cogn. Neurosci. 2012, 2, 152–161. [Google Scholar] [CrossRef]
- Suffren, S.; La Buissonnière-Ariza, V.; Tucholka, A.; Nassim, M.; Séguin, J.R.; Boivin, M.; Singh, M.K.; Foland-Ross, L.C.; Lepore, F.; Gotlib, I.H.; et al. Prefrontal cortex and amygdala anatomy in youth with persistent levels of harsh parenting practices and subclinical anxiety symptoms over time during childhood. Dev. Psychopathol. 2021, 34, 957–968. [Google Scholar] [CrossRef]
- Whittle, S.; Vijayakumar, N.; Dennison, M.; Schwartz, O.; Simmons, J.G.; Sheeber, L.; Allen, N.B. Observed Measures of Negative Parenting Predict Brain Development during Adolescence. PLoS ONE 2016, 11, e0147774. [Google Scholar] [CrossRef]
- Dannlowski, U.; Stuhrmann, A.; Beutelmann, V.; Zwanzger, P.; Lenzen, T.; Grotegerd, D.; Domschke, K.; Hohoff, C.; Ohrmann, P.; Bauer, J.; et al. Limbic Scars: Long-Term Consequences of Childhood Maltreatment Revealed by Functional and Structural Magnetic Resonance Imaging. Biol. Psychiatry 2012, 71, 286–293. [Google Scholar] [CrossRef]
- Bush, N.R.; Wakschlag, L.S.; LeWinn, K.Z.; Hertz-Picciotto, I.; Nozadi, S.S.; Pieper, S.; Lewis, J.; Biezonski, D.; Blair, C.; Deardorff, J.; et al. Family Environment, Neurodevelopmental Risk, and the Environmental Influences on Child Health Outcomes (ECHO) Initiative: Looking Back and Moving Forward. Front. Psychiatry 2020, 11, 547. [Google Scholar] [CrossRef]
- Belsky, J.; de Haan, M. Annual Research Review: Parenting and children’s brain development: The end of the beginning. J. Child Psychol. Psychiatry 2011, 52, 409–428. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A.; Anderson, C.M.; Ohashi, K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016, 17, 652–666. [Google Scholar] [CrossRef]
- Bick, J.; Nelson, C.A. Early Adverse Experiences and the Developing Brain. Neuropsychopharmacology 2016, 41, 177–196. [Google Scholar] [CrossRef]
- Askari, M.S.; Rutherford, C.G.; Mauro, P.M.; Kreski, N.T.; Keyes, K.M. Structure and trends of externalizing and internalizing psychiatric symptoms and gender differences among adolescents in the US from 1991 to 2018. Soc. Psychiatry 2021, 57, 737–748. [Google Scholar] [CrossRef]
- Catani, M.; Dell’Acqua, F.; de Schotten, M.T. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013, 37, 1724–1737. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Tamnes, C.K.; Herting, M.M.; Goddings, A.-L.; Meuwese, R.; Blakemore, S.-J.; Dahl, R.E.; Güroğlu, B.; Raznahan, A.; Sowell, E.R.; Crone, E.; et al. Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness. J. Neurosci. 2017, 37, 3402–3412. [Google Scholar] [CrossRef]
- Habel, U.; Klein, M.; Kellermann, T.; Shah, N.J.; Schneider, F. Same or different? Neural correlates of happy and sad mood in healthy males. NeuroImage 2005, 26, 206–214. [Google Scholar] [CrossRef]
- Fish, A.M.; Nadig, A.; Seidlitz, J.; Reardon, P.K.; Mankiw, C.; McDermott, C.L.; Blumenthal, J.D.; Clasen, L.S.; Lalonde, F.; Lerch, J.P.; et al. Sex-biased trajectories of amygdalo-hippocampal morphology change over human development. NeuroImage 2019, 204, 116122. [Google Scholar] [CrossRef]
- Garavan, H.; Bartsch, H.; Conway, K.; Decastro, A.; Goldstein, R.Z.; Heeringa, S.; Jernigan, T.; Potter, A.; Thompson, W.; Zahs, D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018, 32, 16–22. [Google Scholar] [CrossRef]
- Tottenham, N.; Sheridan, M.A. A review of adversity, the amygdala and the hippocampus: A consideration of developmental timing. Front. Hum. Neurosci. 2010, 3, 68. [Google Scholar] [CrossRef]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P.; Kim, D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003, 27, 33–44. [Google Scholar] [CrossRef]
- Lenroot, R.K.; Gogtay, N.; Greenstein, D.K.; Wells, E.M.; Wallace, G.L.; Clasen, L.S.; Blumenthal, J.D.; Lerch, J.; Zijdenbos, A.P.; Evans, A.C.; et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 2007, 36, 1065–1073. [Google Scholar] [CrossRef]
- Barch, D.M.; Albaugh, M.D.; Avenevoli, S.; Chang, L.; Clark, D.B.; Glantz, M.D.; Hudziak, J.J.; Jernigan, T.L.; Tapert, S.F.; Yurgelun-Todd, D.; et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev. Cogn. Neurosci. 2018, 32, 55–66. [Google Scholar] [CrossRef]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Adult Forms & Profiles; BMC: New York, NY, USA, 2003. [Google Scholar]
- Hagler, D.J.; Hatton, S.; Cornejo, M.D.; Makowski, C.; Fair, D.A.; Dick, A.S.; Sutherland, M.T.; Casey, B.; Barch, D.M.; Harms, M.P.; et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage 2019, 202, 116091. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Vijayakumar, N.; Allen, N.B.; Youssef, G.; Yucel, M.; Simmons, J.G.; Whittle, S. Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum. Brain Mapp. 2016, 37, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Tyborowska, A.; Volman, I.; Niermann, H.C.M.; Pouwels, J.L.; Smeekens, S.; Cillessen, A.H.N.; Toni, I.; Roelofs, K. Early-life and pubertal stress differentially modulate grey matter development in human adolescents. Sci. Rep. 2018, 8, 9201. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.A.; Olson, E.A.; Killgore, W.D.; Pizzagalli, D.A.; Rauch, S.L.; Rosso, I.M. Rostral Anterior Cingulate Cortex Morphology Predicts Treatment Response to Internet-Based Cognitive Behavioral Therapy for Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.; Allen, N.; Whittle, S.; Simmons, J.G.; Yücel, M.; Lubman, D.I. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology 2014, 231, 1731–1742. [Google Scholar] [CrossRef]
- Whittle, S.; Yap, M.B.H.; Yücel, M.; Fornito, A.; Simmons, J.G.; Barrett, A.; Sheeber, L.; Allen, N.B. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent–adolescent interactions. Proc. Natl. Acad. Sci. USA 2008, 105, 3652–3657. [Google Scholar] [CrossRef]
- Meyer, A.; Nelson, B.; Perlman, G.; Klein, D.N.; Kotov, R. A neural biomarker, the error-related negativity, predicts the first onset of generalized anxiety disorder in a large sample of adolescent females. J. Child Psychol. Psychiatry 2018, 59, 1162–1170. [Google Scholar] [CrossRef]
- Ducharme, S.; Albaugh, M.D.; Hudziak, J.J.; Botteron, K.N.; Nguyen, T.-V.; Truong, C.; Evans, A.C.; Karama, S.; Ball, W.S.; Byars, A.W.; et al. Anxious/Depressed Symptoms are Linked to Right Ventromedial Prefrontal Cortical Thickness Maturation in Healthy Children and Young Adults. Cereb. Cortex 2014, 24, 2941–2950. [Google Scholar] [CrossRef]
- Luby, J.L.; Agrawal, A.; Belden, A.; Whalen, D.; Tillman, R.; Barch, D.M. Developmental Trajectories of the Orbitofrontal Cortex and Anhedonia in Middle Childhood and Risk for Substance Use in Adolescence in a Longitudinal Sample of Depressed and Healthy Preschoolers. Am. J. Psychiatry 2018, 175, 1010–1021. [Google Scholar] [CrossRef]
- Cheetham, A.; Allen, N.B.; Whittle, S.; Simmons, J.G.; Yücel, M.; Lubman, D.I. Orbitofrontal Volumes in Early Adolescence Predict Initiation of Cannabis Use: A 4-Year Longitudinal and Prospective Study. Biol. Psychiatry 2012, 71, 684–692. [Google Scholar] [CrossRef]
- Subramaniam, P.; Rogowska, J.; DiMuzio, J.; Lopez-Larson, M.; McGlade, E.; Yurgelun-Todd, D. Orbitofrontal connectivity is associated with depression and anxiety in marijuana-using adolescents. J. Affect. Disord. 2018, 239, 234–241. [Google Scholar] [CrossRef]
- Hanson, J.L.; Nacewicz, B.M.; Sutterer, M.J.; Cayo, A.A.; Schaefer, S.M.; Rudolph, K.D.; Shirtcliff, E.A.; Pollak, S.D.; Davidson, R.J. Behavioral Problems After Early Life Stress: Contributions of the Hippocampus and Amygdala. Biol. Psychiatry 2015, 77, 314–323. [Google Scholar] [CrossRef]
- Rao, U.; Chen, L.-A.; Bidesi, A.S.; Shad, M.U.; Thomas, M.A.; Hammen, C.L. Hippocampal Changes Associated with Early-Life Adversity and Vulnerability to Depression. Biol. Psychiatry 2010, 67, 357–364. [Google Scholar] [CrossRef]
- Calem, M.; Bromis, K.; McGuire, P.; Morgan, C.; Kempton, M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. NeuroImage Clin. 2017, 14, 471–479. [Google Scholar] [CrossRef]
- Luby, J.L.; Barch, D.M.; Belden, A.; Gaffrey, M.S.; Tillman, R.; Babb, C.; Nishino, T.; Suzuki, H.; Botteron, K.N. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. USA 2012, 109, 2854–2859. [Google Scholar] [CrossRef]
- Whittle, S.; Vijayakumar, N.; Simmons, J.G.; Dennison, M.; Schwartz, O.; Pantelis, C.; Sheeber, L.; Byrne, M.L.; Allen, N.B. Role of Positive Parenting in the Association between Neighborhood Social Disadvantage and Brain Development Across Adolescence. JAMA Psychiatry 2017, 74, 824–832. [Google Scholar] [CrossRef]
- Moreno-López, L.; Ioannidis, K.; Askelund, A.D.; Smith, A.J.; Schueler, K.; van Harmelen, A.-L. The Resilient Emotional Brain: A Scoping Review of the Medial Prefrontal Cortex and Limbic Structure and Function in Resilient Adults with a History of Childhood Maltreatment. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 5, 392–402. [Google Scholar] [CrossRef]
- Ioannidis, K.; Askelund, A.D.; Kievit, R.A.; Van Harmelen, A.L. The complex neurobiology of resilient functioning after childhood maltreatment. BMC Med. 2020, 18, 32. [Google Scholar] [CrossRef]
- Meyer, G.J.; Finn, S.E.; Eyde, L.D.; Kay, G.G.; Moreland, K.L.; Dies, R.R.; Eisman, E.J.; Kubiszyn, T.W. Psychological testing and psychological assessment: A review of evidence and issues. Am. Psychol. 2001, 56, 128–165. [Google Scholar] [CrossRef]
- Schoemaker, D.; Buss, C.; Head, K.; Sandman, C.A.; Davis, E.P.; Chakravarty, M.M.; Gauthier, S.; Pruessner, J.C. Hippocampus and amygdala volumes from magnetic resonance images in children: Assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage 2016, 129, 1–14. [Google Scholar] [CrossRef]
- Ahmed, S.P.; Bittencourt-Hewitt, A.; Sebastian, C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015, 15, 11–25. [Google Scholar] [CrossRef]

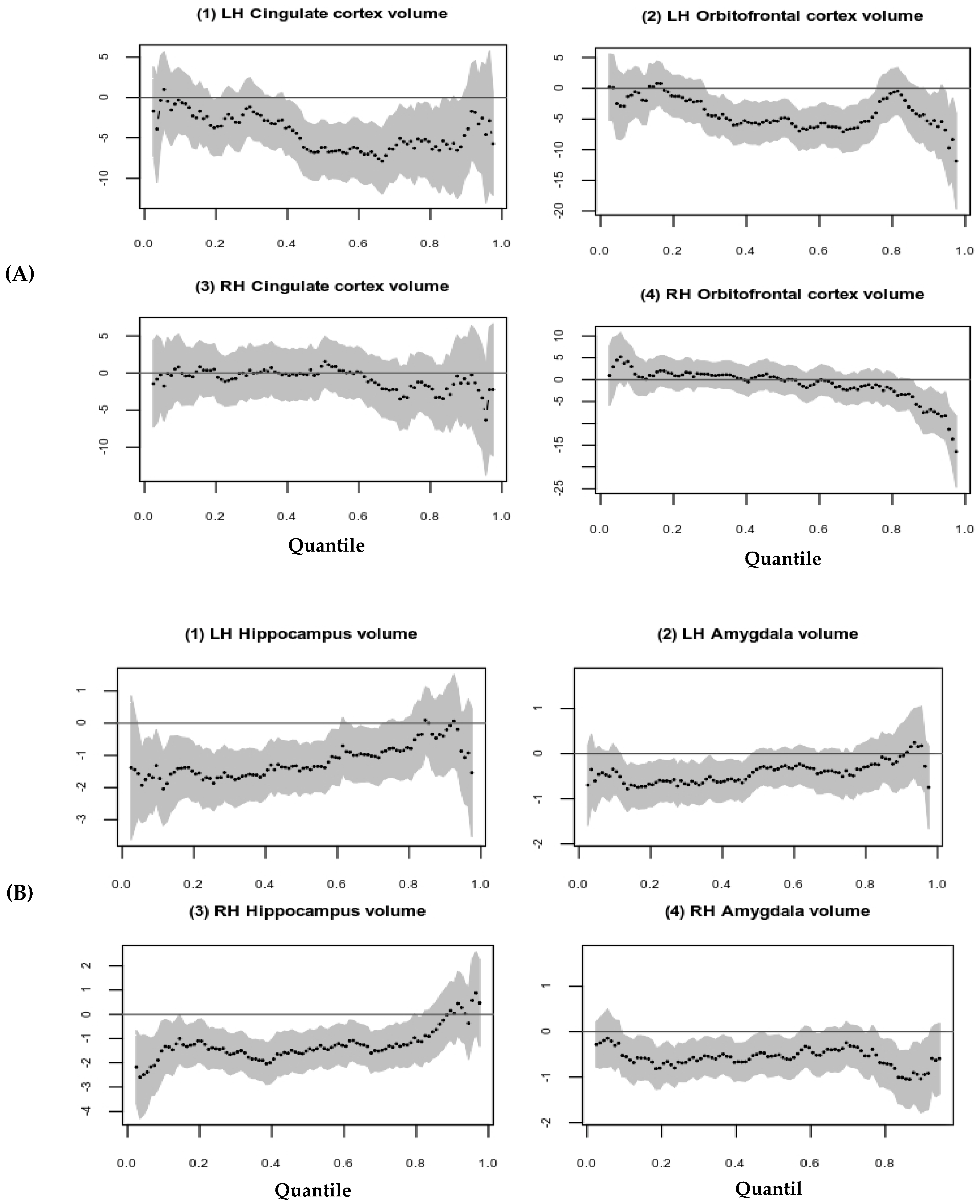
| Variables | Mean (SD) | Median [Q1, Q3] |
|---|---|---|
| Sample size (n) | 11,878 | |
| Age (Years) | 9.91 (0.625) | 9.92 [9.33, 10.50] |
| Female (%) | 5682 (47.80) | |
| BMI | 19.30 (36.61) | 17.65 [15.94, 20.66] |
| Race (%) | ||
| White | 7525 (64.30) | |
| African American | 1869 (16.00) | |
| Others | 2313 (19.80) | |
| Hispanic (Yes, %) | 2292 (19.80) | |
| Marital status (married/partner, %) | 8569 (73.70) | |
| Parent education (%) | ||
| <HS and HS Diploma | 1956 (16.70) | |
| Some college | 3440 (29.40) | |
| Bachelor’s degree | 3316 (28.40) | |
| Postgraduate degree | 2979 (25.50) | |
| Income (%) | ||
| <$50k | 3131 (29.20) | |
| $50k to <$100k | 3046 (28.40) | |
| $100k to <$200k | 3299 (30.80) | |
| >$200k | 1249 (11.60) |
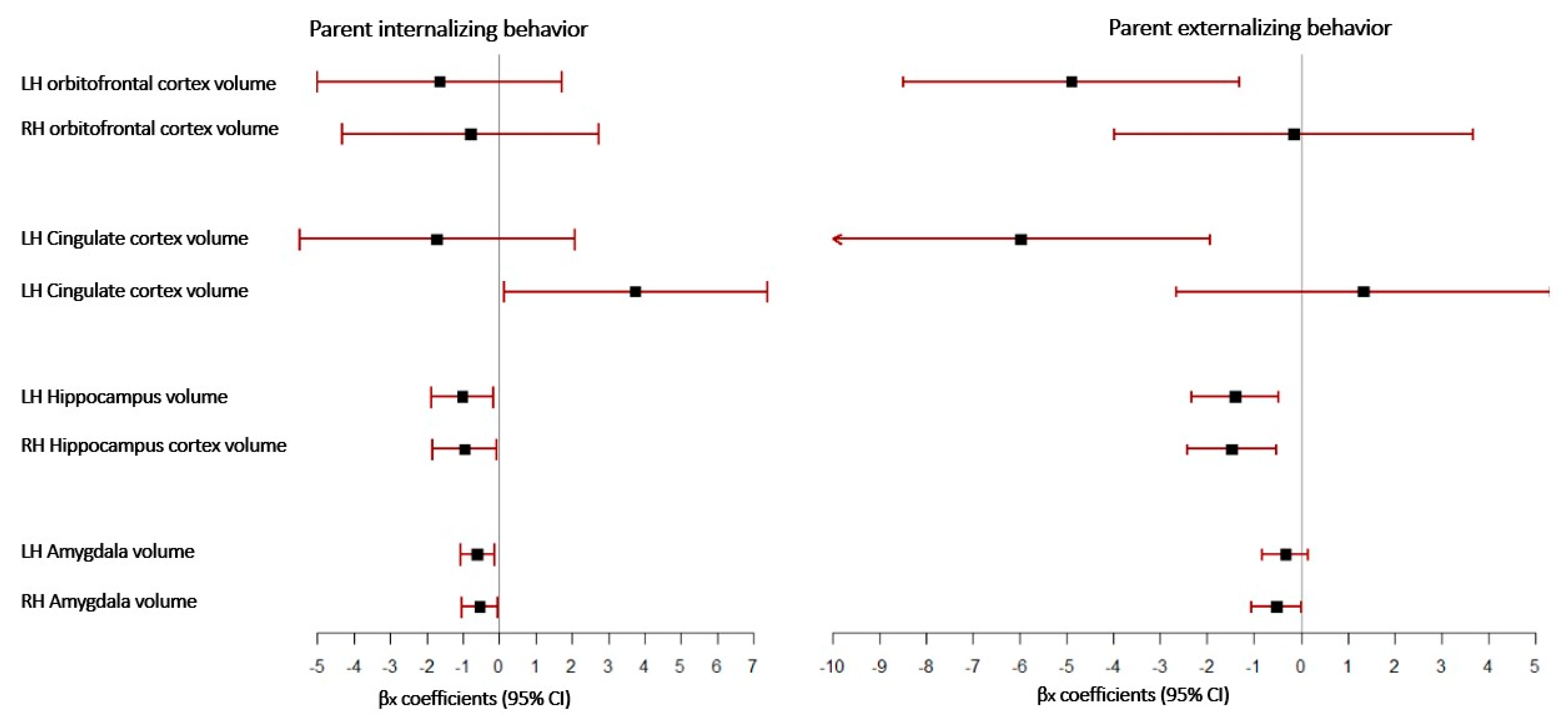
| β | Robust | t | p | 95% CI | ||
|---|---|---|---|---|---|---|
| SE | LL | UL | ||||
| LH Orbitofrontal cortex volume | −1.643 | 1.722 | −0.950 | 0.340 | −5.020 | 1.733 |
| RH Orbitofrontal cortex volume | −0.779 | 1.801 | −0.430 | 0.665 | −4.309 | 2.752 |
| LH Cingulate cortex volume | −1.707 | 1.928 | −0.890 | 0.376 | −5.486 | 2.073 |
| RH Cingulate cortex volume | 3.751 | 1.847 | 2.030 | 0.042 | 0.130 | 7.373 |
| LH Hippocampus volume | −1.008 | 0.439 | −2.290 | 0.022 | −1.869 | −0.147 |
| RH Hippocampus volume | −0.953 | 0.447 | −2.130 | 0.033 | −1.828 | −0.078 |
| LH Amygdala volume | −0.599 | 0.234 | −2.560 | 0.010 | −1.058 | −0.141 |
| RH Amygdala volume | −0.536 | 0.257 | −2.080 | 0.037 | −1.040 | −0.032 |
| β | Robust | t | p | 95% CI | ||
|---|---|---|---|---|---|---|
| SE | LL | UL | ||||
| LH Orbitofrontal cortex volume | −4.899 | 1.829 | −2.68 | 0.007 | −8.484 | −1.313 |
| RH Orbitofrontal cortex volume | −0.156 | 1.955 | −0.08 | 0.936 | −3.989 | 3.676 |
| LH Cingulate cortex volume | −5.989 | 2.07 | −2.89 | 0.004 | −10.046 | −1.931 |
| RH Cingulate cortex volume | 1.329 | 2.037 | 0.65 | 0.514 | −2.663 | 5.322 |
| LH Hippocampus volume | −1.413 | 0.473 | −2.99 | 0.003 | −2.341 | −0.486 |
| RH Hippocampus volume | −1.478 | 0.486 | −3.04 | 0.002 | −2.43 | −0.526 |
| LH Amygdala volume | −0.329 | 0.247 | −1.33 | 0.183 | −0.814 | 0.155 |
| RH Amygdala volume | −0.517 | 0.274 | −1.89 | 0.059 | −1.054 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albar, Z.; Sattar, A. Effects of Parental Internalizing and Externalizing Behavior Problems on Children’s Limbic Brain Structures—An MRI Study. Brain Sci. 2022, 12, 1319. https://doi.org/10.3390/brainsci12101319
Albar Z, Sattar A. Effects of Parental Internalizing and Externalizing Behavior Problems on Children’s Limbic Brain Structures—An MRI Study. Brain Sciences. 2022; 12(10):1319. https://doi.org/10.3390/brainsci12101319
Chicago/Turabian StyleAlbar, Zainab, and Abdus Sattar. 2022. "Effects of Parental Internalizing and Externalizing Behavior Problems on Children’s Limbic Brain Structures—An MRI Study" Brain Sciences 12, no. 10: 1319. https://doi.org/10.3390/brainsci12101319
APA StyleAlbar, Z., & Sattar, A. (2022). Effects of Parental Internalizing and Externalizing Behavior Problems on Children’s Limbic Brain Structures—An MRI Study. Brain Sciences, 12(10), 1319. https://doi.org/10.3390/brainsci12101319






