Profiles of Caregiver-Reported Executive Function in Children with Down Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Measures
2.3.1. Developmental Status
2.3.2. Medical History Questionnaire
2.4. Analytic Approach
2.5. Auxiliary Testing
3. Results
Auxiliary Testing Results
4. Discussion
4.1. Implications
4.2. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laureys, F.; De Waelle, S.; Barendse, M.T.; Lenoir, M.; Deconinck, F.J. The factor structure of executive function in childhood and adolescence. Intelligence 2022, 90, 101600. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendry, A.; Jones, E.J.; Charman, T. Executive function in the first three years of life: Precursors, predictors and patterns. Dev. Rev. 2016, 42, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Schworer, E.; Fidler, D.J.; Kaur, M.; Needham, A.; Prince, M.A.; Daunhauer, L.A. Infant precursors of executive function in Down syndrome. J. Intellect. Disabil. Res. 2022, 66, 108–120. [Google Scholar] [CrossRef]
- Nigg, J.T. Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 2017, 58, 361–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelazo, P.D. Executive function and psychopathology: A neurodevelopmental perspective. Annu. Rev. Clin. Psychol. 2020, 16, 431–454. [Google Scholar] [CrossRef] [Green Version]
- Ozonoff, S.; Jensen, J. Brief Report: Specific Executive Function Profiles in Three Neurodevelopmental Disorders. J. Autism Dev. Disord. 1999, 29, 171–177. [Google Scholar] [CrossRef]
- Fidler, D.; Lanfranchi, S. Executive function and intellectual disability: Innovations, methods and treatment. J. Intellect. Disabil. Res. 2022, 66, 1–8. [Google Scholar] [CrossRef]
- Daunhauer, L.A.; Fidler, D.J.; Hahn, L.; Will, E.; Lee, N.R.; Hepburn, S. Profiles of everyday executive functioning in young children with Down syndrome. Am. J. Intellect. Dev. Disabil. 2014, 119, 303–318. [Google Scholar] [CrossRef] [Green Version]
- Loveall, S.; Conners, F.; Tungate, A.; Hahn, L.; Osso, T. A cross-sectional analysis of executive function in Down syndrome from 2 to 35 years. J. Intellect. Disabil. Res. 2017, 61, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Presson, A.P.; Partyka, G.; Jensen, K.M.; Devine, O.J.; Rasmussen, S.A.; McCabe, L.L.; McCabe, E.R. Current estimate of Down syndrome population prevalence in the United States. J. Pediatr. 2013, 163, 1163–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onnivello, S.; Colaianni, S.; Pulina, F.; Locatelli, C.; Marcolin, C.; Ramacieri, G.; Antonaros, F.; Vione, B.; Piovesan, A.; Lanfranchi, S. Executive functions and adaptive behaviour in individuals with Down syndrome. J. Intellect. Disabil. Res. 2022, 66, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Tungate, A.S.; Conners, F.A. Executive function in Down syndrome: A meta-analysis. Res. Dev. Disabil. 2021, 108, 103802. [Google Scholar] [CrossRef]
- Daunhauer, L.A.; Gerlach-McDonald, B.; Will, E.; Fidler, D.J. Performance and ratings based measures of executive function in school-aged children with Down syndrome. Dev. Neuropsychol. 2017, 42, 351–368. [Google Scholar] [CrossRef]
- Lee, N.R.; Fidler, D.J.; Blakeley-Smith, A.; Daunhauer, L.; Robinson, C.; Hepburn, S.L. Caregiver report of executive functioning in a population-based sample of young children with Down syndrome. Am. J. Intellect. Dev. Disabil. 2011, 116, 290–304. [Google Scholar] [CrossRef] [Green Version]
- Will, E.A.; Schworer, E.K.; Esbensen, A.J. The role of distinct executive functions on adaptive behavior in children and adolescents with Down syndrome. Child Neuropsychol. 2021, 27, 1054–1072. [Google Scholar] [CrossRef]
- Fidler, D.J.; Hepburn, S.; Mankin, G.; Rogers, S.J. Praxis Skills in Young Children With Down Syndrome, Other Developmental Disabilities, and Typically Developing Children. Am. J. Occup. Ther. 2005, 59, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Schworer, E.; Fidler, D.J.; Kaur, M.; Needham, A.; Prince, M.; Daunhauer, L. Goal-directed action planning in infants with Down syndrome. J. Intellect. Disabil. Res. 2020, 64, 713–724. [Google Scholar] [CrossRef]
- Tomaszewski, B.; Fidler, D.J.; Talapatra, D.; Riley, K. Adaptive behaviour, executive function and employment in adults with down syndrome. J. Intellect. Disabil. Res. 2018, 62, 41–52. [Google Scholar] [CrossRef]
- Esbensen, A.J.; Epstein, J.N.; Vincent, L.B.; Kamimura-Nishimura, K.; Wiley, S.; Angkustsiri, K.; Abbeduto, L.; Fidler, D.; Froehlich, T.E. Comparison of Attention-Deficit Hyperactivity Disorder in Typically Developing Children and Children with Down Syndrome. J. Dev. Behav. Pediatr. 2022, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fidler, D.J. The emerging Down syndrome behavioral phenotype in early childhood: Implications for practice. Infants Young Child. 2005, 18, 86–103. [Google Scholar] [CrossRef] [Green Version]
- Prince, M.A.; Fidler, D.J. Analytic approaches to heterogeneity in neurogenetic syndrome research. In International Review of Research in Developmental Disabilities; Elsevier: Amsterdam, The Netherlands, 2021; Volume 60, pp. 55–73. [Google Scholar]
- Freeman, S.B.; Taft, L.F.; Dooley, K.J.; Allran, K.; Sherman, S.L.; Hassold, T.J.; Khoury, M.J.; Saker, D.M. Population-based study of congenital heart defects in Down syndrome. Am. J. Med. Genet. 1998, 80, 213–217. [Google Scholar] [CrossRef]
- Torfs, C.P.; Christianson, R.E. Anomalies in Down syndrome individuals in a large population-based registry. Am. J. Med. Genet. 1998, 77, 431–438. [Google Scholar] [CrossRef]
- Visootsak, J.; Mahle, W.T.; Kirshbom, P.M.; Huddleston, L.; Caron-Besch, M.; Ransom, A.; Sherman, S.L. Neurodevelopmental outcomes in children with Down syndrome and congenital heart defects. Am. J. Med. Genetics. Part A 2011, 155, 2688–2691. [Google Scholar] [CrossRef] [Green Version]
- Alsaied, T.; Marino, B.S.; Esbensen, A.J.; Anixt, J.S.; Epstein, J.N.; Cnota, J.F. Does congenital heart disease affect neurodevelopmental outcomes in children with Down syndrome? Congenit. Heart Dis. 2016, 11, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Rosser, T.C.; Edgin, J.O.; Capone, G.T.; Hamilton, D.R.; Allen, E.G.; Dooley, K.J.; Anand, P.; Strang, J.F.; Armour, A.C.; Frank-Crawford, M.A. Associations between medical history, cognition, and behavior in youth with down syndrome: A report from the down syndrome cognition project. Am. J. Intellect. Dev. Disabil. 2018, 123, 514–528. [Google Scholar] [CrossRef]
- Visootsak, J.; Hess, B.; Bakeman, R.; Adamson, L. Effect of congenital heart defects on language development in toddlers with Down syndrome. J. Intellect. Disabil. Res. 2013, 57, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Visootsak, J.; Huddleston, L.; Buterbaugh, A.; Perkins, A.; Sherman, S.; Hunter, J. Influence of CHDs on psycho-social and neurodevelopmental outcomes in children with Down syndrome. Cardiol. Young 2016, 26, 250–256. [Google Scholar] [CrossRef]
- Aoki, S.; Yamauchi, Y.; Hashimoto, K. Developmental trend of children with Down’s syndrome–How do sex and neonatal conditions influence their developmental patterns? Brain Dev. 2018, 40, 181–187. [Google Scholar] [CrossRef]
- Fidler, D.; Schworer, E.; Will, E.; Patel, L.; Daunhauer, L. Correlates of early cognition in infants with Down syndrome. J. Intellect. Disabil. Res. 2018, 63, 205–214. [Google Scholar] [CrossRef] [PubMed]
- del Hoyo Soriano, L.; Rosser, T.; Hamilton, D.; Wood, T.; Abbeduto, L.; Sherman, S. Gestational age is related to symptoms of attention-deficit/hyperactivity disorder in late-preterm to full-term children and adolescents with down syndrome. Sci. Rep. 2020, 10, 20345. [Google Scholar] [CrossRef]
- Gioia, G.A.; Epsy, K.A.; Isquith, P.K. Behavior Rating Inventory of Executive Function—Preschool Version; Psychological Assessment Resources: Odessa, FL, USA, 2003. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roid, G. Stanford-Binet Intelligence Scales, 5th ed.; Riverside: Itasca, IL, USA, 2003. [Google Scholar]
- Roid, G. Technical Manual. 5; Riverside Publishing: Itasca, IL, USA, 2003. [Google Scholar]
- Sherman, E.M.; Brooks, B.L. Behavior rating inventory of executive function–preschool version (BRIEF-P): Test review and clinical guidelines for use. Child Neuropsychol. 2010, 16, 503–519. [Google Scholar] [CrossRef]
- Berlin, K.S.; Parra, G.R.; Williams, N.A. An introduction to latent variable mixture modeling (part 2): Longitudinal latent class growth analysis and growth mixture models. J. Pediatr. Psychol. 2014, 39, 188–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLachlan, G.J.; Lee, S.X.; Rathnayake, S.I. Finite mixture models. Annu. Rev. Stat. Its Appl. 2019, 6, 355–378. [Google Scholar] [CrossRef]
- Peel, D.; MacLahlan, G. Finite mixture Models; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide: Statistical Analysis with Latent Variables, 8th ed.; Muthén & Muthén Copyright: Los Angeles, CA, USA, 1998–2017. [Google Scholar]
- Lo, Y.; Mendell, N.R.; Rubin, D.B. Testing the number of components in a normal mixture. Biometrika 2001, 88, 767–778. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Muthén, B.; Muthén, L.K. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol. Clin. Exp. Res. 2000, 24, 882–891. [Google Scholar] [CrossRef]
- Jung, T.; Wickrama, K.A. An introduction to latent class growth analysis and growth mixture modeling. Soc. Personal. Psychol. Compass 2008, 2, 302–317. [Google Scholar] [CrossRef]
- Asparouhov, T.; Muthén, B. Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Struct. Equ. Model. 2014, 21, 329–341. [Google Scholar] [CrossRef]
- Lanza, S.T.; Tan, X.; Bray, B.C. Latent class analysis with distal outcomes: A flexible model-based approach. Struct. Equ. Model. A Multidiscip. J. 2013, 20, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, F.; Varuzza, C.; Menghini, D.; Addona, F.; Gianesini, T.; Vicari, S. Executive functions in intellectual disabilities: A comparison between Williams syndrome and Down syndrome. Res. Dev. Disabil. 2013, 34, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Usai, M.C.; Toffalini, E.; Passolunghi, M.C. Meta-analysis on inhibition from childhood to young adulthood in people with Down syndrome. Res. Dev. Disabil. 2021, 109, 103838. [Google Scholar] [CrossRef]
- Traverso, L.; Fontana, M.; Usai, M.C.; Passolunghi, M.C. Response inhibition and interference suppression in individuals with down syndrome compared to typically developing children. Front. Psychol. 2018, 9, 660. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive function in children and adolescents with critical cyanotic congenital heart disease. J. Int. Neuropsychol. Soc. 2015, 21, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Feldmann, M.; Bataillard, C.; Ehrler, M.; Ullrich, C.; Knirsch, W.; Gosteli-Peter, M.A.; Held, U.; Latal, B. Cognitive and Executive Function in Congenital Heart Disease: A Meta-analysis. Pediatrics 2021, 148, e2021050875. [Google Scholar] [CrossRef]
- Memisevic, H.; Sinanovic, O. Executive function in children with intellectual disability–the effects of sex, level and aetiology of intellectual disability. J. Intellect. Disabil. Res. 2014, 58, 830–837. [Google Scholar] [CrossRef]
- Hodapp, R.M.; Fidler, D.J. Down syndrome. In APA Handbook of Intellectual and Developmental Disabilities: Foundations; Glidden, L.M., Abbeduto, L., McIntyre, L.L., Tassé, M.J., Eds.; American Psychological Association: Washington, DC, USA, 2021; pp. 123–150. [Google Scholar]
- Karmiloff-Smith, A.; Al-Janabi, T.; D’Souza, H.; Groet, J.; Massand, E.; Mok, K.; Startin, C.; Fisher, E.; Hardy, J.; Nizetic, D. The importance of understanding individual differences in Down syndrome. F1000Research 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Fidler, D.J.; Philofsky, A.; Hepburn, S.L.; Rogers, S.J. Nonverbal requesting and problem-solving by toddlers with down syndrome. Am. J. Ment. Retard. AJMR 2005, 110, 312–322. [Google Scholar] [CrossRef] [Green Version]
- DiGuiseppi, C.; Hepburn, S.; Davis, J.M.; Fidler, D.J.; Hartway, S.; Lee, N.R.; Miller, L.; Ruttenber, M.; Robinson, C. Screening for autism spectrum disorders in children with Down syndrome: Population prevalence and screening test characteristics. J. Dev. Behav. Pediatr. 2010, 31, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Channell, M.M.; Mattie, L.J.; Hamilton, D.R.; Capone, G.T.; Mahone, E.M.; Sherman, S.L.; Rosser, T.C.; Reeves, R.H.; Kalb, L.G. Capturing cognitive and behavioral variability among individuals with Down syndrome: A latent profile analysis. J. Neurodev. Disord. 2021, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Malak, R.; Kostiukow, A.; Krawczyk-Wasielewska, A.; Mojs, E.; Samborski, W. Delays in motor development in children with Down syndrome. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 1904. [Google Scholar] [CrossRef] [Green Version]
- Berglund, E.; Eriksson, M.; Johansson, I. Parental reports of spoken language skills in children with Down syndrome. J. Speech Lang. Hear. Res. 2001, 44, 179–191. [Google Scholar] [CrossRef]
- Fidler, D.; Prince, M.; Van Deusen, K.; Esbensen, A.; Thurman, A.; Abbeduto, L.; Patel, L.; Mervis, C.; Schworer, E.; Lee, N. Latent profiles of autism symptoms in children and adolescents with Down syndrome. J. Intellect. Disabil. Res. 2022, 66, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.V.; Richmond, J.L. Preschoolers with Down syndrome do not yet show the learning and memory impairments seen in adults with Down syndrome. Dev. Sci. 2015, 18, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Conrad, Z. Age-equivalent scores. In The SAGE Encyclopedia of Educational Research, Measurement, and Evaluation; Frey, B.B., Ed.; Sage Publications: Thousan Oaks, CA, USA, 2018; p. 62. [Google Scholar]
- Hessl, D.; Nguyen, D.V.; Green, C.; Chavez, A.; Tassone, F.; Hagerman, R.J.; Senturk, D.; Schneider, A.; Lightbody, A.; Reiss, A.L. A solution to limitations of cognitive testing in children with intellectual disabilities: The case of fragile X syndrome. J. Neurodev. Disord. 2009, 1, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Sansone, S.M.; Schneider, A.; Bickel, E.; Berry-Kravis, E.; Prescott, C.; Hessl, D. Improving IQ measurement in intellectual disabilities using true deviation from population norms. J. Neurodev. Disord. 2014, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Abbeduto, L.; Warren, S.F.; Conners, F.A. Language development in Down syndrome: From the prelinguistic period to the acquisition of literacy. Ment. Retard. Dev. Disabil. Res. Rev. 2007, 13, 247–261. [Google Scholar] [CrossRef]
- Lubke, G.; Neale, M.C. Distinguishing between latent classes and continuous factors: Resolution by maximum likelihood? Multivar. Behav. Res. 2006, 41, 499–532. [Google Scholar] [CrossRef]
| Child Characteristics | Mean (SD) or % (n) |
|---|---|
| % Male | 44.9 (31) |
| Child Chronological Age (years) | 6.23 (1.91) |
| Child Mental Age (years) | 2.68 (0.89) |
| Race (n = 1 missing) | |
| Asian-American | 5.90 (4) |
| Black/African American | 2.90 (2) |
| White | 88.2 (60) |
| Other | 2.90 (2) |
| Ethnicity (n = 3 missing) | |
| Hispanic | 13.60 (9) |
| Not Hispanic | 86.40 (57) |
| DS Type (n = 1 missing) | |
| Trisomy 21 | 91.2 (62) |
| Mosaicism | 2.9 (2) |
| Translocation | 2.9 (2) |
| Not Sure | 2.9 (2) |
| Premature Birth (% yes; n = 2 missing) | 23.9 (16) |
| Congenital Heart Defects (% yes) | 63.8 (44) |
| Caregiver Characteristics | |
| Primary Caregiver Age (n = 1 missing) | 41.75 (6.43) |
| % Primary Caregiver Education at least 1 year of college/tech training | 98.6 (68) |
| 1-Class | 2-Class | 3-Class | 4-Class | |
|---|---|---|---|---|
| aBIC | 2683.167 | 2582.681 | 2539.631 | 2511.826 |
| Entropy | − | 0.84 | 0.898 | 0.889 |
| ALC-Probabilities | − | 0.96 to 0.96 | 0.93 to 0.96 | 0.91 to 0.99 |
| LMR Test (value, p-value) | − | 102.941, 0.021 | 47.681, 0.413 | 33.013, 0.348 |
| Number in Most Likely Class Count (Proportion) | ||||
| Class 1 | 69 (100%) | 29 (43%) | 18 (26%) | 5 (7%) |
| Class 2 | 40 (57%) | 8 (12%) | 7 (10%) | |
| Class 3 | 43 (62%) | 30 (43%) | ||
| Class 4 | 27 (39%) | |||
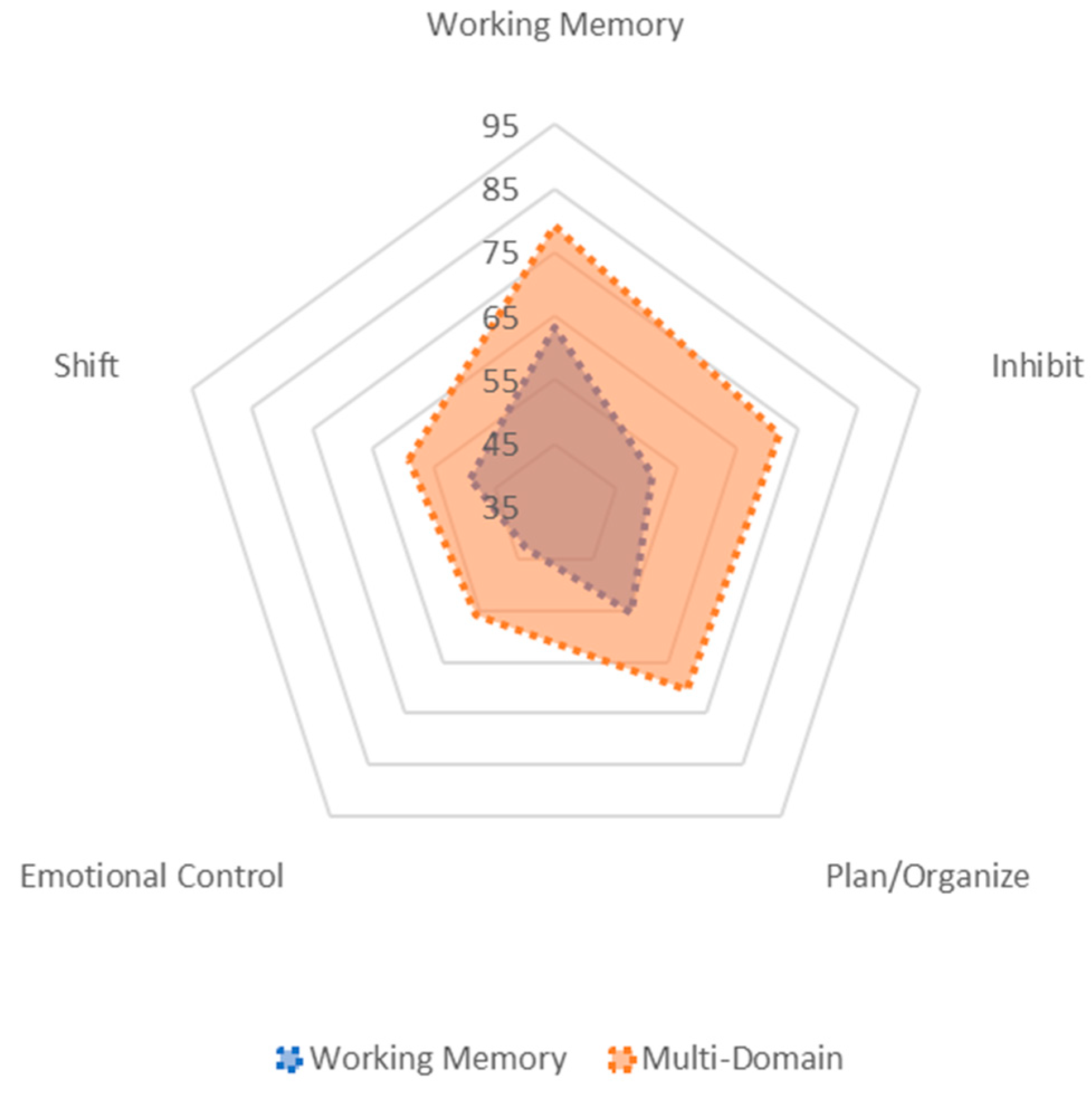
| BRIEF-P Domain Models | ||
|---|---|---|
| Working Memory N = 29 M (SE) | Multi-Domain N = 40 M (SE) | |
| Working Memory | 63.26 (2.66) | 79.11 (2.08) |
| Plan/Organize | 55.41 (2.23) | 70.34 (1.88) |
| Shift | 49.01 (1.94) | 59.09 (1.92) |
| Inhibit | 51.11 (2.26) | 71.75 (2.03) |
| Emotional Control | 43.00 (1.70) | 55.91 (1.62) |
| Auxiliary Testing Mean or Proportion in Each Class | ||
| Chronological Age (Mean in yrs) | 6.77 | 5.83 |
| Sex (proportion) | ||
| Male | 0.56 | 0.36 |
| Female | 0.44 | 0.64 |
| Sensory Composite (Mean) | 0.85 | 1.14 |
| Biomedical Composite (Mean) | 0.92 | 1.14 |
| DS Type (proportion) | ||
| Trisomy 21 | 1.00 | 0.84 |
| Mosaicism | 0 | 0.05 |
| Translocation | 0 | 0.05 |
| Not Sure | 0 | 0.05 |
| Premature Birth (proportion) | ||
| No | 0.80 | 0.71 |
| Yes | 0.20 | 0.26 |
| Do not know | 0 | 0.03 |
| * Congenital Heart Defect (proportion) | ||
| No | 0.52 | 0.24 |
| Yes | 0.48 | 0.76 |
| * Mental Age (MA) Developmental Quotient (MA/CA) | 35.66 0.46 | 29.64 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Deusen, K.; Prince, M.A.; Esbensen, A.J.; Edgin, J.O.; Schworer, E.K.; Thurman, A.J.; Patel, L.R.; Daunhauer, L.A.; Fidler, D.J. Profiles of Caregiver-Reported Executive Function in Children with Down Syndrome. Brain Sci. 2022, 12, 1333. https://doi.org/10.3390/brainsci12101333
Van Deusen K, Prince MA, Esbensen AJ, Edgin JO, Schworer EK, Thurman AJ, Patel LR, Daunhauer LA, Fidler DJ. Profiles of Caregiver-Reported Executive Function in Children with Down Syndrome. Brain Sciences. 2022; 12(10):1333. https://doi.org/10.3390/brainsci12101333
Chicago/Turabian StyleVan Deusen, Kaylyn, Mark A. Prince, Anna J. Esbensen, Jamie O. Edgin, Emily K. Schworer, Angela John Thurman, Lina R. Patel, Lisa A. Daunhauer, and Deborah J. Fidler. 2022. "Profiles of Caregiver-Reported Executive Function in Children with Down Syndrome" Brain Sciences 12, no. 10: 1333. https://doi.org/10.3390/brainsci12101333





