The Effect of Coil Orientation on the Stimulation of the Pre–Supplementary Motor Area: A Combined TMS and EEG Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Experimental Sessions
2.2. Electric Field Modelling
2.3. TMS, Electromyographic Recording and Analysis
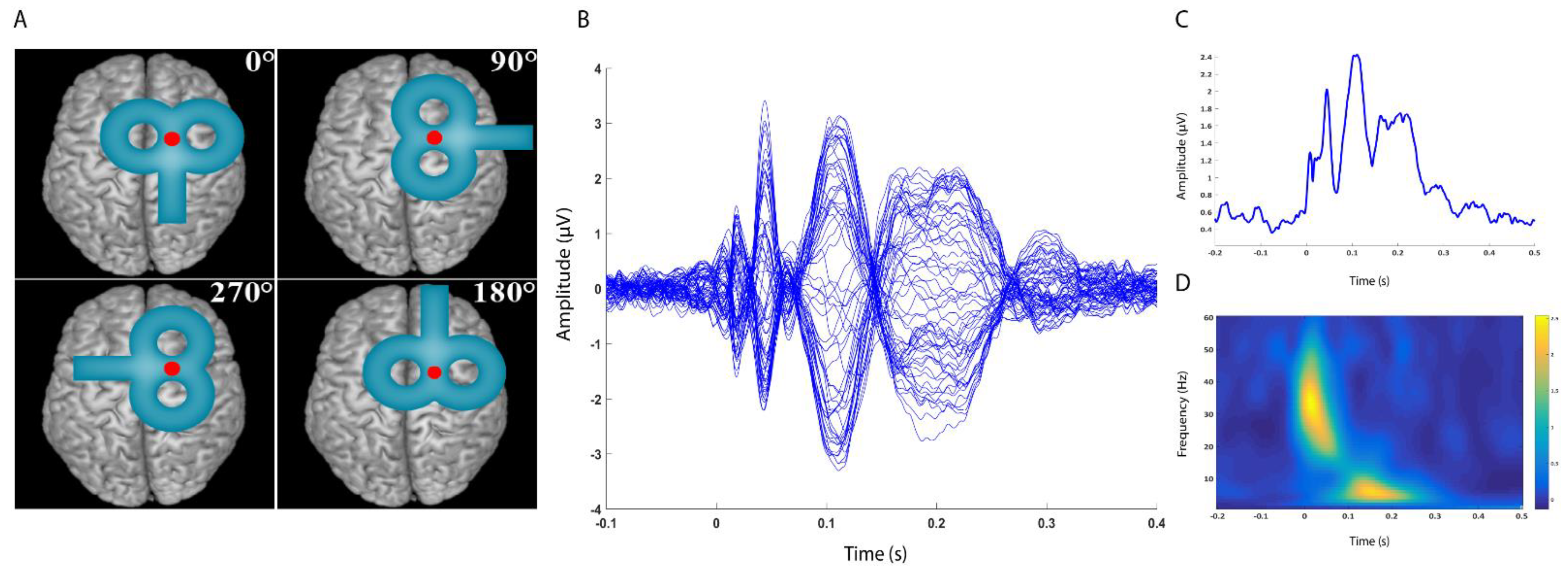
2.4. Electroencephalographic Recording and Analysis
2.5. Statistical Analysis
3. Results
3.1. Electric Field Modelling
3.2. Local Mean Field Potential and TEP Peaks
3.3. TMS–Related Spectral Perturbation
3.4. Motor Evoked Potentials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, B.L.; Dressler, D.; de Noordhout, A.M.; Marsden, C.D.; Nakashima, K.; Rothwell, J.C.; Thompson, P.D. Electric and magnetic stimulation of human motor cortex: Surface EMG and single motor unit responses. J. Physiol. 1989, 412, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Rothwell, J.C. Corticospinal activity evoked and modulated by non–invasive stimulation of the intact human motor cortex. J. Physiol. 2014, 592, 4115–4128. [Google Scholar] [CrossRef] [PubMed]
- Hanajima, R.; Ugawa, Y.; Terao, Y.; Sakai, K.; Furubayashi, T.; Machii, K.; Kanazawa, I. Paired–pulse magnetic stimulation of the human motor cortex: Differences among I waves. J. Physiol. 1998, 509, 607–618. [Google Scholar] [CrossRef] [PubMed]
- D’Ostilio, K.; Goetz, S.M.; Hannah, R.; Ciocca, M.; Chieffo, R.; Chen, J.A.; Peterchev, A.V.; Rothwell, J.C. Effect of coil orientation on strength–duration time constant and I–wave activation with controllable pulse parameter transcranial magnetic stimulation. Clin. Neurophysiol. 2016, 127, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Hannah, R.; Rothwell, J.C. Pulse Duration as Well as Current Direction Determines the Specificity of Transcranial Magnetic Stimulation of Motor Cortex during Contraction. Brain Stimul. 2017, 10, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, L.; Spampinato, D.A.; Pezzopane, V.; Orth, M.; Bisiacchi, P.S.; Rothwell, J.C.; Casula, E.P. Cerebellar noninvasive neuromodulation influences the reactivity of the contralateral primary motor cortex and surrounding areas: A TMS–EMG–EEG study. Cerebellum 2022. [Google Scholar] [CrossRef]
- Casula, E.P.; Rocchi, L.; Hannah, R.; Rothwell, J.C. Effects of pulse width, waveform and current direction in the cortex: A combined cTMS–EEG study. Brain Stimul. 2018, 11, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Hannah, R.; Cavanagh, S.E.; Tremblay, S.; Simeoni, S.; Rothwell, J.C. Selective Suppression of Local Interneuron Circuits in Human Motor Cortex Contributes to Movement Preparation. J. Neurosci. 2018, 38, 1264–1276. [Google Scholar] [CrossRef] [Green Version]
- Rawji, V.; Modi, S.; Rocchi, L.; Jahanshahi, M.; Rothwell, J.C. Proactive inhibition is marked by differences in the pattern of motor cortex activity during movement preparation and execution. J. Neurophysiol. 2022, 127, 819–828. [Google Scholar] [CrossRef]
- Picard, N.; Strick, P.L. Motor areas of the medial wall: A review of their location and functional activation. Cereb. Cortex 1996, 6, 342–353. [Google Scholar] [CrossRef]
- Picard, N.; Strick, P.L. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001, 11, 663–672. [Google Scholar] [CrossRef]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre–supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, M.F.; Hadland, K.A.; Paus, T.; Sipila, P.K. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J. Neurophysiol. 2002, 87, 2577–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Lu, M.S.; Chen, C.Y.; Muggleton, N.G.; Hsu, T.Y.; Juan, C.H. Roles of the pre–SMA and rIFG in conditional stopping revealed by transcranial magnetic stimulation. Behav. Brain Res. 2016, 296, 459–467. [Google Scholar] [CrossRef]
- Obeso, I.; Robles, N.; Marron, E.M.; Redolar-Ripoll, D. Dissociating the Role of the pre–SMA in Response Inhibition and Switching: A Combined Online and Offline TMS Approach. Front. Hum. Neurosci. 2013, 7, 150. [Google Scholar] [CrossRef] [Green Version]
- Obeso, I.; Cho, S.S.; Antonelli, F.; Houle, S.; Jahanshahi, M.; Ko, J.H.; Strafella, A.P. Stimulation of the pre–SMA influences cerebral blood flow in frontal areas involved with inhibitory control of action. Brain Stimul. 2013, 6, 769–776. [Google Scholar] [CrossRef]
- Chen, C.Y.; Muggleton, N.G.; Tzeng, O.J.; Hung, D.L.; Juan, C.H. Control of prepotent responses by the superior medial frontal cortex. Neuroimage 2009, 44, 537–545. [Google Scholar] [CrossRef]
- Ilmoniemi, R.J.; Kicic, D. Methodology for combined TMS and EEG. Brain Topogr. 2010, 22, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Casula, E.P.; Mayer, I.M.S.; Desikan, M.; Tabrizi, S.J.; Rothwell, J.C.; Orth, M. Motor cortex synchronization influences the rhythm of motor performance in premanifest huntington’s disease. Mov. Disord. 2018, 33, 440–448. [Google Scholar] [CrossRef]
- Leodori, G.; De Bartolo, M.I.; Guerra, A.; Fabbrini, A.; Rocchi, L.; Latorre, A.; Paparella, G.; Belvisi, D.; Conte, A.; Bhatia, K.P.; et al. Motor Cortical Network Excitability in Parkinson’s Disease. Mov. Disord. 2022, 37, 734–744. [Google Scholar] [CrossRef]
- Biondi, A.; Rocchi, L.; Santoro, V.; Rossini, P.G.; Beatch, G.N.; Richardson, M.P.; Premoli, I. Spontaneous and TMS–related EEG changes as new biomarkers to measure anti–epileptic drug effects. Sci. Rep. 2022, 12, 1919. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Pellicciari, M.C.; Bonnì, S.; Borghi, I.; Maiella, M.; Assogna, M.; Minei, M.; Motta, C.; D’Acunto, A.; Porrazzini, F.; et al. Decreased Frontal Gamma Activity in Alzheimer Disease Patients. Ann. Neurol. 2022, 92, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, S.; Canali, P.; Rosanova, M.; Pigorini, A.; Fecchio, M.; Mariotti, M.; Lucca, A.; Colombo, C.; Benedetti, F.; Massimini, M. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topogr. 2013, 26, 326–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocchi, L.; Ibanez, J.; Benussi, A.; Hannah, R.; Rawji, V.; Casula, E.; Rothwell, J. Variability and Predictors of Response to Continuous Theta Burst Stimulation: A TMS–EEG Study. Front. Neurosci. 2018, 12, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M. Natural frequencies of human corticothalamic circuits. J. Neurosci. 2009, 29, 7679–7685. [Google Scholar] [CrossRef]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 222–225. [Google Scholar] [CrossRef]
- Windhoff, M.; Opitz, A.; Thielscher, A. Electric field calculations in brain stimulation based on finite elements: An optimized processing pipeline for the generation and usage of accurate individual head models. Hum. Brain Mapp. 2013, 34, 923–935. [Google Scholar] [CrossRef]
- Rocchi, L.; Erro, R.; Antelmi, E.; Berardelli, A.; Tinazzi, M.; Liguori, R.; Bhatia, K.; Rothwell, J. High frequency somatosensory stimulation increases sensori–motor inhibition and leads to perceptual improvement in healthy subjects. Clin. Neurophysiol. 2017, 128, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijevic, M.R.; Hallett, M.; Katayama, Y.; Lucking, C.H.; et al. Non–invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Sharp, D.J.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.F.; James, S.G.; Patel, M.C.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef] [Green Version]
- Li, C.S.; Huang, C.; Constable, R.T.; Sinha, R. Imaging response inhibition in a stop–signal task: Neural correlates independent of signal monitoring and post–response processing. J. Neurosci. 2006, 26, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casarotto, S.; Lauro, L.J.R.; Bellina, V.; Casali, A.G.; Rosanova, M.; Pigorini, A.; Defendi, S.; Mariotti, M.; Massimini, M. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PLoS ONE 2010, 5, e10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herwig, U.; Schonfeldt-Lecuona, C.; Wunderlich, A.P.; von Tiesenhausen, C.; Thielscher, A.; Walter, H.; Spitzer, M. The navigation of transcranial magnetic stimulation. Psychiatry Res. 2001, 108, 123–131. [Google Scholar] [CrossRef]
- Mancuso, M.; Sveva, V.; Cruciani, A.; Brown, K.; Ibáñez, J.; Rawji, V.; Casula, E.; Premoli, I.; D’Ambrosio, S.; Rothwell, J.; et al. Transcranial Evoked Potentials Can Be Reliably Recorded with Active Electrodes. Brain Sci. 2021, 11, 145. [Google Scholar] [CrossRef]
- Rocchi, L.; Di Santo, A.; Brown, K.; Ibáñez, J.; Casula, E.; Rawji, V.; Di Lazzaro, V.; Koch, G.; Rothwell, J. Disentangling EEG responses to TMS due to cortical and peripheral activations. Brain Stimul. 2021, 14, 4–18. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single–trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Rogasch, N.C.; Sullivan, C.; Thomson, R.H.; Rose, N.S.; Bailey, N.W.; Fitzgerald, P.B.; Farzan, F.; Hernandez-Pavon, J.C. Analysing concurrent transcranial magnetic stimulation and electroencephalographic data: A review and introduction to the open–source TESA software. Neuroimage 2017, 147, 934–951. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Thomson, R.H.; Daskalakis, Z.J.; Fitzgerald, P.B. Short–latency artifacts associated with concurrent TMS–EEG. Brain Stimul. 2013, 6, 868–876. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Thomson, R.H.; Farzan, F.; Fitzgibbon, B.M.; Bailey, N.W.; Hernandez-Pavon, J.C.; Daskalakis, Z.J.; Fitzgerald, P.B. Removing artefacts from TMS–EEG recordings using independent component analysis: Importance for assessing prefrontal and motor cortex network properties. Neuroimage 2014, 101, 425–439. [Google Scholar] [CrossRef]
- Pellicciari, M.C.; Brignani, D.; Miniussi, C. Excitability modulation of the motor system induced by transcranial direct current stimulation: A multimodal approach. Neuroimage 2013, 83, 569–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecchio, M.; Pigorini, A.; Comanducci, A.; Sarasso, S.; Casarotto, S.; Premoli, I.; Derchi, C.C.; Mazza, A.; Russo, S.; Resta, F.; et al. The spectral features of EEG responses to transcranial magnetic stimulation of the primary motor cortex depend on the amplitude of the motor evoked potentials. PLoS ONE 2017, 12, e0184910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. TMS–EEG: A window into the neurophysiological effects of transcranial electrical stimulation in non–motor brain regions. Neurosci. Biobehav. Rev. 2016, 64, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Leodori, G.; Rocchi, L.; Mancuso, M.; De Bartolo, M.I.; Baione, V.; Costanzo, M.; Belvisi, D.; Conte, A.; Defazio, G.; Berardelli, A. The effect of stimulation frequency on transcranial evoked potentials. Transl. Neurosci. 2022, 13, 211–217. [Google Scholar] [CrossRef]
- Drummond, N.M.; Cressman, E.K.; Carlsen, A.N. Offline continuous theta burst stimulation over right inferior frontal gyrus and pre–supplementary motor area impairs inhibition during a go/no–go task. Neuropsychologia 2017, 99, 360–367. [Google Scholar] [CrossRef]
- Obeso, I.; Wilkinson, L.; Teo, J.T.; Talelli, P.; Rothwell, J.C.; Jahanshahi, M. Theta burst magnetic stimulation over the pre–supplementary motor area improves motor inhibition. Brain Stimul. 2017, 10, 944–951. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.; Singh, K.D.; Verbruggen, F.; Chambers, C.D. Evidence for parallel activation of the pre–supplementary motor area and inferior frontal cortex during response inhibition: A combined MEG and TMS study. R. Soc. Open Sci. 2018, 5, 171369. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, D.; Rocchi, L.; Tocco, P.; Speekenbrink, M.; Rothwell, J.C.; Jahanshahi, M. Continuous Theta Burst Stimulation Over the Dorsolateral Prefrontal Cortex and the Pre–SMA Alter Drift Rate and Response Thresholds Respectively During Perceptual Decision–Making. Brain Stimul. 2016, 9, 601–608. [Google Scholar] [CrossRef]
- Mendez, J.C.; Rocchi, L.; Jahanshahi, M.; Rothwell, J.; Merchant, H. Probing the timing network: A continuous theta burst stimulation study of temporal categorization. Neuroscience 2017, 356, 167–175. [Google Scholar] [CrossRef]
- Conte, A.; Rocchi, L.; Nardella, A.; Dispenza, S.; Scontrini, A.; Khan, N.; Berardelli, A. Theta–burst stimulation–induced plasticity over primary somatosensory cortex changes somatosensory temporal discrimination in healthy humans. PLoS ONE 2012, 7, e32979. [Google Scholar] [CrossRef]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial magnetic stimulation and cortical evoked potentials: A TMS/EEG co–registration study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Salo, K.S.; Vaalto, S.M.; Mutanen, T.P.; Stenroos, M.; Ilmoniemi, R.J. Individual activation patterns after the stimulation of different motor areas–a TMS–EEG study. Brain Connect. 2018, 8, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, S.; Turco, F.; Comanducci, A.; Perretti, A.; Marotta, G.; Pezzoli, G.; Rosanova, M.; Isaias, I.U. Excitability of the supplementary motor area in Parkinson’s disease depends on subcortical damage. Brain Stimul. 2018, 12, 152–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannah, R.; Rocchi, L.; Tremblay, S.; Rothwell, J.C. Controllable Pulse Parameter TMS and TMS–EEG As Novel Approaches to Improve Neural Targeting with rTMS in Human Cerebral Cortex. Front. Neural Circuits 2016, 10, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escola, L.; Michelet, T.; Macia, F.; Guehl, D.; Bioulac, B.; Burbaud, P. Disruption of information processing in the supplementary motor area of the MPTP–treated monkey: A clue to the pathophysiology of akinesia? Brain 2003, 126, 95–114. [Google Scholar] [CrossRef]
- Clower, W.T.; Alexander, G.E. Movement sequence–related activity reflecting numerical order of components in supplementary and presupplementary motor areas. J. Neurophysiol. 1998, 80, 1562–1566. [Google Scholar] [CrossRef]
- Rogasch, N.C.; Fitzgerald, P.B. Assessing cortical network properties using TMS–EEG. Hum. Brain Mapp. 2013, 34, 1652–1669. [Google Scholar] [CrossRef]
- Premoli, I.; Castellanos, N.; Rivolta, D.; Belardinelli, P.; Bajo, R.; Zipser, C.; Espenhahn, S.; Heidegger, T.; Muller-Dahlhaus, F.; Ziemann, U. TMS–EEG signatures of GABAergic neurotransmission in the human cortex. J. Neurosci. 2014, 34, 5603–5612. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Muller-Dahlhaus, F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef]
- Petrichella, S.; Johnson, N.; He, B. The influence of corticospinal activity on TMS–evoked activity and connectivity in healthy subjects: A TMS–EEG study. PLoS ONE 2017, 12, e0174879. [Google Scholar] [CrossRef] [Green Version]
- Gordon, P.C.; Desideri, D.; Belardinelli, P.; Zrenner, C.; Ziemann, U. Comparison of cortical EEG responses to realistic sham versus real TMS of human motor cortex. Brain Stimul. 2018, 11, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, P.; Biabani, M.; Blumberger, D.M.; Bortoletto, M.; Casarotto, S.; David, O.; Desideri, D.; Etkin, A.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Reproducibility in TMS–EEG studies: A call for data sharing, standard procedures and effective experimental control. Brain Stimul. 2019, 12, 787–790. [Google Scholar] [CrossRef] [Green Version]
- Conde, V.; Tomasevic, L.; Akopian, I.; Stanek, K.; Saturnino, G.B.; Thielscher, A.; Bergmann, T.O.; Siebner, H.R. The non–transcranial TMS–evoked potential is an inherent source of ambiguity in TMS–EEG studies. Neuroimage 2019, 185, 300–312. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Tanji, J. Changing directions of forthcoming arm movements: Neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex. J. Neurophysiol. 1996, 76, 2327–2342. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Hosaka, R.; Mushiake, H.; Tanji, J. Covert representation of second–next movement in the pre–supplementary motor area of monkeys. J. Neurophysiol. 2009, 101, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Hertrich, I.; Muller-Dahlhaus, F.; Ackermann, H.; Belardinelli, P.; Desideri, D.; Seibold, V.C.; Ziemann, U. Reduced Performance During a Sentence Repetition Task by Continuous Theta–Burst Magnetic Stimulation of the Pre–supplementary Motor Area. Front. Neurosci. 2018, 12, 361. [Google Scholar] [CrossRef]
- Badran, B.W.; Glusman, C.E.; Austelle, C.W.; Jenkins, S.; DeVries, W.H.; Galbraith, V.; Thomas, T.; Adams, T.G., Jr.; George, M.S.; Revuelta, G.J. A Double–Blind, Sham–Controlled Pilot Trial of Pre–Supplementary Motor Area (Pre–SMA) 1 Hz rTMS to Treat Essential Tremor. Brain Stimul. 2016, 9, 945–947. [Google Scholar] [CrossRef]




| RMT | 0° | 90° | 180° | 270° |
|---|---|---|---|---|
| 100% RMT | 110 | 108.8 | 90.5 | 90.6 |
| 120% RMT | 132.2 | 130.9 | 108.8 | 108.9 |
| 140% RMT | 154.4 | 152.9 | 127.1 | 127.2 |
| CO | SI | CO × SI | ||||
|---|---|---|---|---|---|---|
| F3,45 | p | F2,30 | p | F6,90 | p | |
| PI | 6.763 | 0.001 | 1.031 | 0.369 | 0.289 | 0.941 |
| PII | 1.709 | 0.179 | 2.646 | 0.087 | 3.520 | 0.004 |
| PIII | 7.697 | <0.001 | 0.475 | 0.626 | 2.355 | 0.037 |
| PIV | 4.131 | 0.011 | 8.899 | 0.001 | 0.283 | 0.944 |
| PV | 7.316 | <0.001 | 4.504 | 0.019 | 0.743 | 0.617 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casula, E.P.; Leodori, G.; Ibáñez, J.; Benussi, A.; Rawji, V.; Tremblay, S.; Latorre, A.; Rothwell, J.C.; Rocchi, L. The Effect of Coil Orientation on the Stimulation of the Pre–Supplementary Motor Area: A Combined TMS and EEG Study. Brain Sci. 2022, 12, 1358. https://doi.org/10.3390/brainsci12101358
Casula EP, Leodori G, Ibáñez J, Benussi A, Rawji V, Tremblay S, Latorre A, Rothwell JC, Rocchi L. The Effect of Coil Orientation on the Stimulation of the Pre–Supplementary Motor Area: A Combined TMS and EEG Study. Brain Sciences. 2022; 12(10):1358. https://doi.org/10.3390/brainsci12101358
Chicago/Turabian StyleCasula, Elias P., Giorgio Leodori, Jaime Ibáñez, Alberto Benussi, Vishal Rawji, Sara Tremblay, Anna Latorre, John C. Rothwell, and Lorenzo Rocchi. 2022. "The Effect of Coil Orientation on the Stimulation of the Pre–Supplementary Motor Area: A Combined TMS and EEG Study" Brain Sciences 12, no. 10: 1358. https://doi.org/10.3390/brainsci12101358
APA StyleCasula, E. P., Leodori, G., Ibáñez, J., Benussi, A., Rawji, V., Tremblay, S., Latorre, A., Rothwell, J. C., & Rocchi, L. (2022). The Effect of Coil Orientation on the Stimulation of the Pre–Supplementary Motor Area: A Combined TMS and EEG Study. Brain Sciences, 12(10), 1358. https://doi.org/10.3390/brainsci12101358









