Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
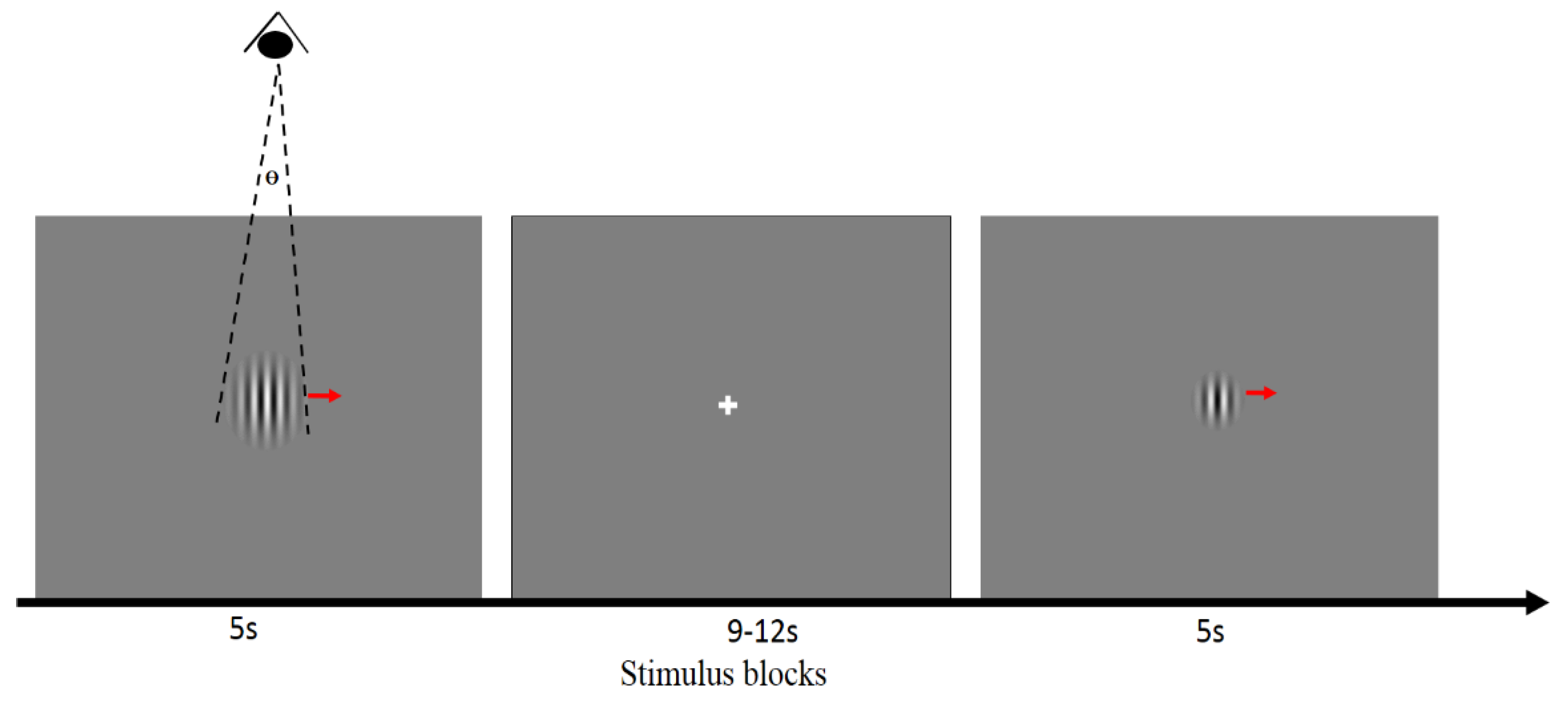
2.2. Visual Stimuli and Task
2.3. MRI Data Acquisition
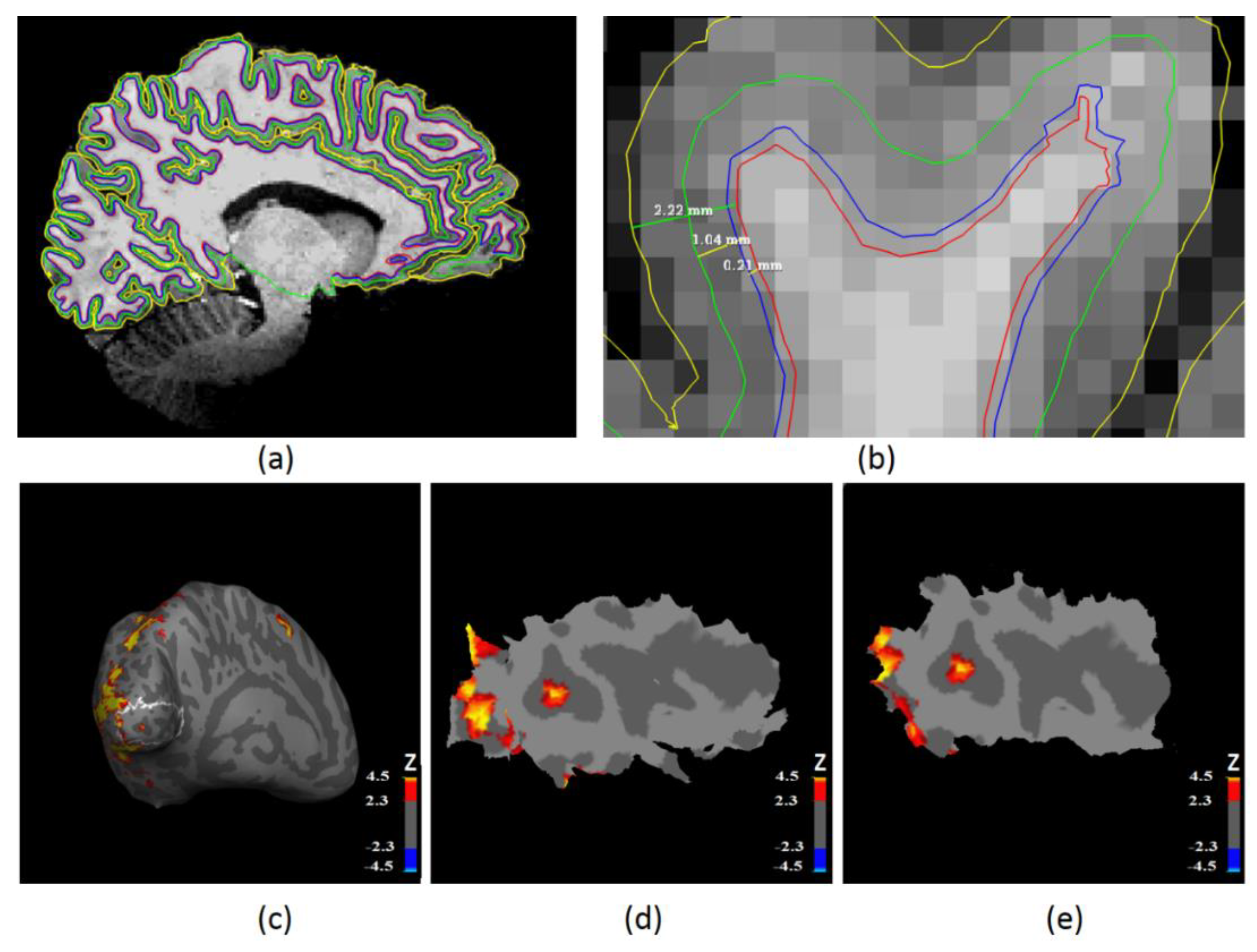
2.4. Laminar Surface Reconstruction Using High-Resolution Anatomical MRI
2.5. Functional MRI Analysis
2.6. Laminar Functional Data Analysis in the Primary Visual Cortex
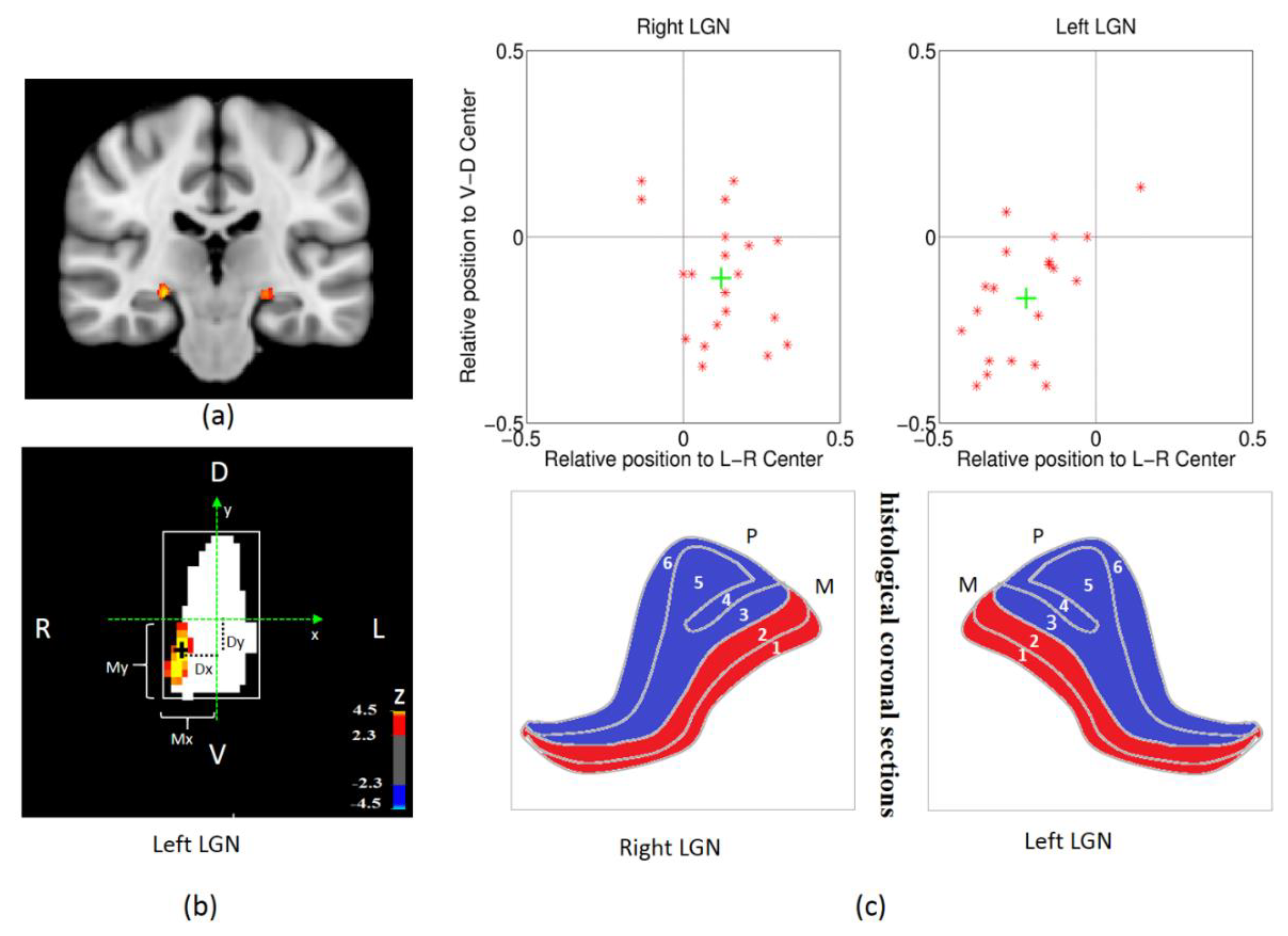
2.7. LGN ROI Definition and Analysis
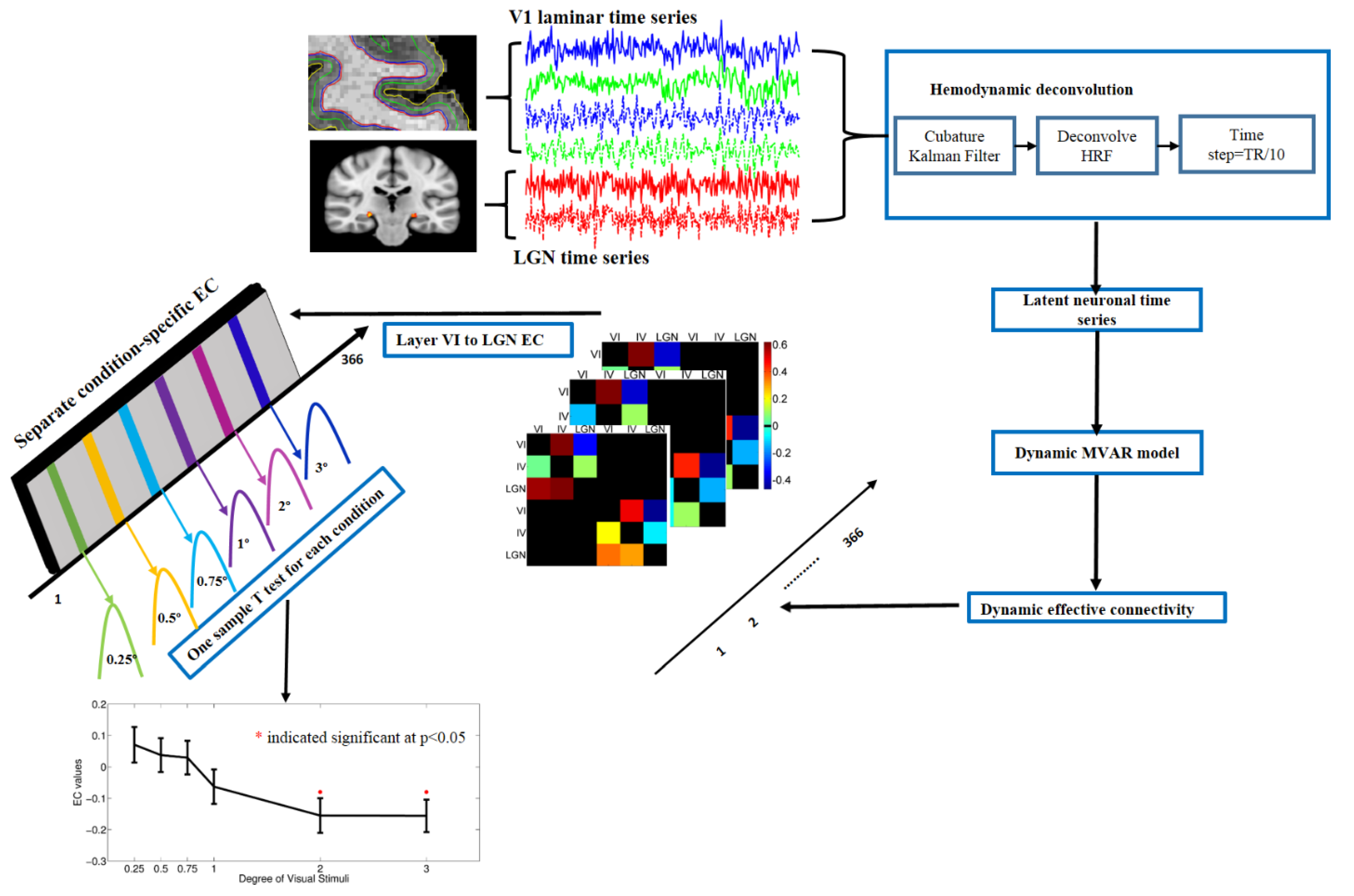
2.8. Dynamic Granger Causality Analysis
3. Results
3.1. The Validity of the Spatial Localization of Magnocellular Neurons in LGN
3.2. The Enhanced Center-Surround Inhibition Effect on LGN
3.3. Center-Surround Inhibition Effects in Different Layers of Primary Visual Cortex
3.4. Dynamic Effective Connectivity
4. Discussion
5. Limitations and Future Work
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briggs, F.; Usrey, W.M. Emerging views of corticothalamic function. Curr. Opin. Neurobiol. 2008, 18, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, F.; Usrey, W.M. Parallel Processing in the Corticogeniculate Pathway of the Macaque Monkey. Neuron 2009, 62, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, F.; Usrey, W.M. Corticogeniculate feedback and visual processing in the primate. J. Physiol. 2011, 589, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.E.; Wilson, J.R.; Ogren, M.P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J. Comp. Neurol. 1978, 182, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Ichida, J.M.; Casagrande, V.A. Organization of the feedback pathway from striate cortex (V1) to the lateral geniculate nucleus (LGN) in the owl monkey (Aotus trivirgatus). J. Comp. Neurol. 2002, 454, 272–283. [Google Scholar] [CrossRef]
- Lund, J.S.; Lund, R.D.; Hendrickson, A.E.; Bunt, A.H.; Fuchs, A.F. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J. Comp. Neurol. 1975, 164, 287–303. [Google Scholar] [CrossRef]
- Conley, M.; Raczkowski, D. Sublaminar organization within layer VI of the striate cortex in Galago. J. Comp. Neurol. 1990, 302, 425–436. [Google Scholar] [CrossRef]
- Merigan, W.H.; Katz, L.M.; Maunsell, J.H. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J. Neurosci. 1991, 11, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, M.; Hubel, D. Segregation of Depth: Form, Anatomy, Color, Physiology, and Movement, and Perception. Sci. New Ser. 1988, 240, 740–749. [Google Scholar] [CrossRef] [Green Version]
- Derrington, A.M.; Lennie, P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J. Physiol. 1984, 357, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Logothetis, N.K. The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 1990, 13, 392–398. [Google Scholar] [CrossRef]
- Nassi, J.J.; Callaway, E.M. Parallel processing strategies of the primate visual system. Nat. Rev. Neurosci. 2009, 10, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Merigan, W.H.; Maunsell, J.H. How parallel are the primate visual pathways? Annu. Rev. Neurosci. 1993, 16, 369–402. [Google Scholar] [CrossRef] [PubMed]
- Grieve, K.L.; Sillito, A.M. Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J. Neurosci. 1995, 15, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.E.; Andolina, I.M.; Grieve, K.L.; Wang, W.; Salt, T.E.; Cudeiro, J.; Sillito, A.M. Responses of primate LGN cells to moving stimuli involve a constant background modulation by feedback from area MT. Neuroscience 2013, 246, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.C.; Sillito, A.M. Corticofugal feedback influences the generation of length tuning in the visual pathway. Nature 1987, 329, 727–729. [Google Scholar] [CrossRef]
- Sillito, A.M.; Jones, H.E. Corticothalamic interactions in the transfer of visual information. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 357, 1739–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wandell, B.A.; Winawer, J.; Kay, K.N. Computational Modeling of Responses in Human Visual Cortex. In Brain Mapping; Elsevier: Amsterdam, The Netherlands, 2015; pp. 651–659. [Google Scholar]
- Sillito, A.M.; Cudeiro, J.; Jones, H.E. Always returning: Feedback and sensory processing in visual cortex and thalamus. Trends Neurosci. 2006, 29, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Angelucci, A.; Sainsbury, K. Contribution of feedforward thalamic afferents and corticogeniculate feedback to the spatial summation area of macaque V1 and LGN. J. Comp. Neurol. 2006, 498, 330–351. [Google Scholar] [CrossRef] [PubMed]
- Andolina, I.M.; Jones, H.E.; Wang, W.; Sillito, A.M. Corticothalamic feedback enhances stimulus response precision in the visual system. Proc. Natl. Acad. Sci. USA 2007, 104, 1685–1690. [Google Scholar] [CrossRef]
- Briggs, F.; Kiley, C.W.; Callaway, E.M.; Usrey, W.M. Morphological Substrates for Parallel Streams of Corticogeniculate Feedback Originating in Both V1 and V2 of the Macaque Monkey. Neuron 2016, 90, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, F.; Usrey, W.M. A fast, reciprocal pathway between the lateral geniculate nucleus and visual cortex in the macaque monkey. J. Neurosci. 2007, 27, 5431–5436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, S.R.; Bortone, D.S.; Adesnik, H.; Scanziani, M. Gain control by layer six in cortical circuits of vision. Nature 2012, 483, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kok, P.; Bains, L.J.; Van Mourik, T.; Norris, D.G.; De Lange, F.P. Selective activation of the deep layers of the human primary visual cortex by top-down feedback. Curr. Biol. 2016, 26, 371–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muckli, L.; De Martino, F.; Vizioli, L.; Petro, L.S.; Smith, F.W.; Ugurbil, K.; Goebel, R.; Yacoub, E. Contextual Feedback to Superficial Layers of V1. Curr. Biol. 2015, 25, 2690–2695. [Google Scholar] [CrossRef] [Green Version]
- Hevner, R.F. Development of connections in the human visual system during fetal mid-gestation: A DiI-tracing study. J. Neuropathol. Exp. Neurol. 2000, 59, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Krubitzer, L. The magnificent compromise: Cortical field evolution in mammals. Neuron 2007, 56, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Stevens, C.F. An evolutionary scaling law for the primate visual system and its basis in cortical function. Nature 2001, 411, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Daugman, J.G. Two-dimensional spectral analysis of cortical receptive field profiles. Vis. Res. 1980, 20, 847–856. [Google Scholar] [CrossRef]
- Gabor, D. Theory of communication. J. Inst. Electr. Eng. 1946, 93, 429–457. [Google Scholar] [CrossRef]
- Jones, J.P.; Palmer, L.A. An evaluation of the two-dimensional Gabor filter model of simple receptive fields in cat striate cortex. J. Neurophysiol. 1987, 58, 1233–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.P.; Palmer, L.A. The two-dimensional spatial structure of simple receptive fields in cat striate cortex. J. Neurophysiol. 1987, 58, 1187–1211. [Google Scholar] [CrossRef]
- Fredericksen, R.E.; Bex, P.J.; Verstraten, F.a. How big is a Gabor patch, and why should we care? J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alitto, H.J.; Moore, B.D.; Rathbun, D.L.; Usrey, W.M. A comparison of visual responses in the lateral geniculate nucleus of alert and anaesthetized macaque monkeys. J. Physiol. 2011, 589, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Alitto, H.J.; Usrey, W.M. Origin and Dynamics of Extraclassical Suppression in the Lateral Geniculate Nucleus of the Macaque Monkey. Neuron 2008, 57, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denison, R.N.; Vu, A.T.; Yacoub, E.; Feinberg, D.A.; Silver, M.A. Functional mapping of the magnocellular and parvocellular subdivisions of human LGN. Neuroimage 2014, 102, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, E.; Benardete, E. The dynamics of primate retinal ganglion cells. Prog. Brain Res. 2001, 134, 17–34. [Google Scholar]
- Mazer, J.A.; Vinje, W.E.; McDermott, J.; Schiller, P.H.; Gallant, J.L. Spatial frequency and orientation tuning dynamics in area V1. Proc. Natl. Acad. Sci. USA 2002, 99, 1645–1650. [Google Scholar] [CrossRef] [Green Version]
- Sceniak, M.P.; Hawken, M.J.; Shapley, R. Contrast-dependent changes in spatial frequency tuning of macaque V1 neurons: Effects of a changing receptive field size. J. Neurophysiol. 2002, 88, 1363–1373. [Google Scholar] [CrossRef]
- Sillito, A.M.; Cudeiro, J.; Murphy, P.C. Orientation sensitive elements in the corticofugal influence on centre-surround interactions in the dorsal lateral geniculate nucleus. Exp. Brain Res. 1993, 93, 6–16. [Google Scholar] [CrossRef]
- Wang, W.; Jones, H.E.; Andolina, I.M.; Salt, T.E.; Sillito, A.M. Functional alignment of feedback effects from visual cortex to thalamus. Nat. Neurosci. 2006, 9, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, P.J.; Barth, M.; Norris, D.G. Layer-specific BOLD activation in human V1. Hum. Brain Mapp. 2010, 31, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, D.A.; Moeller, S.; Smith, S.M.; Auerbach, E.; Ramanna, S.; Glasser, M.F.; Miller, K.L.; Ugurbil, K.; Yacoub, E. Multiplexed echo planar imaging for sub-second whole brain fmri and fast diffusion imaging. PLoS ONE 2010, 5, e15710. [Google Scholar] [CrossRef] [Green Version]
- Yacoub, E.; Harel, N.; Ugurbil, K. High-field fMRI unveils orientation columns in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 10607–10612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olman, C.A.; Harel, N.; Feinberg, D.A.; He, S.; Zhang, P.; Ugurbil, K.; Yacoub, E. Layer-specific fmri reflects different neuronal computations at different depths in human V1. PLoS ONE 2012, 7, e32536. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, J.R.; Fischl, B.; Greve, D.N.; Wald, L.L. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 2010, 52, 1334–1346. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.E.; Buchbinder, B.R.; Aharon, I. Three-dimensional mapping of cortical thickness using Laplace’s equation. Hum. Brain Mapp. 2000, 11, 12–32. [Google Scholar] [CrossRef]
- Waehnert, M.D.; Dinse, J.; Weiss, M.; Streicher, M.N.; Waehnert, P.; Geyer, S.; Turner, R.; Bazin, P.L. Anatomically motivated modeling of cortical laminae. Neuroimage 2014, 93, 210–220. [Google Scholar] [CrossRef]
- Havlicek, M.; Jan, J.; Brazdil, M.; Calhoun, V.D. Dynamic Granger causality based on Kalman filter for evaluation of functional network connectivity in fMRI data. Neuroimage 2010, 53, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Katwal, S.; Rogers, B.; Gore, J.; Deshpande, G. Experimental Validation of Dynamic Granger Causality for Inferring Stimulus-evoked Sub-100ms Timing Differences from fMRI. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 25, 539–546. [Google Scholar] [CrossRef]
- Bellucci, G.; Chernyak, S.; Hoffman, M.; Deshpande, G.; Dal Monte, O.; Knutson, K.M.; Grafman, J.; Krueger, F. Effective connectivity of brain regions underlying third-party punishment: Functional MRI and Granger causality evidence. Soc. Neurosci. 2016, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hampstead, B.M.; Khoshnoodi, M.; Yan, W.; Deshpande, G.; Sathian, K. Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. Neuroimage 2016, 124, 997–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Deshpande, G.; Liu, C.; Gu, R.; Luo, Y.J.; Krueger, F. Diffusion of responsibility attenuates altruistic punishment: A functional magnetic resonance imaging effective connectivity study. Hum. Brain Mapp. 2016, 37, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Lüsebrink, F.; Wollrab, A.; Speck, O. Cortical thickness determination of the human brain using high resolution 3T and 7T MRI data. Neuroimage 2013, 70, 122–131. [Google Scholar] [CrossRef]
- Hinds, O.P.; Rajendran, N.; Polimeni, J.R.; Augustinack, J.C.; Wiggins, G.; Wald, L.L.; Diana Rosas, H.; Potthast, A.; Schwartz, E.L.; Fischl, B. Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage 2008, 39, 1585–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M. Fast Robust Automated Brain Extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Woolrich, M.W.; Ripley, B.D.; Brady, M.; Smith, S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001, 14, 1370–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, M.; Polimeni, J.R.; Schwartz, E.L. Near-isometric flattening of brain surfaces. Neuroimage 2010, 51, 694–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürgel, U.; Schormann, T.; Schleicher, A.; Zilles, K. Mapping of Histologically Identified Long Fiber Tracts in Human Cerebral Hemispheres to the MRI Volume of a Reference Brain: Position and Spatial Variability of the Optic Radiation. Neuroimage 1999, 10, 489–499. [Google Scholar] [CrossRef]
- Bolz, J.; Gilbert, C.D.; Gilbert, C.D. Generation of end-inhibition in the visual cortex via interlaminar connections. Nature 1986, 320, 362–365. [Google Scholar] [CrossRef]
- Murphy, P.C.; Duckett, S.G.; Sillito, A.M. Feedback Connections to the Lateral Geniculate Nucleus and Cortical Response Properties. Science 1999, 286, 1552–1554. [Google Scholar] [CrossRef] [Green Version]
- Spillmann, L. Receptive fields of visual neurons: The early years. Perception 2014, 43, 1145–1176. [Google Scholar] [CrossRef]
- Deshpande, G.; Sathian, K.; Hu, X.; Buckhalt, J.A. A rigorous approach for testing the constructionist hypotheses of brain function. Behav. Brain Sci. 2012, 35, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Hu, X.; Stilla, R.; Sathian, K. Effective connectivity during haptic perception: A study using Granger causality analysis of functional magnetic resonance imaging data. Neuroimage 2008, 40, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, G.; Hu, X. Investigating effective brain connectivity from fMRI data: Past findings and current issues with reference to Granger causality analysis. Brain Connect. 2012, 2, 235–245. [Google Scholar] [CrossRef]
- Granger, C.W.J. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica 1969, 37, 424–438. [Google Scholar] [CrossRef]
- Deshpande, G.; LaConte, S.; James, G.A.; Peltier, S.; Hu, X. Multivariate granger causality analysis of fMRI data. Hum. Brain Mapp. 2009, 30, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Hu, X.; Lacey, S.; Stilla, R.; Sathian, K. Object familiarity modulates effective connectivity during haptic shape perception. Neuroimage 2010, 49, 1991–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampstead, B.M.; Stringer, A.Y.; Stilla, R.F.; Deshpande, G.; Hu, X.; Moore, A.B.; Sathian, K. Activation and effective connectivity changes following explicit-memory training for face-name pairs in patients with mild cognitive impairment: A pilot study. Neurorehabilit. Neural Repair 2011, 25, 210–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, S.; Hagtvedt, H.; Patrick, V.M.; Anderson, A.; Stilla, R.; Deshpande, G.; Hu, X.; Sato, J.R.; Reddy, S.; Sathian, K. Art for reward’s sake: Visual art recruits the ventral striatum. Neuroimage 2011, 55, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Krueger, F.; Landgraf, S.; Van Der Meer, E.; Deshpande, G.; Hu, X. Effective connectivity of the multiplication network: A functional MRI and multivariate granger causality mapping study. Hum. Brain Mapp. 2011, 32, 1419–1431. [Google Scholar] [CrossRef]
- Preusse, F.; Elke, V.D.M.; Deshpande, G.; Krueger, F.; Wartenburger, I. Fluid intelligence allows flexible recruitment of the parieto-frontal network in analogical reasoning. Front. Hum. Neurosci. 2011, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- SSathian, K.; Lacey, S.; Stilla, R.; Gibson, G.O.; Deshpande, G.; Hu, X.; LaConte, S.; Glielmi, C. Dual pathways for haptic and visual perception of spatial and texture information. Neuroimage 2011, 57, 462–475. [Google Scholar] [CrossRef] [Green Version]
- SStrenziok, M.; Krueger, F.; Deshpande, G.; Lenroot, R.K.; Van der meer, E.; Grafman, J. Fronto-parietal regulation of media violence exposure in adolescents: A multi-method study. Soc. Cogn. Affect. Neurosci. 2011, 6, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Gaschler-Markefski, B.; Baumgart, F.; Tempelmann, C.; Schindler, F.; Stiller, D.; Heinze, H.J.; Scheich, H. Statistical methods in functional magnetic resonance imaging with respect to nonstationary time-series: Auditory cortex activity. Magn. Reson. Med. 1997, 38, 811–820. [Google Scholar] [CrossRef]
- Sakata, S.; Harris, K.D. Laminar Structure of Spontaneous and Sensory-Evoked Population Activity in Auditory Cortex. Neuron 2009, 64, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Kapogiannis, D.; Deshpande, G.; Krueger, F.; Thornburg, M.P.; Grafman, J.H. Brain networks shaping religious belief. Brain Connect. 2014, 4, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Sathian, K.; Deshpande, G.; Stilla, R. Neural changes with tactile learning reflect decision-level reweighting of perceptual readout. J. Neurosci. 2013, 33, 5387–5398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.M.; White, D.; Hadley, J.; Hutcheson, N.; Shelton, R.; Sreenivasan, K.; Deshpande, G. Early life trauma and directional brain connectivity within major depression. Hum. Brain Mapp. 2014, 35, 4815–4826. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, M.D.; Sreenivasan, K.R.; Wood, K.H.; Ver Hoef, L.W.; Deshpande, G.; Knight, D.C. Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. Neuroimage 2014, 102, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Lacey, S.; Stilla, R.; Sreenivasan, K.; Deshpande, G.; Sathian, K. Spatial imagery in haptic shape perception. Neuropsychologia 2014, 60, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Grant, M.M.; Wood, K.; Sreenivasan, K.; Wheelock, M.; White, D.; Thomas, J.; Knight, D.C.; Deshpande, G. Influence of Early Life Stress on Intra- and Extra-Amygdaloid Causal Connectivity. Neuropsychopharmacology 2015, 40, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Libero, L.E.; DeRamus, T.P.; Lahti, A.C.; Deshpande, G.; Kana, R.K. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex 2015, 66, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Hutcheson, N.L.; Sreenivasan, K.R.; Deshpande, G.; Reid, M.A.; Hadley, J.; White, D.M.; Ver Hoef, L.; Lahti, A.C. Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Hum. Brain Mapp. 2015, 36, 1442–1457. [Google Scholar] [CrossRef]
- Deshpande, G.; Sathian, K.; Hu, X. Assessing and compensating for zero-lag correlation effects in time-lagged granger causality analysis of fMRI. IEEE Trans. Biomed. Eng. 2010, 57, 1446–1456. [Google Scholar] [CrossRef] [Green Version]
- Büchel, C.; Friston, K.J. Dynamic changes in effective connectivity characterized by variable parameter regression and Kalman filtering. Hum. Brain Mapp. 1998, 6, 403–408. [Google Scholar] [CrossRef]
- Arnold, M.; Miltner, W.H.R.; Witte, H.; Bauer, R.; Braun, C. Adaptive AR modeling of nonstationary time series by means of kaiman filtering. IEEE Trans. Biomed. Eng. 1998, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- David, O.; Guillemain, I.; Saillet, S.; Reyt, S.; Deransart, C.; Segebarth, C.; Depaulis, A. Identifying neural drivers with functional MRI: An electrophysiological validation. PLoS Biol. 2008, 6, 2683–2697. [Google Scholar] [CrossRef] [PubMed]
- Ryali, S.; Chen, T.; Supekar, K.; Tu, T.; Kochlka, J.; Cai, W.; Menon, V. Multivariate dynamical systems-based estimation of causal brain interactions in fMRI: Group-level validation using benchmark data, neurophysiological models and human connectome project data. J. Neurosci. Methods 2016, 268, 142–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryali, S.; Shih, Y.Y.I.; Chen, T.; Kochalka, J.; Albaugh, D.; Fang, Z.; Supekar, K.; Lee, J.H.; Menon, V. Combining optogenetic stimulation and fMRI to validate a multivariate dynamical systems model for estimating causal brain interactions. Neuroimage 2016, 132, 398–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryali, S.; Supekar, K.; Chen, T.; Menon, V. Multivariate dynamical systems models for estimating causal interactions in fMRI. Neuroimage 2011, 54, 807–823. [Google Scholar] [CrossRef] [Green Version]
- Goodyear, K.; Parasuraman, R.; Chernyak, S.; de Visser, E.; Madhavan, P.; Deshpande, G.; Krueger, F. An fMRI and effective connectivity study investigating miss errors during advice utilization from human and machine agents. Soc. Neurosci. 2016, 12, 1–12. [Google Scholar] [CrossRef]
- Deshpande, G.; Wang, Y.; Robinson, J. Resting state fMRI connectivity is sensitive to laminar connectional architecture in the human brain. Brain Inform. 2022, 9, 2. [Google Scholar] [CrossRef]
- Sreenivasan, K.R.; Havlicek, M.; Deshpande, G. Nonparametric hemodynamic deconvolution of fMRI using homomorphic filtering. IEEE Trans. Med. Imaging 2015, 34, 1155–1163. [Google Scholar] [CrossRef]
- Dumoulin, S.O.; Wandell, B.A. Population receptive field estimates in human visual cortex. Neuroimage 2007, 39, 647–660. [Google Scholar] [CrossRef] [Green Version]
- Horton, J.C.; Sherk, H. Receptive field properties in the cat’s lateral geniculate nucleus in the absence of on-center retinal input. J. Neurosci. 1984, 4, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Grieve, K.L.; Sillito, A.M. The length summation properties of layer VI cells in the visual cortex and hypercomplex cell end zone inhibition. Exp. Brain Res. 1991, 84, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, X.H. Correlation of activation sizes between lateral geniculate nucleus and primary visual cortex in humans. Magn. Reson. Med. 2001, 45, 202–205. [Google Scholar] [CrossRef]
- Schneider, K.A.; Kastner, S. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J. Neurosci. 2009, 29, 1784–1795. [Google Scholar] [CrossRef] [Green Version]
- Schneider, K.A. Subcortical mechanisms of feature-based attention. J. Neurosci. 2011, 31, 8643–8653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uğurbil, K.; Hu, X.; Chen, W.; Zhu, X.H.; Kim, S.G.; Georgopoulos, A. Functional mapping in the human brain using high magnetic fields. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1195–1213. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, K.; Schneider, K.A.; Kastner, S. Neural correlates of binocular rivalry in the human lateral geniculate nucleus. Nat. Neurosci. 2005, 8, 1595–1602. [Google Scholar] [CrossRef] [Green Version]
- Schneider, K.A.; Richter, M.C.; Kastner, S. Retinotopic organization and functional subdivisions of the human lateral geniculate nucleus: A high-resolution functional magnetic resonance imaging study. J. Neurosci. 2004, 24, 8975–8985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, D.H.F.; Hess, R.F.; Mullen, K.T. Color responses and their adaptation in human superior colliculus and lateral geniculate nucleus. Neuroimage 2016, 138, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wen, W.; Sun, X.; He, S. Selective reduction of fMRI responses to transient achromatic stimuli in the magnocellular layers of the LGN and the superficial layer of the SC of early glaucoma patients. Hum. Brain Mapp. 2016, 37, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Selemon, L.D.L.; Begovic, A. Stereologic analysis of the lateral geniculate nucleus of the thalamus in normal and schizophrenic subjects. Psychiatry Res. 2007, 151, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Jin, C.-H.; Jiang, S.; Li, M.-M.; Wang, Z.-L.; Zhu, H.; Chen, C.-Y.; Hua, T.-M. Effects of surround suppression on response adaptation of V1 neurons to visual stimuli. Dongwuxue Yanjiu 2014, 35, 411–419. [Google Scholar] [PubMed]
- Barbas, H.; García-Cabezas, M.Á.; Zikopoulos, B. Frontal-thalamic circuits associated with language. Brain Lang. 2013, 126, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Sereno, M.I.; Lutti, A.; Weiskopf, N.; Dick, F. Mapping the human cortical surface by combining quantitative T1 with retinotopy. Cereb. Cortex 2013, 23, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Trampel, R.; Bazin, P.-L.; Schäfer, A.; Heidemann, R.M.; Ivanov, D.; Lohmann, G.; Geyer, S.; Turner, R. Laminar-specific fingerprints of different sensorimotor areas obtained during imagined and actual finger tapping. Proc. Int. Soc. Magn. Reson. Med. 2012, 20, 663. [Google Scholar]
- Markuerkiaga, I.; Barth, M.; Norris, D.G. A cortical vascular model for examining the specificity of the laminar BOLD signal. Neuroimage 2016, 132, 491–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, T.J.; Halpern, S.D.; Purves, D. Correlated size variations in human visual cortex, lateral geniculate nucleus, and optic tract. J. Neurosci. 1997, 17, 2859–2868. [Google Scholar] [CrossRef] [Green Version]
- Kyathanahally, S.; Franco-Watkins, A.; Zhang, X.; Calhoun, V.; Deshpande, G. A realistic framework for investigating decision-making in the brain with high spatio-temporal resolution using simultaneous EEG/fMRI and joint ICA. IEEE J. Biomed. Health Inform. 2016, 2194, 1. [Google Scholar]


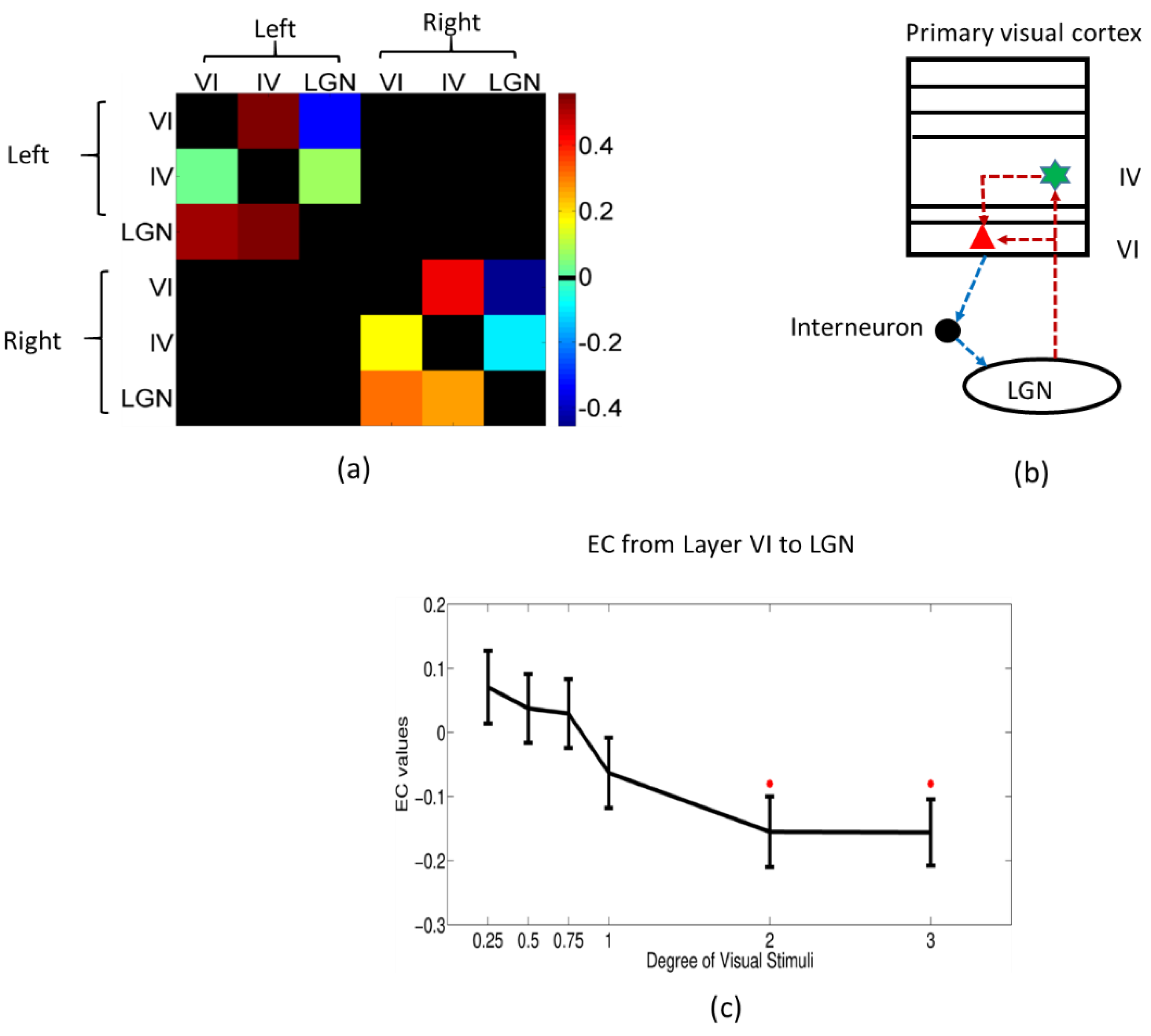
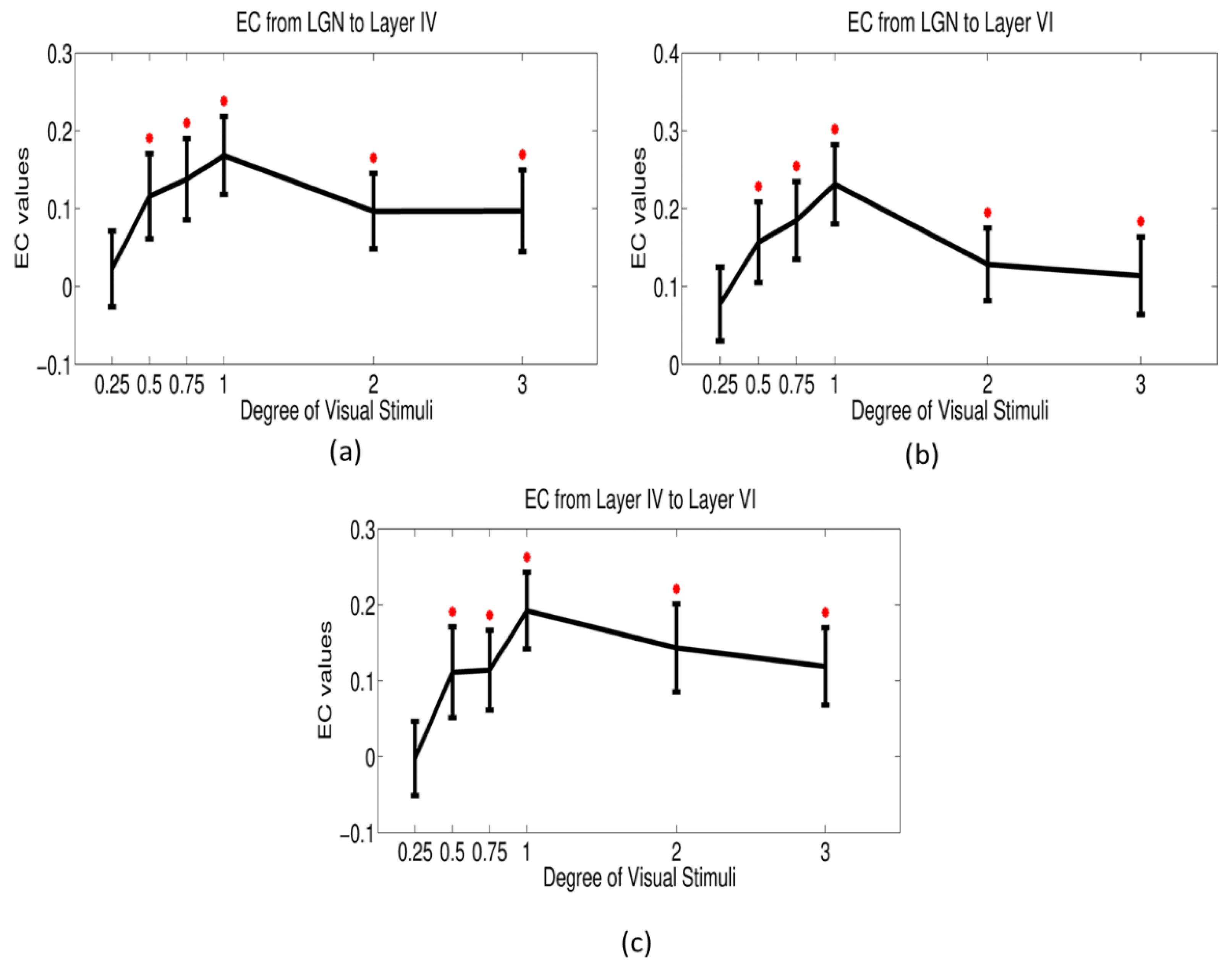
| (a) | ||
| Paired t-Test for Condition A < Condition B at Alpha = 0.05 in Left M Type LGN | ||
| A | B | p Value |
| 0.25° | 0.75° | 2.40 × 10−3 |
| 0.5° | 0.75° | 3.60 × 10−3 |
| 2° | 0.75° | 8.99 × 10−5 |
| 3° | 0.75° | 9.32 × 10−4 |
| 0.25° | 1° | 2.07 × 10−8 |
| 0.5° | 1° | 2.07 × 10−8 |
| 2° | 1° | 2.21 × 10−8 |
| 3° | 1° | 3.07 × 10−8 |
| (b) | ||
| Paired t-Test for Condition A < Condition B at Alpha = 0.05 in Right M Type LGN | ||
| A | B | p Value |
| 0.25° | 0.75° | 1.10 × 10−3 |
| 0.5° | 0.75° | 6.37 × 10−7 |
| 2° | 0.75° | 1.00 × 10−4 |
| 3° | 0.75° | 5.50 × 10−8 |
| 0.25° | 1° | 4.06 × 10−6 |
| 0.5° | 1° | 7.17 × 10−6 |
| 2° | 1° | 2.10 × 10−3 |
| 3° | 1° | 1.90 × 10−3 |
| (a) | |||||
| Paired t-Test for Condition A < Condition B in Layer VI of Left Primary Visual Cortex | |||||
| A | B | p Value | |||
| 0.25° | 0.5° | 1.98 × 10−7 | |||
| 0.25° | 0.75° | 2.07 × 10−8 | |||
| 0.25° | 1° | 2.39 × 10−8 | |||
| 2° | 0.5° | 3.67 × 10−5 | |||
| 2° | 0.75° | 2.07 × 10−4 | |||
| 2° | 1° | 2.09 × 10−8 | |||
| 3° | 0.5° | 7.18 × 10−6 | |||
| 3° | 0.75° | 2.55 × 10−8 | |||
| 3° | 1° | 1.87 × 10−7 | |||
| (b) | |||||
| Paired t-Test for Condition A < Condition B in Layer IV of Left Primary Visual Cortex | |||||
| A | B | p Value | |||
| 0.25° | 0.5° | 1.98 × 10−7 | |||
| 0.25° | 0.75° | 2.07 × 10−8 | |||
| 0.25° | 1° | 2.39 × 10−8 | |||
| 2° | 0.5° | 3.67 × 10−5 | |||
| 2° | 0.75° | 2.07 × 10−4 | |||
| 2° | 1° | 2.09 × 10−8 | |||
| 3° | 0.5° | 7.18 × 10−6 | |||
| 3° | 0.75° | 2.55 × 10−8 | |||
| 3° | 1° | 1.87 × 10−7 | |||
| (c) | |||||
| p-Value of Paired t-Test for Layer IV > VI under Six Conditions | |||||
| 0.25° | 0.5° | 0.75° | 1° | 2° | 3° |
| 4.71 × 10−12 | 7.45 × 10−5 | 3.53 × 10−8 | 2.39 × 10−8 | 5.54 × 10−12 | 2.69 × 10−10 |
| (a) | |||||
| Paired t-Test for Condition A < Condition B in Layer VI of Right Primary Visual Cortex | |||||
| A | B | p Value | |||
| 0.25° | 0.5° | 2.07 × 10−8 | |||
| 0.25° | 0.75° | 2.06 × 10−8 | |||
| 0.25° | 1° | 2.06 × 10−8 | |||
| 2° | 0.5° | 2.06 × 10−5 | |||
| 2° | 0.75° | 2.06 × 10−4 | |||
| 2° | 1° | 2.05 × 10−8 | |||
| 3° | 0.5° | 2.08 × 10−6 | |||
| 3° | 0.75° | 2.07 × 10−8 | |||
| 3° | 1° | 3.10 × 10−7 | |||
| (b) | |||||
| Paired t-Test for Condition A < Condition B in Layer IV of Right Primary Visual Cortex | |||||
| A | B | p Value | |||
| 0.25° | 0.5° | 2.44 × 10−8 | |||
| 0.25° | 0.75° | 2.10 × 10−8 | |||
| 0.25° | 1° | 2.28 × 10−8 | |||
| 2° | 0.5° | 2.13 × 10−8 | |||
| 2° | 0.75° | 4.87 × 10−7 | |||
| 2° | 1° | 2.07 × 10−8 | |||
| 3° | 0.5° | 1.19 × 10−4 | |||
| 3° | 0.75° | 1.65 × 10−5 | |||
| 3° | 1° | 4.60 × 10−3 | |||
| (c) | |||||
| p-Value of Paired t-Test for Layer IV > VI under Six Conditions | |||||
| 0.25° | 0.5° | 0.75° | 1° | 2° | 3° |
| 1.50 × 10−14 | 8.06 × 10−9 | 1.49 × 10−9 | 9.75 × 10−9 | 1.24 × 10−14 | 5.84 × 10−12 |
| Left Primary Visual Cortex | Right Primary Visual Cortex | |
|---|---|---|
| Visual Stimuli Factor | 3.02 × 10−14 | 3.10 × 10−14 |
| Layer Factor | 2.07 × 10−6 | 9.97 × 10−20 |
| Interaction | 0.87 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshpande, G.; Wang, Y. Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI. Brain Sci. 2022, 12, 1361. https://doi.org/10.3390/brainsci12101361
Deshpande G, Wang Y. Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI. Brain Sciences. 2022; 12(10):1361. https://doi.org/10.3390/brainsci12101361
Chicago/Turabian StyleDeshpande, Gopikrishna, and Yun Wang. 2022. "Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI" Brain Sciences 12, no. 10: 1361. https://doi.org/10.3390/brainsci12101361





