Postoperative Delirium in Neurosurgical Patients: Recent Insights into the Pathogenesis
Abstract
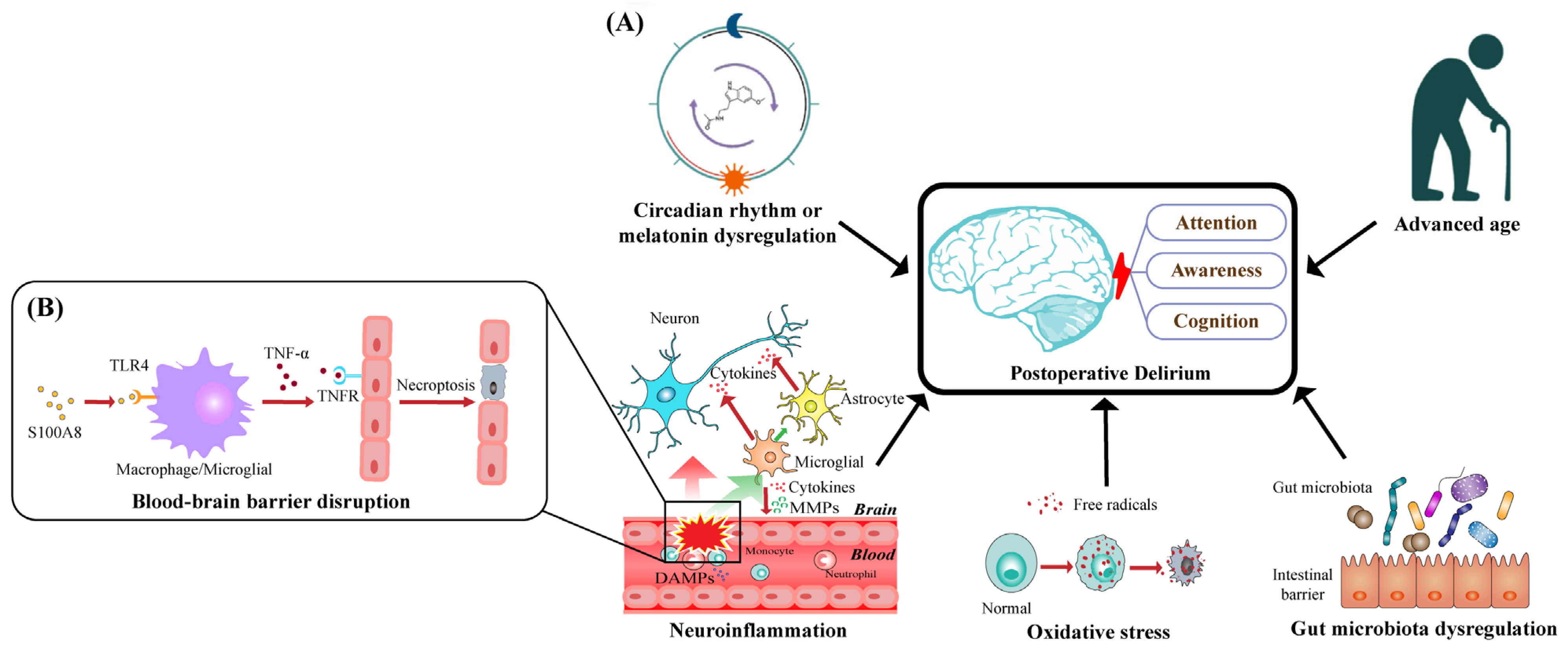
:1. Introduction
2. Risk Factors
2.1. Anesthesia
2.2. Age
2.3. Cognitive Condition
2.4. Intrinsic and Extrinsic Factors
3. Pathological Theories
3.1. Neuroinflammation
3.2. Oxidative Stress
3.3. Circadian Rhythm or Melatonin Dysregulation
3.4. Older Age
3.5. Dysregulation of the Gut Microbiota
4. Postoperative Delirium in Neurosurgical Patients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), 5th ed.; American Psychiatric Association Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Evered, L.; Silbert, B.; Knopman, D.S.; Scott, D.A.; DeKosky, S.T.; Rasmussen, L.S.; Oh, E.S.; Crosby, G.; Berger, M.; Eckenhoff, R.G.; et al. Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology 2018, 129, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Migirov, A.; Chahar, P.; Maheshwari, K. Postoperative delirium and neurocognitive disorders. Curr. Opin. Crit. Care 2021, 27, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Olin, K.; Eriksdotter-Jonhagen, M.; Jansson, A.; Herrington, M.K.; Kristiansson, M.; Permert, J. Postoperative delirium in elderly patients after major abdominal surgery. Br. J. Surg. 2005, 92, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Smulter, N.; Lingehall, H.C.; Gustafson, Y.; Olofsson, B.; Engstrom, K.G. Delirium after cardiac surgery: Incidence and risk factors. Interact. Cardiovasc Thorac. Surg. 2013, 17, 790–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Card, E.; Pandharipande, P.; Tomes, C.; Lee, C.; Wood, J.; Nelson, D.; Graves, A.; Shintani, A.; Ely, E.W.; Hughes, C. Emergence from general anaesthesia and evolution of delirium signs in the post-anaesthesia care unit. Br. J. Anaesth. 2015, 115, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudzwaard, J.A.; de Ronde-Tillmans, M.; de Jager, T.A.J.; Lenzen, M.J.; Nuis, R.J.; van Mieghem, N.M.; Daemen, J.; de Jaegere, P.P.T.; Mattace-Raso, F.U.S. Incidence, determinants and consequences of delirium in older patients after transcatheter aortic valve implantation. Age Ageing 2020, 49, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Sipahi, N.F.; Mehdiani, A.; Petrov, G.; Awe, M.; Minol, J.P.; Boeken, U.; Korbmacher, B.; Lichtenberg, A.; Dalyanoglu, H. Risk and Consequences of Postoperative Delirium in Cardiac Surgery. Thorac. Cardiovasc. Surg. 2020, 68, 417–424. [Google Scholar] [CrossRef]
- Wu, J.; Gao, S.; Zhang, S.; Yu, Y.; Liu, S.; Zhang, Z.; Mei, W. Perioperative risk factors for recovery room delirium after elective non-cardiovascular surgery under general anaesthesia. Perioper. Med. 2021, 10, 3. [Google Scholar] [CrossRef]
- Budenas, A.; Tamasauskas, S.; Sliauzys, A.; Navickaite, I.; Sidaraite, M.; Pranckeviciene, A.; Deltuva, V.P.; Tamasauskas, A.; Bunevicius, A. Incidence and clinical significance of postoperative delirium after brain tumor surgery. Acta Neurochir. 2018, 160, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Kappen, P.R.; Kakar, E.; Dirven, C.M.F.; van der Jagt, M.; Klimek, M.; Osse, R.J.; Vincent, A. Delirium in neurosurgery: A systematic review and meta-analysis. Neurosurg. Rev. 2022, 45, 329–341. [Google Scholar] [CrossRef]
- Barbateskovic, M.; Krauss, S.R.; Collet, M.O.; Larsen, L.K.; Jakobsen, J.C.; Perner, A.; Wetterslev, J. Pharmacological interventions for prevention and management of delirium in intensive care patients: A systematic overview of reviews and meta-analyses. BMJ Open 2019, 9, e024562. [Google Scholar] [CrossRef] [Green Version]
- Turan, A.; Duncan, A.; Leung, S.; Karimi, N.; Fang, J.; Mao, G.; Hargrave, J.; Gillinov, M.; Trombetta, C.; Ayad, S.; et al. Dexmedetomidine for reduction of atrial fibrillation and delirium after cardiac surgery (DECADE): A randomised placebo-controlled trial. Lancet 2020, 396, 177–185. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Liu, M.; Zou, Z.; Wang, L.; Xu, F.Y.; Shi, X.Y. Strategies for prevention of postoperative delirium: A systematic review and meta-analysis of randomized trials. Crit. Care 2013, 17, R47. [Google Scholar] [CrossRef] [Green Version]
- Weldon, B.C.; Bell, M.; Craddock, T. The effect of caudal analgesia on emergence agitation in children after sevoflurane versus halothane anesthesia. Anesth. Analg. 2004, 98, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Aouad, M.T.; Kanazi, G.E.; Siddik-Sayyid, S.M.; Gerges, F.J.; Rizk, L.B.; Baraka, A.S. Preoperative caudal block prevents emergence agitation in children following sevoflurane anesthesia. Acta Anaesthesiol. Scand. 2005, 49, 300–304. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, C.S.; Kim, S.D.; Lee, J.R. Fascia iliaca compartment block reduces emergence agitation by providing effective analgesic properties in children. J. Clin. Anesth. 2011, 23, 119–123. [Google Scholar] [CrossRef]
- Shin, J.E.; Kyeong, S.; Lee, J.S.; Park, J.Y.; Lee, W.S.; Kim, J.J.; Yang, K.H. A personality trait contributes to the occurrence of postoperative delirium: A prospective study. BMC Psychiatry 2016, 16, 371. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Dong, T.; Cui, Y.; Meng, X.; Dai, Z. Effect of regional anesthesia on the postoperative delirium: A systematic review and meta-analysis of randomized controlled trials. Front. Surg. 2022, 9, 937293. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, H.; Chen, B.; Fan, L.; Shi, W.; Jin, Y.; Ren, X.; Lang, L.; Zhu, F. The Incidence and Predictors of Postoperative Delirium after Brain Tumor Resection in Adults: A Cross-Sectional Survey. World Neurosurg. 2020, 140, e129–e139. [Google Scholar] [CrossRef]
- Rizk, P.; Morris, W.; Oladeji, P.; Huo, M. Review of Postoperative Delirium in Geriatric Patients Undergoing Hip Surgery. Geriatr. Orthop. Surg. Rehabil. 2016, 7, 100–105. [Google Scholar] [CrossRef]
- Kang, T.; Park, S.Y.; Lee, J.H.; Lee, S.H.; Park, J.H.; Kim, S.K.; Suh, S.W. Incidence & Risk Factors of Postoperative Delirium after Spinal Surgery in Older Patients. Sci. Rep. 2020, 10, 9232. [Google Scholar] [PubMed]
- Inouye, S.K.; Westendorp, R.G.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Gross, A.L.; Jones, R.N.; Habtemariam, D.A.; Fong, T.G.; Tommet, D.; Quach, L.; Schmitt, E.; Yap, L.; Inouye, S.K. Delirium and Long-term Cognitive Trajectory among Persons with Dementia. Arch. Intern. Med. 2012, 172, 1324–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, T.G.; Jones, R.N.; Marcantonio, E.R.; Tommet, D.; Gross, A.L.; Habtemariam, D.; Schmitt, E.; Yap, L.; Inouye, S.K. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann. Intern. Med. 2012, 156, 848–856, W296. [Google Scholar] [CrossRef] [Green Version]
- Medrzycka-Dabrowska, W.; Lange, S.; Religa, D.; Dabrowski, S.; Friganovic, A.; Oomen, B.; Krupa, S. Delirium in ICU Patients after Cardiac Arrest: A Scoping Review. J. Pers. Med. 2022, 12, 1047. [Google Scholar] [CrossRef]
- Punjasawadwong, Y.; Chau-In, W.; Laopaiboon, M.; Punjasawadwong, S.; Pin-On, P. Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non-cardiac and non-neurosurgical procedures in adults. Cochrane Database Syst. Rev. 2018, 5, CD011283. [Google Scholar] [CrossRef]
- Wildes, T.S.; Mickle, A.M.; Ben Abdallah, A.; Maybrier, H.R.; Oberhaus, J.; Budelier, T.P.; Kronzer, A.; McKinnon, S.L.; Park, D.; Torres, B.A.; et al. Effect of Electroencephalography-Guided Anesthetic Administration on Postoperative Delirium among Older Adults Undergoing Major Surgery: The ENGAGES Randomized Clinical Trial. JAMA 2019, 321, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [Green Version]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Hessian, P.A.; Edgeworth, J.; Hogg, N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J. Leukoc. Biol. 1993, 53, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, C.; Klempt, M.; Sorg, C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim. Biophys. Acta 1998, 1448, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Schelbergen, R.F.; Blom, A.B.; van den Bosch, M.H.; Sloetjes, A.; Abdollahi-Roodsaz, S.; Schreurs, B.W.; Mort, J.S.; Vogl, T.; Roth, J.; van den Berg, W.B.; et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012, 64, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Yu, C.J.; Liu, Y.H.; Dong, H.Q.; Zhang, X.; Zhang, S.S.; Hu, L.Q.; Zhang, F.; Qian, Y.N.; Gui, B. S100A8 contributes to postoperative cognitive dysfunction in mice undergoing tibial fracture surgery by activating the TLR4/MyD88 pathway. Brain Behav. Immun. 2015, 44, 221–234. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Buchanan, M.M.; Hutchinson, M.; Watkins, L.R.; Yin, H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Liu, H.; Chen, F.; Wang, H.; Chen, S.; Xie, J.; Zheng, Z.; Li, Z. Alarmins S100A8/A9 promote intervertebral disc degeneration and inflammation-related pain in a rat model through toll-like receptor-4 and activation of the NF-kappaB signaling pathway. Osteoarthr. Cartil. 2022, 30, 998–1011. [Google Scholar] [CrossRef]
- Saribal, D.; Hocaoglu-Emre, F.S.; Erdogan, S.; Bahtiyar, N.; Caglar Okur, S.; Mert, M. Inflammatory cytokines IL-6 and TNF-alpha in patients with hip fracture. Osteoporos. Int. 2019, 30, 1025–1031. [Google Scholar] [CrossRef]
- Hirsch, J.; Vacas, S.; Terrando, N.; Yuan, M.; Sands, L.P.; Kramer, J.; Bozic, K.; Maze, M.M.; Leung, J.M. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J. Neuroinflam. 2016, 13, 211. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-alpha mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Pan, H.; Najafov, A.; Yuan, J. Necroptosis in development and diseases. Genes Dev. 2018, 32, 327–340. [Google Scholar] [CrossRef]
- Takata, F.; Dohgu, S.; Matsumoto, J.; Takahashi, H.; Machida, T.; Wakigawa, T.; Harada, E.; Miyaji, H.; Koga, M.; Nishioku, T.; et al. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J. Neuroinflam. 2011, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, S.T.; Otu, H.H.; Ngo, L.H.; Fong, T.G.; Vasunilashorn, S.M.; Xie, Z.; Kunze, L.J.; Vlassakov, K.V.; Abdeen, A.; Lange, J.K.; et al. Patterns and Persistence of Perioperative Plasma and Cerebrospinal Fluid Neuroinflammatory Protein Biomarkers After Elective Orthopedic Surgery Using SOMAscan. Anesth. Analg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Feng, X.; Valdearcos, M.; Lutrin, D.; Uchida, Y.; Koliwad, S.K.; Maze, M. Interleukin-6 is both necessary and sufficient to produce perioperative neurocognitive disorder in mice. Br. J. Anaesth. 2018, 120, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Sauri, A.; Orduna-Valls, J.M.; Blasco-Serra, A.; Tornero-Tornero, C.; Cedeno, D.L.; Bejarano-Quisoboni, D.; Valverde-Navarro, A.A.; Benyamin, R.; Vallejo, R. Glia to neuron ratio in the posterior aspect of the human spinal cord at thoracic segments relevant to spinal cord stimulation. J. Anat. 2019, 235, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012, 13, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Mackaness, G.B. Cellular immunity and the parasite. Adv. Exp. Med. Biol. 1977, 93, 65–73. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Minagar, A.; Shapshak, P.; Fujimura, R.; Ownby, R.; Heyes, M.; Eisdorfer, C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 2002, 202, 13–23. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Witcher, K.G.; Bray, C.E.; Chunchai, T.; Zhao, F.; O’Neil, S.M.; Gordillo, A.J.; Campbell, W.A.; McKim, D.B.; Liu, X.; Dziabis, J.E.; et al. Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J. Neurosci. 2021, 41, 1597–1616. [Google Scholar] [CrossRef]
- Rocha, S.M.; Cristovão, A.C.; Campos, F.L.; Fonseca, C.P.; Baltazar, G. Astrocyte-derived GDNF is a potent inhibitor of microglial activation. Neurobiol. Dis. 2012, 47, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shen, Q.; Zhang, H.; Xiao, X.; Lv, C.; Chu, Y.; Shen, Y.; Wang, D.; Shen, Q. The Potential Protective Effect of Mesencephalic Astrocyte-Derived Neurotrophic Factor on Post-Operative Delirium via Inhibiting Inflammation and Microglia Activation. J. Inflamm. Res. 2021, 14, 2781–2791. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Reis, P.A.; Castro-Faria-Neto, H.C. Systemic Response to Infection Induces Long-Term Cognitive Decline: Neuroinflammation and Oxidative Stress as Therapeutical Targets. Front. Neurosci. 2021, 15, 742158. [Google Scholar] [CrossRef]
- Anasooya Shaji, C.; Robinson, B.D.; Yeager, A.; Beeram, M.R.; Davis, M.L.; Isbell, C.L.; Huang, J.H.; Tharakan, B. The Tri-phasic Role of Hydrogen Peroxide in Blood-Brain Barrier Endothelial cells. Sci. Rep. 2019, 9, 133. [Google Scholar] [CrossRef]
- Schreibelt, G.; Kooij, G.; Reijerkerk, A.; van Doorn, R.; Gringhuis, S.I.; van der Pol, S.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Piontek, J.; et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007, 21, 3666–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochhead, J.J.; McCaffrey, G.; Quigley, C.E.; Finch, J.; DeMarco, K.M.; Nametz, N.; Davis, T.P. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J. Cereb. Blood Flow Metab. 2010, 30, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lischper, M.; Beuck, S.; Thanabalasundaram, G.; Pieper, C.; Galla, H.J. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010, 1326, 114–127. [Google Scholar] [CrossRef]
- Wu, M.Y.; Gao, F.; Yang, X.M.; Qin, X.; Chen, G.Z.; Li, D.; Dang, B.Q.; Chen, G. Matrix metalloproteinase-9 regulates the blood brain barrier via the hedgehog pathway in a rat model of traumatic brain injury. Brain Res. 2020, 1727, 146553. [Google Scholar] [CrossRef]
- Lopez, M.G.; Hughes, C.G.; DeMatteo, A.; O’Neal, J.B.; McNeil, J.B.; Shotwell, M.S.; Morse, J.; Petracek, M.R.; Shah, A.S.; Brown, N.J.; et al. Intraoperative Oxidative Damage and Delirium after Cardiac Surgery. Anesthesiology 2020, 132, 551–561. [Google Scholar] [CrossRef]
- Petronilli, V.; Costantini, P.; Scorrano, L.; Colonna, R.; Passamonti, S.; Bernardi, P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J. Biol. Chem. 1994, 269, 16638–16642. [Google Scholar] [CrossRef]
- Gouriou, Y.; Demaurex, N.; Bijlenga, P.; De Marchi, U. Mitochondrial calcium handling during ischemia-induced cell death in neurons. Biochimie 2011, 93, 2060–2067. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dong, Y.; Xu, Z.; Xie, Z. Propofol and magnesium attenuate isoflurane-induced caspase-3 activation via inhibiting mitochondrial permeability transition pore. Med. Gas Res. 2012, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Peng, M.; Zhang, C.; Dong, Y.; Zhang, Y.; Nakazawa, H.; Kaneki, M.; Zheng, H.; Shen, Y.; Marcantonio, E.R.; Xie, Z. Battery of behavioral tests in mice to study postoperative delirium. Sci. Rep. 2016, 6, 29874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Gao, J.; Guo, G.; Li, S.; Zhan, G.; Xie, Z.; Yang, C.; Luo, A. Anesthesia and surgery induce delirium-like behavior in susceptible mice: The role of oxidative stress. Am. J. Transl. Res. 2018, 10, 2435–2444. [Google Scholar]
- Prolo, C.; Alvarez, M.N.; Radi, R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. Biofactors 2014, 40, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Li, J.; Jimenez, D.A.; Levitan, E.S.; Aizenman, E.; Rosenberg, P.A. Peroxynitrite-induced neuronal apoptosis is mediated by intracellular zinc release and 12-lipoxygenase activation. J. Neurosci. 2004, 24, 10616–10627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, M.G.; Pandharipande, P.; Morse, J.; Shotwell, M.S.; Milne, G.L.; Pretorius, M.; Shaw, A.D.; Roberts, L.J., 2nd; Billings, F.T.t. Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic. Biol. Med. 2017, 103, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Zhang, W.; Liu, J.; Song, Y.; Liu, T.; Li, Z.; Wang, X.; Yang, N.; Li, Y.; Han, D.; et al. Metabolomic and Lipidomic Profiling of Preoperative CSF in Elderly Hip Fracture Patients with Postoperative Delirium. Front. Aging Neurosci. 2020, 12, 570210. [Google Scholar] [CrossRef]
- Kazmierski, J.; Miler, P.; Pawlak, A.; Jerczynska, H.; Wozniak, J.; Frankowska, E.; Brzezinska, A.; Nowakowska, K.; Wozniak, K.; Krejca, M.; et al. Oxidative stress and soluble receptor for advanced glycation end-products play a role in the pathophysiology of delirium after cardiac surgery. Sci. Rep. 2021, 11, 23646. [Google Scholar] [CrossRef]
- Egberts, A.; Fekkes, D.; Wijnbeld, E.H.; van der Ploeg, M.A.; van Saase, J.L.; Ziere, G.; van der Cammen, T.J.; Mattace-Raso, F.U. Disturbed Serotonergic Neurotransmission and Oxidative Stress in Elderly Patients with Delirium. Dement. Geriatr. Cogn. Dis. Extra 2015, 5, 450–458. [Google Scholar] [CrossRef]
- Coppola, S.; Caccioppola, A.; Chiumello, D. Internal clock and the surgical ICU patient. Curr. Opin. Anaesthesiol. 2020, 33, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, R.C.; Warman, G.R.; Sleigh, J.; Ludin, N.M.; Cheeseman, J.F. How does general anaesthesia affect the circadian clock? Sleep Med. Rev. 2018, 37, 35–44. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Yuan, Y.; Jia, X.; Zhang, W.; Wang, G.; Jia, Y.; Wang, X.; Liu, L.; Li, W.; et al. Effects of general versus subarachnoid anaesthesia on circadian melatonin rhythm and postoperative delirium in elderly patients undergoing hip fracture surgery: A prospective cohort clinical trial. EBioMedicine 2021, 70, 103490. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Speh, J.C. GABA is the principal neurotransmitter of the circadian system. Neurosci. Lett. 1993, 150, 112–116. [Google Scholar] [CrossRef]
- Gao, B.; Fritschy, J.M.; Moore, R.Y. GABAA-receptor subunit composition in the circadian timing system. Brain Res. 1995, 700, 142–156. [Google Scholar] [CrossRef]
- Novak, C.M.; Albers, H.E. Novel phase-shifting effects of GABAA receptor activation in the suprachiasmatic nucleus of a diurnal rodent. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R820–R825. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, B.F.; Andretic, R.; Heller, H.C.; Carter, D.B.; Kilduff, T.S. GABAA, GABAC, and NMDA receptor subunit expression in the suprachiasmatic nucleus and other brain regions. Brain Res. Mol. Brain Res. 1995, 28, 239–250. [Google Scholar] [CrossRef]
- Mihara, T.; Kikuchi, T.; Kamiya, Y.; Koga, M.; Uchimoto, K.; Kurahashi, K.; Goto, T. Day or night administration of ketamine and pentobarbital differentially affect circadian rhythms of pineal melatonin secretion and locomotor activity in rats. Anesth. Analg. 2012, 115, 805–813. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Jang, J.S.; Hwang, S.M.; Tark, H.; Kim, J.H.; Lee, J.J. Effects of surgery start time on postoperative cortisol, inflammatory cytokines, and postoperative hospital day in hip surgery: Randomized controlled trial. Medicine 2019, 98, e15820. [Google Scholar] [CrossRef]
- Meagher, D.J.; Moran, M.; Raju, B.; Gibbons, D.; Donnelly, S.; Saunders, J.; Trzepacz, P.T. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. Br. J. Psychiatry 2007, 190, 135–141. [Google Scholar] [CrossRef] [Green Version]
- BaHammam, A. Sleep in acute care units. Sleep Breath 2006, 10, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Cirelli, C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef] [Green Version]
- Terrando, N.; Yang, T.; Ryu, J.K.; Newton, P.T.; Monaco, C.; Feldmann, M.; Ma, D.; Akassoglou, K.; Maze, M. Stimulation of the alpha7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol. Med. 2015, 20, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Xue, R.; Wan, Y.; Sun, X.; Zhang, X.; Gao, W.; Wu, W. Nicotinic Mitigation of Neuroinflammation and Oxidative Stress after Chronic Sleep Deprivation. Front. Immunol. 2019, 10, 2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewy, A.J. Melatonin as a marker and phase-resetter of circadian rhythms in humans. Adv. Exp. Med. Biol. 1999, 460, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, J.; Zhu, R.; Gao, S.; Fan, J.; Xia, M.; Zhao, R.C.; Zhang, J. Melatonin protects blood-brain barrier integrity and permeability by inhibiting matrix metalloproteinase-9 via the NOTCH3/NF-kappaB pathway. Aging 2019, 11, 11391–11415. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Dou, Y.; Tian, X.; Liu, C.; Li, H.; Shen, H.; Chen, G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury. Transl. Stroke Res. 2018, 9, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.M.; Axon, D.R.; Martin, J.R.; Slack, M.K.; Mollon, L.; Lee, J.K. Melatonin for the prevention of postoperative delirium in older adults: A systematic review and meta-analysis. BMC Geriatr. 2019, 19, 272. [Google Scholar] [CrossRef] [Green Version]
- Khaing, K.; Nair, B.R. Melatonin for delirium prevention in hospitalized patients: A systematic review and meta-analysis. J. Psychiatr. Res. 2021, 133, 181–190. [Google Scholar] [CrossRef]
- Blodgett, T.J.; Blodgett, N.P. Melatonin and melatonin-receptor agonists to prevent delirium in hospitalized older adults: An umbrella review. Geriatr. Nurs. 2021, 42, 1562–1568. [Google Scholar] [CrossRef]
- Leung, J.M.; Sands, L.P.; Newman, S.; Meckler, G.; Xie, Y.; Gay, C.; Lee, K. Preoperative Sleep Disruption and Postoperative Delirium. J. Clin. Sleep. Med. 2015, 11, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Saito, N.; Miyawaki, I.; Shiotani, H. Preoperative circadian physical activity rhythm and postoperative delirium in cardiovascular surgery patients. Chronobiol. Int. 2020, 37, 1059–1066. [Google Scholar] [CrossRef]
- Chen, H.; Mo, L.; Hu, H.; Ou, Y.; Luo, J. Risk factors of postoperative delirium after cardiac surgery: A meta-analysis. J. Cardiothorac. Surg. 2021, 16, 113. [Google Scholar] [CrossRef]
- Pinho, C.; Cruz, S.; Santos, A.; Abelha, F.J. Postoperative delirium: Age and low functional reserve as independent risk factors. J. Clin. Anesth. 2016, 33, 507–513. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Lovett, N.G.; Smith, S.C.; Dutta, N.; Bendon, C.; Lloyd-Lavery, A.; Mehta, Z.; Rothwell, P.M. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: Age-specific rates and associated factors, mortality and re-admission. BMJ Open 2015, 5, e007808. [Google Scholar] [CrossRef]
- Streit, W.J.; Sammons, N.W.; Kuhns, A.J.; Sparks, D.L. Dystrophic microglia in the aging human brain. Glia 2004, 45, 208–212. [Google Scholar] [CrossRef]
- Streit, W.J. Microglia and neuroprotection: Implications for Alzheimer’s disease. Brain Res. Brain Res. Rev. 2005, 48, 234–239. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bisht, K.; Tremblay, M.E. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Lyons, A.; Downer, E.J.; Crotty, S.; Nolan, Y.M.; Mills, K.H.; Lynch, M.A. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: A role for IL-4. J. Neurosci. 2007, 27, 8309–8313. [Google Scholar] [CrossRef] [Green Version]
- Wynne, A.M.; Henry, C.J.; Huang, Y.; Cleland, A.; Godbout, J.P. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav. Immun. 2010, 24, 1190–1201. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.G.; Barrientos, R.M.; Biedenkapp, J.C.; Rudy, J.W.; Watkins, L.R.; Maier, S.F. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol. Aging 2006, 27, 717–722. [Google Scholar] [CrossRef]
- Safaiyan, S.; Kannaiyan, N.; Snaidero, N.; Brioschi, S.; Biber, K.; Yona, S.; Edinger, A.L.; Jung, S.; Rossner, M.J.; Simons, M. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 2016, 19, 995–998. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Munch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [Green Version]
- Liufu, N.; Liu, L.; Shen, S.; Jiang, Z.; Dong, Y.; Wang, Y.; Culley, D.; Crosby, G.; Cao, M.; Shen, Y.; et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging 2020, 12, 1965–1986. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, J.J.; Guo, G.J.; Yang, L.; Zhu, B.; Zhan, G.F.; Li, S.; Huang, N.N.; Hashimoto, K.; Yang, C.; et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci. Ther. 2019, 25, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Yoshitani, K.; Yahagi, M.; Asahara, T.; Shishido, Y.; Fukushima, S.; Tadokoro, N.; Fujita, T.; Ohnishi, Y. Association between postoperative changes in the gut microbiota and pseudopsia after cardiac surgery: Prospective observational study. BMC Surg. 2020, 20, 247. [Google Scholar] [CrossRef]
- Lederer, A.K.; Pisarski, P.; Kousoulas, L.; Fichtner-Feigl, S.; Hess, C.; Huber, R. Postoperative changes of the microbiome: Are surgical complications related to the gut flora? A systematic review. BMC Surg. 2017, 17, 125. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, M.; Koutsogiannaki, S.; Schaefers, M.; Babazada, H.; Liu, R.; Yuki, K. The Differential Effects of Anesthetics on Bacterial Behaviors. PLoS ONE 2017, 12, e0170089. [Google Scholar] [CrossRef] [Green Version]
- Pejcic, A.V. Delirium associated with the use of macrolide antibiotics: A review. Int. J. Psychiatry Clin. Pract. 2022, 26, 29–42. [Google Scholar] [CrossRef]
- Teng, C.; Frei, C.R. Delirium Associations with Antibiotics: A Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). Drugs Real World Outcomes 2022, 9, 23–29. [Google Scholar] [CrossRef]
- Ekmekciu, I.; von Klitzing, E.; Fiebiger, U.; Escher, U.; Neumann, C.; Bacher, P.; Scheffold, A.; Kuhl, A.A.; Bereswill, S.; Heimesaat, M.M. Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice. Front. Immunol. 2017, 8, 397. [Google Scholar] [CrossRef] [Green Version]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis. 2020, 11, 1029–1045. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, G.; Xu, Y.L.; Fang, Q.; Ye, L.; Wang, C.H.; Liu, X.S. Preoperative Status of Gut Microbiota Predicts Postoperative Delirium in Patients with Gastric Cancer. Front. Psychiatry 2022, 13, 852269. [Google Scholar] [CrossRef] [PubMed]
- Mossad, O.; Batut, B.; Yilmaz, B.; Dokalis, N.; Mezo, C.; Nent, E.; Nabavi, L.S.; Mayer, M.; Maron, F.J.M.; Buescher, J.M.; et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N(6)-carboxymethyllysine. Nat. Neurosci. 2022, 25, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhu, Q.; Sun, L.; Cheng, Y. Effect of Anesthesia/Surgery on Gut Microbiota and Fecal Metabolites and Their Relationship with Cognitive Dysfunction. Front. Syst. Neurosci. 2021, 15, 655695. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Ye, M.; Luo, F.; Hu, B.; Wang, A.; Chen, J.; Yan, J.; He, Z.; Chen, F.; Qian, C.; et al. Probiotics treatment improves cognitive impairment in patients and animals: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 120, 159–172. [Google Scholar] [CrossRef]
- Mao, L.; Zeng, Q.; Su, W.; Song, M.; Li, J.; Xie, M. Elevation of miR-146a Inhibits BTG2/BAX Expression to Ameliorate Postoperative Cognitive Dysfunction Following Probiotics (VSL#3) Treatment. Mol. Neurobiol. 2021, 58, 3457–3470. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Xue, L.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef]
- Yang, X.D.; Wang, L.K.; Wu, H.Y.; Jiao, L. Effects of prebiotic galacto-oligosaccharide on postoperative cognitive dysfunction and neuroinflammation through targeting of the gut-brain axis. BMC Anesthesiol. 2018, 18, 177. [Google Scholar] [CrossRef] [Green Version]
- Flanigan, P.M.; Jahangiri, A.; Weinstein, D.; Dayani, F.; Chandra, A.; Kanungo, I.; Choi, S.; Sankaran, S.; Molinaro, A.M.; McDermott, M.W.; et al. Postoperative Delirium in Glioblastoma Patients: Risk Factors and Prognostic Implications. Neurosurgery 2018, 83, 1161–1172. [Google Scholar] [CrossRef]
- Viderman, D.; Brotfain, E.; Bilotta, F.; Zhumadilov, A. Risk Factors and Mechanisms of Postoperative Delirium after Intracranial Neurosurgical Procedures. Asian J. Anesthesiol. 2020, 58, 5–13. [Google Scholar] [CrossRef]
- Bjornsson, G.L.; Thorsteinsson, L.; Gudmundsson, K.O.; Jonsson, H., Jr.; Gudmundsson, S.; Gudbjornsson, B. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand. J. Immunol. 2007, 65, 99–105. [Google Scholar] [CrossRef]
- Boehme, A.K.; Hays, A.N.; Kicielinski, K.P.; Arora, K.; Kapoor, N.; Lyerly, M.J.; Gadpaille, A.; Shiue, H.; Albright, K.; Miller, D.; et al. Systemic Inflammatory Response Syndrome and Outcomes in Intracerebral Hemorrhage. Neurocrit. Care 2016, 25, 133–140. [Google Scholar] [CrossRef]
- Yu, Y.P.; Chi, X.L.; Liu, L.J. A hypothesis: Hydrogen sulfide might be neuroprotective against subarachnoid hemorrhage induced brain injury. Sci. World J. 2014, 2014, 432318. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radtke, F.M.; Franck, M.; MacGuill, M.; Seeling, M.; Lutz, A.; Westhoff, S.; Neumann, U.; Wernecke, K.D.; Spies, C.D. Duration of fluid fasting and choice of analgesic are modifiable factors for early postoperative delirium. Eur. J. Anaesthesiol. 2010, 27, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Pincus, D.; Choi, S.; Jenkinson, R.; Wasserstein, D.N.; Redelmeier, D.A. Association of Duration of Surgery with Postoperative Delirium Among Patients Receiving Hip Fracture Repair. JAMA Netw. Open 2019, 2, e190111. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ma, Q.; Du, H.; Yang, C.; Lin, G. Postoperative Delirium in Neurosurgical Patients: Recent Insights into the Pathogenesis. Brain Sci. 2022, 12, 1371. https://doi.org/10.3390/brainsci12101371
Xu Y, Ma Q, Du H, Yang C, Lin G. Postoperative Delirium in Neurosurgical Patients: Recent Insights into the Pathogenesis. Brain Sciences. 2022; 12(10):1371. https://doi.org/10.3390/brainsci12101371
Chicago/Turabian StyleXu, Yinuo, Qianquan Ma, Haiming Du, Chenlong Yang, and Guozhong Lin. 2022. "Postoperative Delirium in Neurosurgical Patients: Recent Insights into the Pathogenesis" Brain Sciences 12, no. 10: 1371. https://doi.org/10.3390/brainsci12101371
APA StyleXu, Y., Ma, Q., Du, H., Yang, C., & Lin, G. (2022). Postoperative Delirium in Neurosurgical Patients: Recent Insights into the Pathogenesis. Brain Sciences, 12(10), 1371. https://doi.org/10.3390/brainsci12101371





