Intra-Arrest Therapeutic Hypothermia and Neurologic Outcome in Patients Admitted after Out-of-Hospital Cardiac Arrest: A Post Hoc Analysis of the Princess Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Patients
2.3. Randomization and Trial Intervention
2.4. Data Collection
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Primary Outcome
3.3. Secondary Outcomes
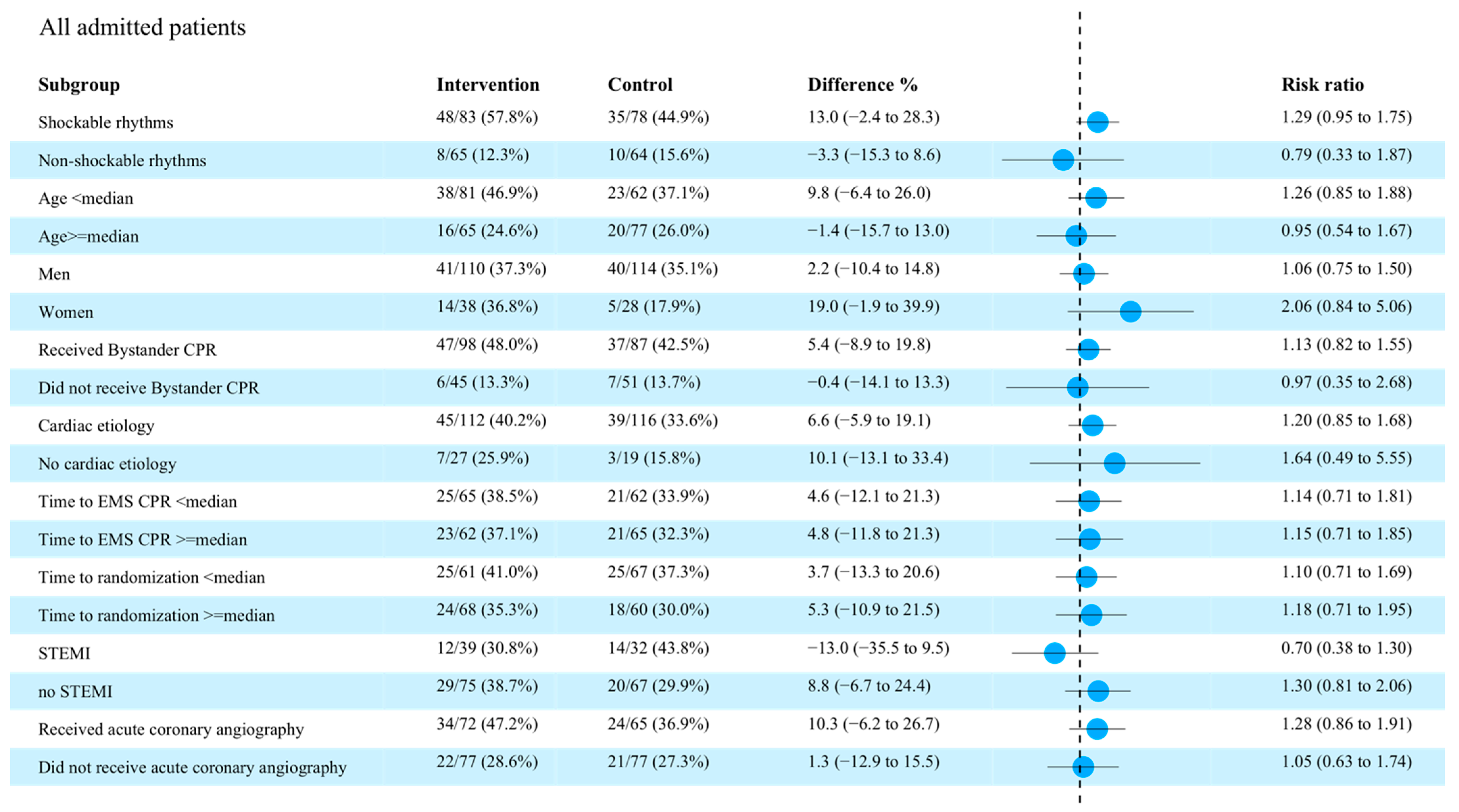
3.4. Subgroup Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Panchal, A.R.; Bartos, J.A.; Cabañas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142 (Suppl 2), S366–S468. [Google Scholar] [CrossRef] [PubMed]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lascarrou, J.-B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.-F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Picetti, E.; Vincent, J.-L. High Quality Targeted Temperature Management (TTM) After Cardiac Arrest. Crit. Care 2020, 24, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abella, B.S.; Zhao, D.; Alvarado, J.; Hamann, K.; Hoek, T.L.V.; Becker, L.B. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004, 109, 2786–2791. [Google Scholar] [CrossRef] [Green Version]
- Nozari, A.; Safar, P.; Stezoski, S.W.; Wu, X.; Kostelnik, S.; Radovsky, A.; Tisherman, S.; Kochanek, P. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation 2006, 113, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Riter, H.G.; Brooks, L.A.; Pretorius, A.M.; Ackermann, L.W.; Kerber, R.E. Intra-arrest hypothermia: Both cold liquid ventilation with perfluorocarbons and cold intravenous saline rapidly achieve hypothermia, but only cold liquid ventilation improves resumption of spontaneous circulation. Resuscitation 2009, 80, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staffey, K.S.; Dendi, R.; Brooks, L.A.; Pretorius, A.M.; Ackermann, L.W.; Zamba, K.; Dickson, E.; Kerber, R.E. Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation 2008, 78, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castrén, M.; Nordberg, P.; Svensson, L.; Taccone, F.; Vincent, J.; Desruelles, D.; Eichwede, F.; Mols, P.; Schwab, T.; Vergnion, M.; et al. Intra-arrest transnasal evaporative cooling: A randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation 2010, 122, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, P.; Taccone, F.S.; Truhlar, A.; Forsberg, S.; Hollenberg, J.; Jonsson, M.; Cuny, J.; Goldstein, P.; Vermeersch, N.; Higuet, A.; et al. Effect of Trans-Nasal Evaporative Intra-arrest Cooling on Functional Neurologic Outcome in Out-of-Hospital Cardiac Arrest: The PRINCESS Randomized Clinical Trial. JAMA 2019, 321, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Hollenberg, J.; Forsberg, S.; Truhlar, A.; Jonsson, M.; Annoni, F.; Gryth, D.; Ringh, M.; Cuny, J.; Busch, H.J.; et al. Effect of intra-arrest trans-nasal evaporative cooling in out-of-hospital cardiac arrest: A pooled individual participant data analysis. Crit. Care 2021, 25, 198. [Google Scholar] [CrossRef]
- Nordberg, P.; Taccone, F.S.; Castren, M.; Truhlář, A.; Desruelles, D.; Forsberg, S.; Hollenberg, J.; Vincent, J.-L.; Svensoon, L. Design of the PRINCESS trial: Pre-hospital resuscitation intra-nasal cooling effectiveness survival study (PRINCESS). BMC Emerg. Med. 2013, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.J.R.; Nolan, J.P. The role of adrenaline in cardiopulmonary resuscitation. Crit. Care 2018, 22, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef]
- Haywood, K.; Whitehead, L.; Nadkarni, V.M.; Achana, F.; Beesems, S.; Böttiger, B.W.; Brooks, A.; Castrén, M.; Ong, M.E.; Hazinski, M.F.; et al. COSCA (Core Outcome Set for Cardiac Arrest) in Adults: An Advisory Statement From the International Liaison Committee on Resuscitation. Circulation 2018, 137, e783–e801. [Google Scholar] [CrossRef]
- Hsu, C.; Li, J.; Cinousis, M.J.; Sheak, K.R.; Gaieski, D.F.; Abella, B.; Leary, M. Cerebral performance category at hospital discharge predicts long-term survival of cardiac arrest survivors receiving targeted temperature management. Crit. Care Med. 2014, 42, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Cariou, A.; Claessens, Y.E.; Pène, F.; Marx, J.S.; Spaulding, C.; Hababou, C.; Casadevall, N.; Mira, J.-P.; Carli, P.; Hermine, O. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: A matched control study. Resuscitation 2008, 76, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Cariou, A.; Deye, N.; Vivien, B.; Richard, O.; Pichon, N.; Bourg, A.; Huet, L.; Buleon, C.; Frey, J.; Asfar, P.; et al. Early High-Dose Erythropoietin Therapy After Out-of-Hospital Cardiac Arrest: A Multicenter, Randomized Controlled Trial. J. Am. Coll. Cardiol. 2016, 68, 40–49. [Google Scholar] [CrossRef] [PubMed]


| Intervention (n = 149) | Control (n = 142) | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 62 [53–70] | 65 [57–71] | 0.13 |
| Male sex, n (%) | 110 (73.8%) | 114 (80.2%) | 0.23 |
| Height, cm | 177 [170–180] | 178 [170–180] | 0.78 |
| Weight, Kg | 81 [70–90] | 85 [75–95] | 0.16 |
| Resuscitation Characteristics, No./Total (%) | |||
| Location at home, n (%) | 70 (47.0%) | 86 (60.5%) | 0.02 |
| Presumed cardiac cause, n (%) | 112 (75.2%) | 116 (81.7%) | 0.24 |
| Shockable rhythm, n (%) | 83 (55.7%) | 78 (54.9%) | 0.84 |
| Bystander CPR performed, n (%) | 98 (65.8%) | 87 (61.3%) | 0.33 |
| CPR by first responder, n (%) | 79 (53.0%) | 92 (64.8%) | 0.06 |
| Medical history | 0.33 | ||
| Coronary artery disease, n (%) | 24 (16.1%) | 33 (23.2%) | |
| Hypertension, n (%) | 29 (19.4%) | 31 (21.8%) | |
| COPD, n (%) | 9 (6.0%) | 4 (2.8%) | |
| Heart failure, n (%) | 4 (2.8%) | 3 (2.1%) | |
| Pulmonary embolism, n (%) | 0 (0%) | 2 (1.4%) | |
| Key median time (IQR), min | |||
| Time to CPR by EMS, min | 8 [6–11] | 8 [6–12] | 0.26 |
| Time to ALS arrival, min | 11 [8–17] | 11 [8–16] | 0.53 |
| Time to airway established, min | 13 [10–17] | 12 [10–16] | 0.66 |
| Time to randomization, min | 15 [12–20] | 14 [11–19] | 0.15 |
| Intervention (n = 149) | Control (n = 142) | p-Value | |
|---|---|---|---|
| Prehospital characteristics | |||
| Adrenaline, median [IQR], mg | 5 [3–7] | 4 [2–7] | 0.12 |
| Amiodarone, median [IQR], mg | 300 [300–300] | 300 [300–300] | 0.4 |
| Duration CPR by EMS, median [IQR], min | 18 [7–30] | 13 [6–21] | 0.01 |
| Ongoing CPR to hospital, No./total (%) | 7 (4.7%) | 11 (7.7%) | 0.72 |
| New prehospital cardiac arrest, No./total (%) | 15 (10.0%) | 14 (10%) | 0.96 |
| Time to start of EMS cooling, median [IQR], min | 17 [14–23] | - | - |
| Time to ROSC, median [IQR], min | 29 [30–35] | 25 [19–35] | 0.98 |
| Tympanic temperature at ROSC—°C, median [IQR] | 35.8 [34.8–36.4] | 36 [35.5–36.5] | 0.06 |
| Time to hospital arrival, median [IQR], min | 51 [43–63] | 54 [40–64] | 0.98 |
| Characteristics at hospital admission | |||
| Tympanic temperature at ED, median [IQR] | 34.8 [34.2–35.5] | 35.8 [35.4–36.2] | 0.002 |
| Glasgow Coma Scale, median [IQR] | 3 [3–3] | 3 [3,4] | 0.25 |
| PaCO2, median [IQR], mmHg | 33 [27–39] | 34 [26–41] | 0.66 |
| Arterial pH—value, median [IQR] | 7.15 [6.98–7.24] | 7.17 [7.03–7.29] | 0.06 |
| Base excess, median [IQR], mmol/l | −14 [−20–−9] | −12 [−16–−10] | 0.14 |
| Lactate, median [IQR], mmol/l | 10.2 [7.7–14.4] | 10.3 [7.4–13.9] | 0.82 |
| Heart rate, median [IQR], mmol/l | 87 [72–103] | 86 [68–102] | 1.00 |
| Systolic blood pressure, median [IQR], mmHg | 115 [92–143] | 113 [98–133] | 0.68 |
| Mean arterial pressure, median [IQR], mmHg | 78 [63–96] | 79 [68–94] | 0.78 |
| SpO2, median [IQR], mmHg | 98 [93–99] | 98 [95–99] | 0.25 |
| Spontaneous breathing, No./total (%) | 33 (22.1%) | 35 (24.6%) | 0.80 |
| ST-elevation/new LBBB on ECG—No./total (%) | 39 (26.2%) | 32 (22.5%) | 0.77 |
| ST-depression >1 mm on ECG—No./total (%) | 26 (17.4%) | 35 (24.6%) | 0.045 |
| Revascularization and circulatory support during ICU stay, n (%) | |||
| Angiography after hospital admission | 72 (48.3%) | 65 (45.8%) | 0.96 |
| Angiography during ICU stay | 10 (6.7%) | 8 (5.6%) | 0.72 |
| Angiography after ICU stay | 4 (2.6%) | 4 (2.8%) | 0.67 |
| PCI performed | 50 (33.6%) | 41 (28.9%) | 0.52 |
| CABG performed | 5 (3.4%) | 1 (0.7%) | 0.12 |
| IABP performed | 5 (3.4%) | 6 (4.2%) | 0.70 |
| Primary Outcome | Intervention | Control | Difference (95% CI) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Survival with CPC 1–2 at 90 days, n (%) | |||||
| All patients | 56/149 (37.6%) | 45/142 (31.7%) | 5.9 [−5.2–16.8] | 1.19 [0.86–1.63] | 0.29 |
| Shockable rhythm | 48/83 (57.8%) | 35/78 (44.9%) | 12.9 [−2.4–28.3] | 1.29 [0.95–1.75] | 0.10 |
| Non-shockable rhythm | 8/66 (12.1%) | 10/64 (15.6%) | −3.3 [−15.3–8.6] | 0.79 [0.33–1.87] | 0.58 |
| Secondary Outcome | Intervention | Control | Difference (95% CI) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Overall survival at 90 days, no (%) | |||||
| All patients | 60/149 (40.3%) | 52/142 (36.6%) | 3.7 [−7.5–14.8] | 1.10 [0.82–1.47] | 0.09 |
| Shockable rhythm | 51/83 (61.4%) | 41/78 (52.6%) | 8.8 [−6.4–24.1] | 1.17 [0.89–1.53] | 0.25 |
| Non-shockable rhythm | 9/66 (13.6%) | 11/64 (17.1%) | −3.5 [−15.8–9.1] | 0.81 [0.36–1.81] | 0.36 |
| Survival with CPC 1 at 90 days, no (%) | |||||
| All patients | 50/149 (33.6%) | 35/142 (24.6%) | 9.0 [−1.5–19.3] | 1.36 [0.94–1.96] | 0.11 |
| Shockable rhythm | 45/83 (54.2%) | 27/78 (34.6%) | 19.6 [4.6–34.6] | 1.57 [1.09–2.25] | 0.01 |
| Non-shockable rhythm | 5/66 (7.6%) | 8/64 (12.5%) | −5.0 [−15.2–5.6] | 0.62 [0.21–1.78] | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MACCHINI, E.; DILLENBECK, E.; JONSSON, M.; ANNONI, F.; FORSBERG, S.; HOLLENBERG, J.; TRUHLAR, A.; SVENSSON, L.; NORDBERG, P.; TACCONE, F.S. Intra-Arrest Therapeutic Hypothermia and Neurologic Outcome in Patients Admitted after Out-of-Hospital Cardiac Arrest: A Post Hoc Analysis of the Princess Trial. Brain Sci. 2022, 12, 1374. https://doi.org/10.3390/brainsci12101374
MACCHINI E, DILLENBECK E, JONSSON M, ANNONI F, FORSBERG S, HOLLENBERG J, TRUHLAR A, SVENSSON L, NORDBERG P, TACCONE FS. Intra-Arrest Therapeutic Hypothermia and Neurologic Outcome in Patients Admitted after Out-of-Hospital Cardiac Arrest: A Post Hoc Analysis of the Princess Trial. Brain Sciences. 2022; 12(10):1374. https://doi.org/10.3390/brainsci12101374
Chicago/Turabian StyleMACCHINI, Elisabetta, Emelie DILLENBECK, Martin JONSSON, Filippo ANNONI, Sune FORSBERG, Jacob HOLLENBERG, Anatolij TRUHLAR, Leif SVENSSON, Per NORDBERG, and Fabio Silvio TACCONE. 2022. "Intra-Arrest Therapeutic Hypothermia and Neurologic Outcome in Patients Admitted after Out-of-Hospital Cardiac Arrest: A Post Hoc Analysis of the Princess Trial" Brain Sciences 12, no. 10: 1374. https://doi.org/10.3390/brainsci12101374
APA StyleMACCHINI, E., DILLENBECK, E., JONSSON, M., ANNONI, F., FORSBERG, S., HOLLENBERG, J., TRUHLAR, A., SVENSSON, L., NORDBERG, P., & TACCONE, F. S. (2022). Intra-Arrest Therapeutic Hypothermia and Neurologic Outcome in Patients Admitted after Out-of-Hospital Cardiac Arrest: A Post Hoc Analysis of the Princess Trial. Brain Sciences, 12(10), 1374. https://doi.org/10.3390/brainsci12101374







