Cannabis-Based Medicinal Products in the Management of Emotionally Unstable Personality Disorder (EUPD): A Narrative Review and Case Series
Abstract
:1. Introduction
1.1. EUPD
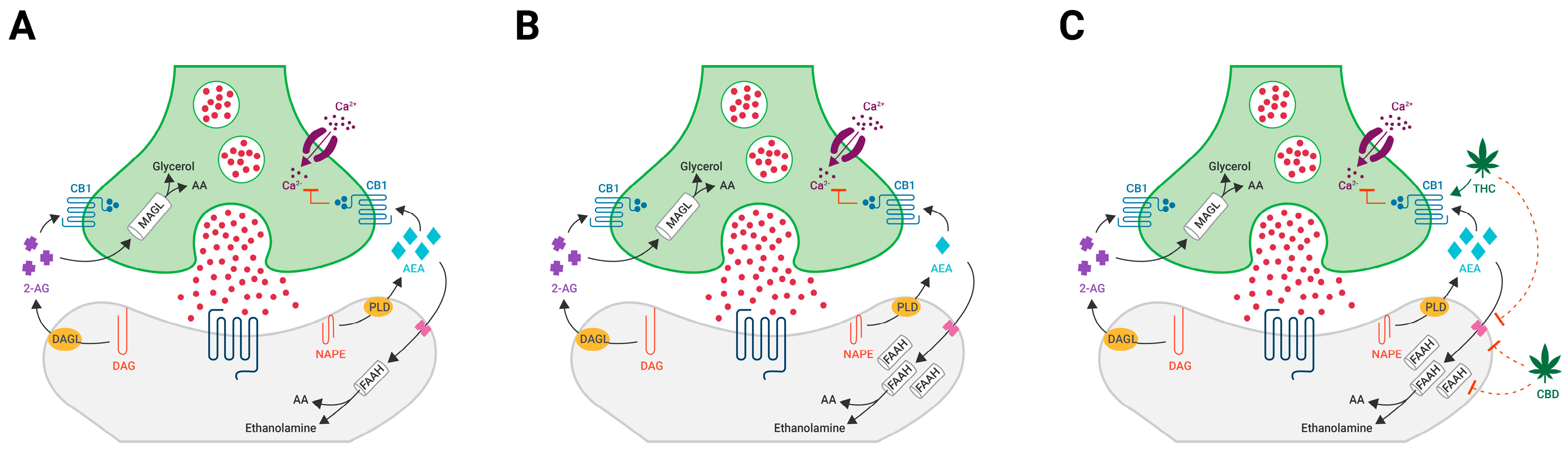
1.2. The Neuromodulating Effects of Cannabinoids
1.3. The Endocannabinoid System as a Therapeutic Target for EUPD
2. Materials and Methods
2.1. Participants
2.2. Cannabis-Based Medicinal Products
2.3. Outcome Measures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roughley, M.; Maguire, A.; Wood, G.; Lee, T. Referral of patients with emotionally unstable personality disorder for specialist psychological therapy: Why, when and how? BJPsych Bull. 2020, 45, 52–58. [Google Scholar] [CrossRef]
- Gamlin, C.; Varney, A.; Agius, M. Emotionally Unstable PersonalityDisorder in Primary Care: A Thematic Review and Novel Toolkit. Psychiatr. Danub. 2019, 31, 282–289. [Google Scholar]
- Holm, A.L.; Severinsson, E. The emotional pain and distress of borderline personality disorder: A review of the literature. Int. J. Ment. Health Nurs. 2008, 17, 27–35. [Google Scholar] [CrossRef]
- Distel, M.A.; Trull, T.J.; Derom, C.; Thiery, E.W.; Grimmer, M.A.; Martin, N.; Willemsen, G.; Boomsma, D.I. Heritability of borderline personality disorder features is similar across three countries. Psychol. Med. 2007, 38, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Fonagy, P.; Luyten, P. A developmental, mentalization-based approach to the understanding and treatment of borderline personality disorder. Dev. Psychopathol. 2009, 21, 1355–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torgersen, S.; Myers, J.; Reichborn-Kjennerud, T.; Roysamb, E.; Kubarych, T.S.; Kendler, K.S. The Heritability of Cluster B Personality Disorders Assessed Both by Personal Interview and Questionnaire. J. Pers. Disord. 2012, 26, 848–866. [Google Scholar] [CrossRef]
- Witt, S.H.; Streit, F.; Jungkunz, M.; Frank, J.; Awasthi, S.; Reinbold, C.S.; Treutlein, J.; Degenhardt, F.; Forstner, A.J.; Heilmann-Heimbach, S.; et al. Genome-wide association study of borderline personality disorder reveals genetic overlap with bipolar disorder, major depression and schizophrenia. Transl. Psychiatry 2017, 7, e1155. [Google Scholar] [CrossRef] [Green Version]
- Paris, J. Clinical Trials of Treatment for Personality Disorders. Psychiatr. Clin. N. Am. 2008, 31, 517–526. [Google Scholar] [CrossRef]
- Skodol, A.E.; Siever, L.J.; Livesley, W.; Gunderson, J.G.; Pfohl, B.; Widiger, T.A. The borderline diagnosis II: Biology, genetics, and clinical course. Biol. Psychiatry 2002, 51, 951–963. [Google Scholar] [CrossRef]
- Gurvits, I.G.; Koenigsberg, H.W.; Siever, L.J. Neurotransmitter Dysfunction in Patients with Borderline Personality Disorder. Psychiatr. Clin. N. Am. 2000, 23, 27–40. [Google Scholar] [CrossRef]
- Calati, R.; Gressier, F.; Balestri, M.; Serretti, A. Genetic modulation of borderline personality disorder: Systematic review and meta-analysis. J. Psychiatr. Res. 2013, 47, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Kolla, N.J.; Mizrahi, R.; Karas, K.; Wang, C.; Bagby, R.M.; Mc Main, S.; Simpson, A.I.; Rusjan, P.M.; Tyndale, R.; Houle, S.; et al. Elevated fatty acid amide hydrolase in the prefrontal cortex of borderline personality disorder: A [11C]CURB positron emission tomography study. Neuropsychopharmacology 2020, 45, 1834–1841. [Google Scholar] [CrossRef]
- Storebø, O.J.; Stoffers-Winterling, J.M.; Völlm, B.A.; Kongerslev, M.T.; Mattivi, J.T.; Jørgensen, M.S.; Faltinsen, E.; Todorovac, A.; Sales, C.P.; E Callesen, H.; et al. Psychological therapies for people with borderline personality disorder. Cochrane Database Syst. Rev. 2020, 2020, CD012955. [Google Scholar] [CrossRef] [Green Version]
- Guidance|Borderline Personality Disorder: Recognition and Management|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/cg78/chapter/1-Guidance (accessed on 25 September 2022).
- Gartlehner, G.; Crotty, K.; Kennedy, S.; Edlund, M.J.; Ali, R.; Siddiqui, M.; Fortman, R.; Wines, R.; Persad, E.; Viswanathan, M. Pharmacological Treatments for Borderline Personality Disorder: A Systematic Review and Meta-Analysis. CNS Drugs 2021, 35, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Zanarini, M.C.; Frankenburg, F.R.; Reich, D.B.; Harned, A.L.; Fitzmaurice, G.M. Rates of Psychotropic Medication Use Reported by Borderline Patients and Axis II Comparison Subjects Over 16 Years of Prospective Follow-Up. J. Clin. Psychopharmacol. 2015, 35, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Paton, C.; Crawford, M.; Bhatti, S.F.; Patel, M.X.; Barnes, T.R.E. The Use of Psychotropic Medication in Patients with Emotionally Unstable Personality Disorder under the Care of UK Mental Health Services. J. Clin. Psychiatry 2015, 76, e512–e518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.F.; Sharma, M.S.; Brunoni, A.R.; Vieta, E.; Fava, G.A. The Safety, Tolerability and Risks Associated with the Use of Newer Generation Antidepressant Drugs: A Critical Review of the Literature. Psychother. Psychosom. 2016, 85, 270–288. [Google Scholar] [CrossRef]
- Orsolini, L.; Tomasetti, C.; Valchera, A.; Vecchiotti, R.; Matarazzo, I.; Vellante, F.; Iasevoli, F.; Buonaguro, E.F.; Fornaro, M.; Fiengo, A.L.C.; et al. An update of safety of clinically used atypical antipsychotics. Expert Opin. Drug Saf. 2016, 15, 1329–1347. [Google Scholar] [CrossRef]
- Ashton, C.H. Pharmacology and effects of cannabis: A brief review. Br. J. Psychiatry 2001, 178, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30 (Suppl. 1), S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.L.; Thakur, G.A.; Makriyannis, A. Cannabinergic ligands. Chem. Phys. Lipids 2002, 121, 3–19. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Carrier, E.J.; McLaughlin, R.J.; Morrish, A.C.; Meier, S.E.; Hillard, C.J.; Gorzalka, B.B. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J. Neurochem. 2008, 106, 2322–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- De Meij, J.; Alfanek, Z.; Morel, L.; Decoeur, F.; Leyrolle, Q.; Picard, K.; Carrier, M.; Aubert, A.; Séré, A.; Lucas, C.; et al. Microglial Cannabinoid Type 1 Receptor Regulates Brain Inflammation in a Sex-Specific Manner. Cannabis Cannabinoid Res. 2021, 6, 488–507. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Patel, S.; Bains, J.; Hill, M.N. Neurobiological Interactions between Stress and the Endocannabinoid System. Neuropsychopharmacology 2015, 41, 80–102. [Google Scholar] [CrossRef] [Green Version]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [Green Version]
- Rupprecht, R.; Rupprecht, C.; Di Benedetto, B.; Rammes, G. Neuroinflammation and psychiatric disorders: Relevance of C1q, translocator protein (18 kDa) (TSPO), and neurosteroids. World J. Biol. Psychiatry 2021, 1–7. [Google Scholar] [CrossRef]
- MacDowell, K.S.; Marsá, M.D.; Buenache, E.; Villatoro, J.M.L.; Moreno, B.; Leza, J.C.; Carrasco, J.L. Inflammatory and antioxidant pathway dysfunction in borderline personality disorder. Psychiatry Res. 2020, 284, 112782. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Futur. Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Croxford, J.L.; Pryce, G.; Jackson, S.J.; Ledent, C.; Giovannoni, G.; Pertwee, R.G.; Yamamura, T.; Baker, D. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J. Neuroimmunol. 2008, 193, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Baker, D.; Cuzner, M.L.; Diemel, L.T. Cannabinoid-mediated neuroprotection following interferon-gamma treatment in a three-dimensional mouse brain aggregate cell culture. Eur. J. Neurosci. 2004, 20, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Molina-Holgado, E.; Guaza, C.; Rothwell, N.J. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J. Neurosci. Res. 2002, 67, 829–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nutt, D. Why doctors have a moral imperative to prescribe and support medical cannabis—An essay by David Nutt. BMJ 2022, 376, n3114. [Google Scholar] [CrossRef]
- Moreno-Sanz, G.; Madiedo, A.; Lynskey, M.; Brown, M.R.D. “Flower Power”: Controlled Inhalation of THC-Predominant Cannabis Flos Improves Health-Related Quality of Life and Symptoms of Chronic Pain and Anxiety in Eligible UK Patients. Biomedicines 2022, 10, 2576. [Google Scholar] [CrossRef]
- Moreno-Sanz, G.; Madiedo, A.; Hernandez, P.; Kratz, J.; Aizpurua-Olaizola, O.; Brown, M.R.D.; López, J.R.; Patiño, J.; Mendivelso, F.O. Sex-Dependent Prescription Patterns and Clinical Outcomes Associated With the Use of Two Oral Cannabis Formulations in the Multimodal Management of Chronic Pain Patients in Colombia. Front. Pain Res. 2022, 3, 854795. [Google Scholar] [CrossRef]
- LaFrance, E.M.; Glodosky, N.C.; Bonn-Miller, M.; Cuttler, C. Short and Long-Term Effects of Cannabis on Symptoms of Post-Traumatic Stress Disorder. J. Affect. Disord. 2020, 274, 298–304. [Google Scholar] [CrossRef]
- Bahorik, A.L.; Sterling, S.A.; Campbell, C.I.; Weisner, C.; Ramo, D.; Satre, D.D. Medical and non-medical marijuana use in depression: Longitudinal associations with suicidal ideation, everyday functioning, and psychiatry service utilization. J. Affect. Disord. 2018, 241, 8–14. [Google Scholar] [CrossRef]
- Kolla, N.J.; Boileau, I.; Bagby, R.M. Higher trait neuroticism is associated with greater fatty acid amide hydrolase binding in borderline and antisocial personality disorders. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty Acid-binding Proteins (FABPs) Are Intracellular Carriers for Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef] [Green Version]
- Davies, C.; Bhattacharyya, S. Cannabidiol as a potential treatment for psychosis. Ther. Adv. Psychopharmacol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Machado-Vieira, R.; Baumann, J.; Wheeler-Castillo, C.; Latov, D.; Henter, I.; Salvadore, G.; Zarate, J.C.A. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals 2010, 3, 19–41. [Google Scholar] [CrossRef]
- I Velligan, D.; Sajatovic, M.; Hatch, A.; Kramata, P.; Docherty, J.P. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer. Adherence 2017, 11, 449–468. [Google Scholar] [CrossRef]
| # | Sex | Age | Occupation | Psychiatric Diagnosis | Comorbidities (List) | Past/Present Substance Use |
|---|---|---|---|---|---|---|
| 1 | F | 24 | Housekeeper | EUPD | Ehlers-Danlos syndrome | Occasional alcohol Non-smoker Current illicit cannabis use |
| 2 | M | 24 | Global Immigration Assistant | EUPD Anxiety Depression OCD | Migraines | Occasional alcohol Non-smoker Previous illicit/medical cannabis use Previous BZD dependency |
| 3 | F | 34 | Unemployed | EUPD | HTN | No alcohol Non-smoker Current illicit cannabis use Previous opiate dependency |
| 4 | M | 28 | Unemployed | EUPD GAD | None | No alcohol Non-smoker Previous illicit/medical cannabis use |
| 5 | F | 46 | Unemployed | EUPD Depression Treatment-resistant OCD | HTN, T2DM | No alcohol Non-smoker No history of cannabis use |
| 6 | F | 25 | Phlebotomist | EUPD Depression Anxiety | None | No alcohol Non-smoker Current illicit cannabis use |
| 7 | F | 29 | College student | EUPD Bipolar disorder Insomnia | None | No alcohol Non-smoker No history of cannabis use |
| # | Current Psychotropic Medication | Past Psychotropic Medication History | Talking Therapies Trialed |
|---|---|---|---|
| 1 | Citalopram 30 mg O.D. | DBT | |
| 2 | None prescribed | Mirtazapine Duloxetine Sertraline Lamotrigine Quetiapine Sodium valproate Zopiclone | DBT |
| 3 | None prescribed | Sulpride Quetiapine Melatonin | CBT |
| 4 | Mirtazapine 30 mg O.N. Quetiapine 100 mg O.N. | Zopiclone Diazepam Propranolol | CBT Counselling |
| 5 | Duloxetine 90 mg O.D. Pregabalin 200 mg T.D.S Olanzapine 5 mg O.N. | Lorazepam Clomipramine Sertraline Fluoxetine | CBT |
| 6 | Fluoxetine 60 mg O.D. | Sertraline Escitalopram Citalopram | CBT Counselling |
| 7 | Paroxetine 12.5 mg O.D. Trazodone 50 mg O.D. | Lamotrigine | CBT |
| # | Cannabis Naïve | Cannabis-Based Medicinal Product Used | Adverse Effects | CGI-I | PGIC |
|---|---|---|---|---|---|
| 1 | No | Chemotype 1 dried flower 15–20% THC; 30 g per month | No side effects reported | 2 | 6 |
| 2 | No | Chemotype 2 Oral extract 10 mg/mL THC: 15 mg/mL CBD 0.5 mL B.D. Chemotype 1 dried flower 20% THC; 20 g per month | No side effects reported | 2 | 5 |
| 3 | No | Chemotype 1 dried flower 20% THC; 30 g per month | No side effects reported | 2 | 6 |
| 4 | No | Chemotype 1 dried flower 15–20% THC; 60 g per month | No side effects reported | 2 | 6 |
| 5 | Yes | Chemotype 2 Oral extract 10 mg/mL THC:12.5 mg/mL CBD 0.3 mL T.D.S. | No side effects reported | 4 | 1 |
| 6 | No | Chemotype 1 dried flower 20% THC; 30 g per month | No side effects reported | 2 | 6 |
| 7 | Yes | Chemotype 3 Oral extract 100 mg/mL CBD 0.4 mL O.D. | No side effects reported | 2 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sultan, W.; Mathew, A.; Brown, M.R.D.; Gálvez-Flórez, J.F.; Moreno-Sanz, G. Cannabis-Based Medicinal Products in the Management of Emotionally Unstable Personality Disorder (EUPD): A Narrative Review and Case Series. Brain Sci. 2022, 12, 1467. https://doi.org/10.3390/brainsci12111467
Sultan W, Mathew A, Brown MRD, Gálvez-Flórez JF, Moreno-Sanz G. Cannabis-Based Medicinal Products in the Management of Emotionally Unstable Personality Disorder (EUPD): A Narrative Review and Case Series. Brain Sciences. 2022; 12(11):1467. https://doi.org/10.3390/brainsci12111467
Chicago/Turabian StyleSultan, Waseem, Anup Mathew, Matthew R. D. Brown, Juan F. Gálvez-Flórez, and Guillermo Moreno-Sanz. 2022. "Cannabis-Based Medicinal Products in the Management of Emotionally Unstable Personality Disorder (EUPD): A Narrative Review and Case Series" Brain Sciences 12, no. 11: 1467. https://doi.org/10.3390/brainsci12111467
APA StyleSultan, W., Mathew, A., Brown, M. R. D., Gálvez-Flórez, J. F., & Moreno-Sanz, G. (2022). Cannabis-Based Medicinal Products in the Management of Emotionally Unstable Personality Disorder (EUPD): A Narrative Review and Case Series. Brain Sciences, 12(11), 1467. https://doi.org/10.3390/brainsci12111467






