Mobile Brain Imaging to Examine Task-Related Cortical Correlates of Reactive Balance: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Review Objectives
2.2. Review Methodology
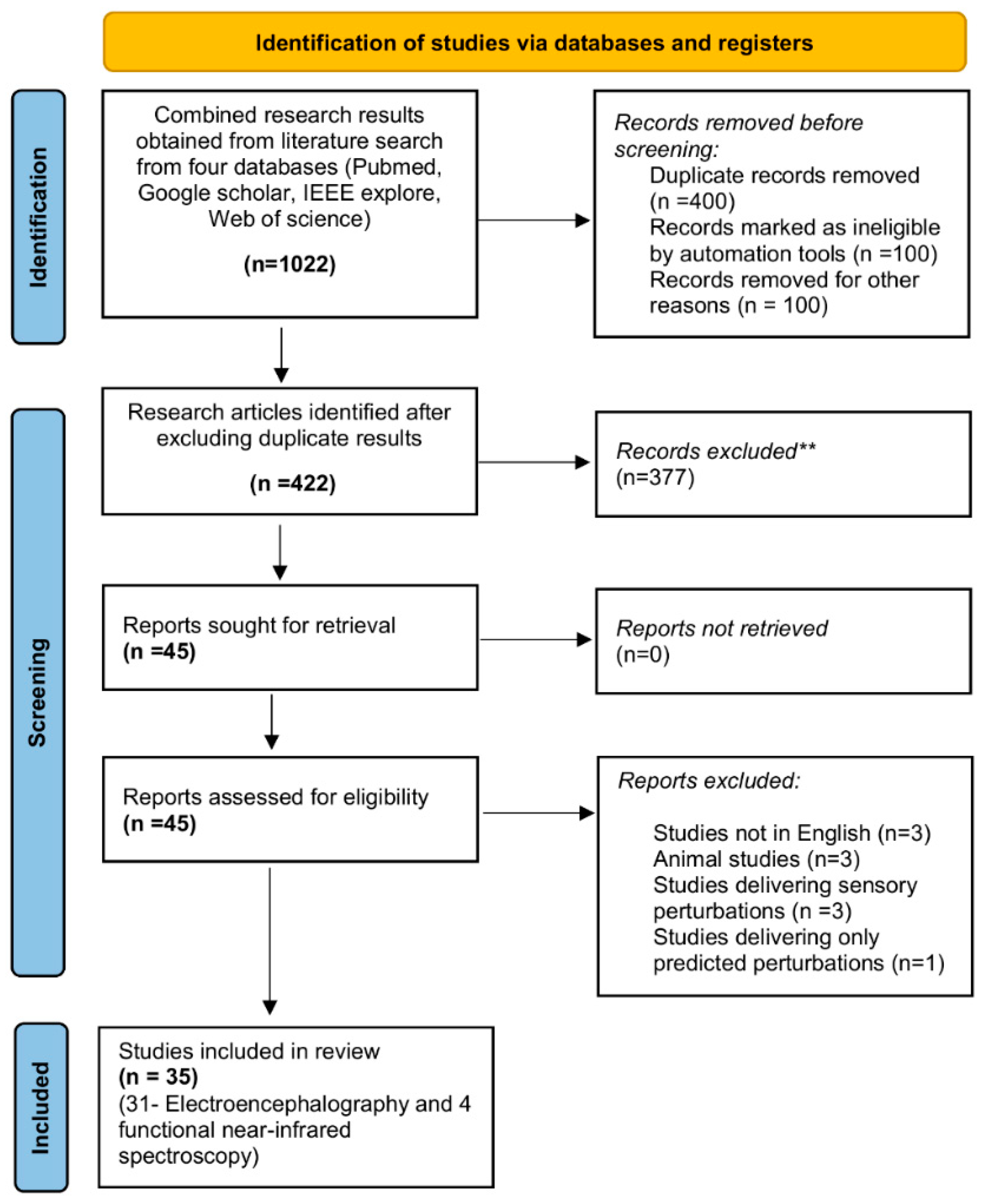
2.2.1. Search Process and Strategy
2.2.2. Eligibility Criteria
2.2.3. Quality Assessment
2.2.4. Data Extraction
2.2.5. Data Analysis
3. Results
3.1. Spatial-Temporal Characteristics of Cortical Activity: Novel Perturbations (RQ1)
3.1.1. EEG Studies—PEPs
3.1.2. EEG Studies—Changes in Frequency Spectrums
3.1.3. fNIRS Studies—Changes in Hemodynamic Responses
3.2. Spatial-Temporal Characteristics of Cortical Activity: Repeated Perturbations (RQ2)
3.2.1. EEG Studies: PEPs
3.2.2. EEG Studies: Changes in Frequency Spectrums
3.2.3. fNIRS Studies: Changes in Hemodynamic Responses
3.3. Behavioral and Kinematic Correlates of Cortical Activity (RQ3)
3.3.1. EEG Studies: PEPs
3.3.2. EEG Studies: Changes in Frequency Spectrums
3.3.3. EEG Studies: Changes in Hemodynamic Responses
4. Discussion
4.1. Spatial-Temporal Characteristics of Cortical Activity: Novel Perturbations (RQ1)
4.2. Spatial-Temporal Characteristics of Cortical Activity: Repeated Perturbations (RQ2)
4.3. Behavioral and Biomechanical/Kinematic Correlates of Cortical Activity (RQ3)
5. Limitations of the Review
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. 2), ii7–ii11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliagina, T.G.; Orlovsky, G.N.; Zelenin, P.V.; Beloozerova, I.N. Neural bases of postural control. Physiology 2006, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B. Adaptation of automatic postural responses. In The Acquisition of Motor Behavior in Vertebrates; The MIT Press: Cambridge, MA, USA, 1996; pp. 57–85. [Google Scholar]
- Massion, J. Postural control system. Curr. Opin. Neurobiol. 1994, 4, 877–887. [Google Scholar] [CrossRef]
- Sousa, A.S.; Silva, A.; Tavares, J.M. Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: A review. Somatosens. Mot. Res. 2012, 29, 131–143. [Google Scholar] [CrossRef]
- Forbes, P.A.; Chen, A.; Blouin, J.-S. Sensorimotor control of standing balance. Handb. Clin. Neurol. 2018, 159, 61–83. [Google Scholar]
- MacKinnon, C.D. Sensorimotor anatomy of gait, balance, and falls. Handb. Clin. Neurol. 2018, 159, 3–26. [Google Scholar]
- Page, P. Sensorimotor training: A “global” approach for balance training. J. Bodyw. Mov. Ther. 2006, 10, 77–84. [Google Scholar] [CrossRef]
- Stelmach, E.G.; Worringham, C.J. Sensorimotor deficits related to postural stability: Implications for falling in the elderly. Clin. Geriatr. Med. 1985, 1, 679–694. [Google Scholar] [CrossRef]
- Li, K.Z.; Bherer, L.; Mirelman, A.; Maidan, I.; Hausdorff, J.M. Cognitive involvement in balance, gait and dual-tasking in aging: A focused review from a neuroscience of aging perspective. Front. Neurol. 2018, 9, 913. [Google Scholar] [CrossRef] [Green Version]
- Maki, B.E.; McIlroy, W.E. Cognitive demands and cortical control of human balance-recovery reactions. J. Neural Transm. 2007, 114, 1279–1296. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.H.; Sowman, P.F.; Brock, J.; Johnson, B.W. Neuromagnetic brain activity associated with anticipatory postural adjustments for bimanual load lifting. Neuroimage 2013, 66, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, J.; Horak, F.B.; Fujiwara, K.; Tomita, H.; Furune, N.; Kunita, K. Changes in activity at the cerebral cortex associate with the optimization of responses to external postural perturbations when given prior warning. Gait Posture Suppl. 2007. [Google Scholar]
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Boxtel, G.; Brunia, C.H. Motor and non-motor aspects of slow brain potentials. Biol. Psychol. 1994, 38, 37–51. [Google Scholar] [CrossRef]
- Saitou, K.; Washimi, Y.; Koike, Y.; Takahashi, A.; Kaneoke, Y. Slow negative cortical potential preceding the onset of postural adjustment. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 449–455. [Google Scholar] [CrossRef]
- Slobounov, S.; Hallett, M.; Stanhope, S.; Shibasaki, H. Role of cerebral cortex in human postural control: An EEG study. Clin. Neurophysiol. 2005, 116, 315–323. [Google Scholar] [CrossRef]
- Bolton, D.A. The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci. Biobehav. Rev. 2015, 57, 142–155. [Google Scholar] [CrossRef]
- Kahya, M.; Moon, S.; Ranchet, M.; Vukas, R.R.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.; Devos, H. Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: A systematic review. Exp. Gerontol. 2019, 128, 110756. [Google Scholar] [CrossRef]
- Wittenberg, E.; Thompson, J.; Nam, C.S.; Franz, J.R. Neuroimaging of Human Balance Control: A Systematic Review. Front. Hum. Neurosci. 2017, 11, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Maki, B.E.; Mcilroy, W.E.; Fernie, G.R. Change-in-support reactions for balance recovery. IEEE Eng. Med. Biol. Mag. 2003, 22, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. The role of limb movements in maintaining upright stance: The “change-in-support” strategy. Phys. Ther. 1997, 77, 488–507. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural. Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. Postural control in the older adult. Clin. Geriatr. Med. 2007, 12, 635–658. [Google Scholar] [CrossRef]
- Chan, C.W.; Jones, G.M.; Kearney, R.E.; Watt, D.G.D. The ‘late’ electromyographic response to limb displacement in man. I. Evidence for supraspinal contribution. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 173–181. [Google Scholar] [CrossRef]
- Taube, W.; Schubert, M.; Gruber, M.; Beck, S.; Faist, M.; Gollhofer, A. Direct corticospinal pathways contribute to neuromuscular control of perturbed stance. J. Appl. Physiol. 2006, 101, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Beloozerova, I.N.; Sirota, M.G.; Swadlow, H.A.; Orlovsky, G.N.; Popova, L.B.; Deliagina, T.G. Activity of different classes of neurons of the motor cortex during postural corrections. J. Neurosci. 2003, 23, 7844–7853. [Google Scholar] [CrossRef] [Green Version]
- Brauer, S.; Woollacott, M.; Shumway-Cook, A. The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait Posture 2002, 15, 83–93. [Google Scholar] [CrossRef]
- Maki, B.E.; Zecevic, A.; Bateni, H.; Kirshenbaum, N.; McIlroy, W.E. Cognitive demands of executing postural reactions: Does aging impede attention switching? Neuroreport 2001, 12, 3583–3587. [Google Scholar] [CrossRef]
- McIlroy, W.E.; Norrie, R.G.; Brooke, J.D.; Bishop, D.C.; Nelson, A.J.; Maki, B.E. Temporal properties of attention sharing consequent to disturbed balance. Neuroreport 1999, 10, 2895–2899. [Google Scholar] [CrossRef]
- Kim, H.; Nnodim, J.O.; Richardson, J.K.; Ashton-Miller, J.A. Effect of age on the ability to recover from a single unexpected underfoot perturbation during gait: Kinematic responses. Gait Posture 2013, 38, 853–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavol, M.J.; Owings, T.M.; Foley, K.T.; Grabiner, M.D. Mechanisms leading to a fall from an induced trip in healthy older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M428–M437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, P.-F.; Woollacott, M.H.; Chong, R.K. Control of reactive balance adjustments in perturbed human walking: Roles of proximal and distal postural muscle activity. Exp. Brain Res. 1998, 119, 141–152. [Google Scholar] [CrossRef]
- Tseng, S.-C.; Stanhope, S.J.; Morton, S.M. Impaired reactive stepping adjustments in older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2009, 64, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Woollacott, M.H.; Tang, P.-F. Balance control during walking in the older adult: Research and its implications. Phys. Ther. 1997, 77, 646–660. [Google Scholar] [CrossRef]
- Kajrolkar, T.; Bhatt, T. Falls-risk post-stroke: Examining contributions from paretic versus non paretic limbs to unexpected forward gait slips. J. Biomech. 2016, 49, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Wong, J.S.; McIlroy, W.E.; Biasin, L.; Brunton, K.; Bayley, M.; Inness, E.L. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy 2015, 101, 373–380. [Google Scholar] [CrossRef]
- Patel, P.J.; Bhatt, T. Does aging with a cortical lesion increase fall-risk: Examining effect of age versus stroke on intensity modulation of reactive balance responses from slip-like perturbations. Neuroscience 2016, 333, 252–263. [Google Scholar] [CrossRef]
- Salot, P.; Patel, P.; Bhatt, T. Reactive Balance in Individuals with Chronic Stroke: Biomechanical Factors Related to Perturbation-Induced Backward Falling. Phys. Ther. 2016, 96, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Adkin, A.L.; Campbell, A.D.; Chua, R.; Carpenter, M.G. The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci. Lett. 2008, 435, 120–125. [Google Scholar] [CrossRef]
- Mochizuki, G.; Sibley, K.M.; Cheung, H.J.; Camilleri, J.M.; McIlroy, W.E. Generalizability of perturbation-evoked cortical potentials: Independence from sensory, motor and overall postural state. Neurosci. Lett. 2009, 451, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M.; Ting, L.H. Balance perturbation-evoked cortical N1 responses are larger when stepping and not influenced by motor planning. J. Neurophysiol. 2020, 124, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M.; Ting, L.H. Worse balance is associated with larger perturbation-evoked cortical responses in healthy young adults. Gait Posture 2020, 80, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Quant, S.; Adkin, A.L.; Staines, W.R.; McIlroy, W.E. Cortical activation following a balance disturbance. Exp. Brain Res. 2004, 155, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Marlin, A.; Mochizuki, G.; Staines, W.R.; McIlroy, W.E. Localizing evoked cortical activity associated with balance reactions: Does the anterior cingulate play a role? J. Neurophysiol. 2014, 111, 2634–2643. [Google Scholar] [CrossRef]
- Bolton, D.A.; Williams, L.; Staines, W.R.; McIlroy, W.E. Contribution of primary motor cortex to compensatory balance reactions. BMC Neurosci. 2012, 13, 102. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.M.; Ferris, D.P. Differentiation in Theta and Beta Electrocortical Activity between Visual and Physical Perturbations to Walking and Standing Balance. eNeuro 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Solis-Escalante, T.; de Kam, D.; Weerdesteyn, V. Classification of Rhythmic Cortical Activity Elicited by Whole-Body Balance Perturbations Suggests the Cortical Representation of Direction-Specific Changes in Postural Stability. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2566–2574. [Google Scholar] [CrossRef]
- Koren, Y.; Parmet, Y.; Bar-Haim, S. Treading on the unknown increases prefrontal activity: A pilot fNIRS study. Gait Posture 2019, 69, 96–100. [Google Scholar] [CrossRef]
- Lee, B.C.; Choi, J.; Martin, B.J. Roles of the prefrontal cortex in learning to time the onset of pre-existing motor programs. PLoS ONE 2020, 15, e0241562. [Google Scholar] [CrossRef]
- Bultmann, U.; Pierscianek, D.; Gizewski, E.R.; Schoch, B.; Fritsche, N.; Timmann, D.; Maschke, M.; Frings, M. Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait Posture 2014, 39, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Petsas, N.; Raz, E.; Sbardella, E.; Tona, F.; Mancinelli, C.R.; Pozzilli, C.; Pantano, P. Balance deficit with opened or closed eyes reveals involvement of different structures of the central nervous system in multiple sclerosis. Mult. Scler. J. 2014, 20, 81–90. [Google Scholar] [CrossRef]
- Prosperini, L.; Sbardella, E.; Raz, E.; Cercignani, M.; Tona, F.; Bozzali, M.; Petsas, N.; Pozzilli, C.; Pantano, P. Multiple sclerosis: White and gray matter damage associated with balance deficit detected at static posturography. Radiology 2013, 268, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, T.; Patel, P.; Dusane, S.; DelDonno, S.R.; Langenecker, S.A. Neural mechanisms involved in mental imagery of slip-perturbation while walking: A preliminary fMRI study. Front. Behav. Neurosci. 2018, 12, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.J.; Bhatt, T.; DelDonno, S.R.; Langenecker, S.A.; Dusane, S. Examining neural plasticity for slip-perturbation training: An fMRI study. Front. Neurol. 2019, 9, 1181. [Google Scholar]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Nobezawa, S.; Futatsubashi, M. Brain activation during maintenance of standing postures in humans. Brain 1999, 122, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, Y.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Nobezawa, S. Absolute changes in regional cerebral blood flow in association with upright posture in humans: An orthostatic PET study. J. Nucl. Med. 2001, 42, 707–712. [Google Scholar]
- Adkin, A.L.; Quant, S.; Maki, B.E.; McIlroy, W.E. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp. Brain Res. 2006, 172, 85–93. [Google Scholar] [CrossRef]
- Dietz, V.; Quintern, J.; Berger, W. Cerebral evoked potentials associated with the compensatory reactions following stance and gait perturbation. Neurosci. Lett. 1984, 50, 181–186. [Google Scholar] [CrossRef]
- Dietz, V.; Quintern, J.; Berger, W. Afferent control of human stance and gait: Evidence for blocking of group I afferents during gait. Exp. Brain Res. 1985, 61, 153–163. [Google Scholar] [CrossRef]
- Dietz, V.; Quintern, J.; Berger, W.; Schenck, E. Cerebral potentials and leg muscle e.m.g. responses associated with stance perturbation. Exp. Brain Res. 1985, 57, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Duckrow, R.B.; Abu-Hasaballah, K.; Whipple, R.; Wolfson, L. Stance perturbation-evoked potentials in old people with poor gait and balance. Clin. Neurophysiol. 1999, 110, 2026–2032. [Google Scholar] [CrossRef]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. Neuroimage 2008, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hattori, N.; Hatakenaka, M.; Yagura, H.; Kawano, T.; Kubota, K. Cortical control of postural balance in patients with hemiplegic stroke. Neuroreport 2012, 23, 314–319. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Goense, J.; Bohraus, Y.; Logothetis, N.K. fMRI at high spatial resolution: Implications for BOLD-models. Front. Comput. Neurosci. 2016, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, B.W.; Bekkers, E.M.; Gilat, M.; de Rond, V.; Hardwick, R.M.; Nieuwboer, A. Functional neuroimaging of human postural control: A systematic review with meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 351–362. [Google Scholar] [CrossRef]
- Varghese, J.P.; McIlroy, R.E.; Barnett-Cowan, M. Perturbation-evoked potentials: Significance and application in balance control research. Neurosci. Biobehav. Rev. 2017, 83, 267–280. [Google Scholar] [CrossRef]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional Magnetic Resonance Imaging and Functional Near-Infrared Spectroscopy: Insights from Combined Recording Studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef]
- Wilcox, T.; Biondi, M. fNIRS in the developmental sciences. Wiley Interdiscip. Rev. Cogn. Sci. 2015, 6, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.M.; Ting, L.H.; Hajcak, G. Do sensorimotor perturbations to standing balance elicit an error-related negativity? Psychophysiology 2019, 56, e13359. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ditz, J.C.; Schwarz, A.; Muller-Putz, G.R. Perturbation-evoked potentials can be classified from single-trial EEG. J. Neural Eng. 2020, 17, 036008. [Google Scholar] [CrossRef]
- Varghese, J.P.; Marlin, A.; Beyer, K.B.; Staines, W.R.; Mochizuki, G.; McIlroy, W.E. Frequency characteristics of cortical activity associated with perturbations to upright stability. Neurosci. Lett. 2014, 578, 33–38. [Google Scholar] [CrossRef]
- Goel, R.; Ozdemir, R.A.; Nakagome, S.; Contreras-Vidal, J.L.; Paloski, W.H.; Parikh, P.J. Effects of speed and direction of perturbation on electroencephalographic and balance responses. Exp. Brain Res. 2018, 236, 2073–2083. [Google Scholar] [CrossRef]
- Varghese, J.P.; Staines, W.R.; McIlroy, W.E. Activity in Functional Cortical Networks Temporally Associated with Postural Instability. Neuroscience 2019, 401, 43–58. [Google Scholar] [CrossRef]
- Quant, S.; Maki, B.E.; McIlroy, W.E. The association between later cortical potentials and later phases of postural reactions evoked by perturbations to upright stance. Neurosci. Lett. 2005, 381, 269–274. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; Van Der Cruijsen, J.; De Kam, D.; Van Kordelaar, J.; Weerdesteyn, V.; Schouten, A.C. Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. Neuroimage 2019, 188, 557–571. [Google Scholar] [CrossRef]
- Ghosn, N.J.; Palmer, J.A.; Borich, M.R.; Ting, L.H.; Payne, A.M. Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability. Brain Sci. 2020, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Shenoy Handiru, V.; Alivar, A.; Hoxha, A.; Saleh, S.; Suviseshamuthu, E.S.; Yue, G.H.; Allexandre, D. Graph-theoretical analysis of EEG functional connectivity during balance perturbation in traumatic brain injury: A pilot study. Hum. Brain Mapp. 2021, 42, 4427–4447. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, G.; Sibley, K.M.; Esposito, J.G.; Camilleri, J.M.; McIlroy, W.E. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clin. Neurophysiol. 2008, 119, 1626–1637. [Google Scholar] [CrossRef]
- Palmer, J.A.; Payne, A.M.; Ting, L.H.; Borich, M.R. Cortical Engagement Metrics during Reactive Balance Are Associated with Distinct Aspects of Balance Behavior in Older Adults. Front. Aging Neurosci. 2021, 13, 684743. [Google Scholar] [CrossRef]
- An, J.; Yoo, D.; Lee, B.C. Electrocortical activity changes in response to unpredictable trip perturbations induced by a split-belt treadmill. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 110–113. [Google Scholar] [PubMed]
- Sibley, K.M.; Mochizuki, G.; Frank, J.S.; McIlroy, W.E. The relationship between physiological arousal and cortical and autonomic responses to postural instability. Exp. Brain Res. 2010, 203, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, G.; Aprigliano, F.; Micera, S.; Monaco, V.; De Venuto, D. Cortical reactive balance responses to unexpected slippages while walking: A pilot study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 6868–6871. [Google Scholar]
- Bogost, M.D.; Burgos, P.I.; Little, C.E.; Woollacott, M.H.; Dalton, B.H. Electrocortical Sources Related to Whole-Body Surface Translations during a Single- and Dual-Task Paradigm. Front. Hum. Neurosci. 2016, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Little, C.E.; Woollacott, M. EEG measures reveal dual-task interference in postural performance in young adults. Exp. Brain Res. 2015, 233, 27–37. [Google Scholar] [CrossRef]
- Mochizuki, G.; Boe, S.G.; Marlin, A.; McIlroy, W.E. Performance of a concurrent cognitive task modifies pre- and post-perturbation-evoked cortical activity. Neuroscience 2017, 348, 143–152. [Google Scholar] [CrossRef]
- Quant, S.; Adkin, A.L.; Staines, W.R.; Maki, B.E.; McIlroy, W.E. The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neurosci. 2004, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, J.V.; Roy, C.L.; Hitt, J.R.; Popov, R.E.; Henry, S.M. Neural mechanisms and functional correlates of altered postural responses to perturbed standing balance with chronic low back pain. Neuroscience 2016, 339, 511–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierau, A.; Hulsdunker, T.; Struder, H.K. Changes in cortical activity associated with adaptive behavior during repeated balance perturbation of unpredictable timing. Front. Behav. Neurosci. 2015, 9, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allexandre, D.; Hoxha, A.; Handiru, V.S.; Saleh, S.; Selvan, S.E.; Yue, G.H. Altered Cortical and Postural Response to Balance Perturbation in Traumatic Brain Injury—An EEG Pilot Study. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2019, 2019, 1543–1546. [Google Scholar]
- Payne, A.M.; Hajcak, G.; Ting, L.H. Dissociation of muscle and cortical response scaling to balance perturbation acceleration. J. Neurophysiol. 2019, 121, 867–880. [Google Scholar] [CrossRef]
- Shirazi, S.Y.; Huang, H.J. Differential Theta-Band Signatures of the Anterior Cingulate and Motor Cortices during Seated Locomotor Perturbations. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 468–477. [Google Scholar] [CrossRef]
- Fujimoto, H.; Mihara, M.; Hattori, N.; Hatakenaka, M.; Kawano, T.; Yagura, H.; Miyai, I.; Mochizuki, H. Cortical changes underlying balance recovery in patients with hemiplegic stroke. Neuroimage 2014, 85, 547–554. [Google Scholar] [CrossRef]
- Palve, S.S.; Palve, S.B. Impact of Aging on Nerve Conduction Velocities and Late Responses in Healthy Individuals. J. Neurosci. Rural Pract. 2018, 9, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Yang, Y.; Xi, J.H.; Chen, Z.Q. Structural and functional connectivity in traumatic brain injury. Neural Regen. Res. 2015, 10, 2062–2071. [Google Scholar]
- Pai, Y.C.; Bhatt, T.S. Repeated-slip training: An emerging paradigm for prevention of slip-related falls among older adults. Phys. Ther. 2007, 87, 1478–1491. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Varas-Diaz, G.; Bhatt, T. Muscle synergy differences between voluntary and reactive backward stepping. Sci. Rep. 2021, 11, 15462. [Google Scholar] [CrossRef]
- Gangwani, R.; Dusane, S.; Wang, S.; Kannan, L.; Wang, E.; Fung, J.; Bhatt, T. Slip-Fall Predictors in Community-Dwelling, Ambulatory Stroke Survivors: A Cross-sectional Study. J. Neurol. Phys. Ther. 2020, 44, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bhatt, T.; Wang, S.; Yang, F.; Pai, Y.C.C. Retention of the “first-trial effect” in gait-slip among community-living older adults. Geroscience 2017, 39, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Reschechtko, S.; Wang, S.; Pai, Y.C.C. The recovery response to a novel unannounced laboratory-induced slip: The “first trial effect” in older adults. Clin. Biomech. 2017, 48, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Pavol, M.J.; Runtz, E.F.; Edwards, B.J.; Pai, Y.C. Age influences the outcome of a slipping perturbation during initial but not repeated exposures. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M496–M503. [Google Scholar] [CrossRef]
| Study | Quality Assessment | Study | Quality Assessment | ||
|---|---|---|---|---|---|
| Author 1 | Author 2 | Author 1 | Author 2 | ||
| Ditz et al. [76] | M | M | Varghese et al. [77] | L | M |
| Goel et al. [78] | M | M | Varghese et al. [79] | L | M |
| Quant et al. [80] | M | M | Solis-Escalante et al. [81] | M | M |
| Payne and Ting [43] | M | H | Ghosn et al. [82] | L | M |
| Payne and Ting [44] | H | H | Solis-Escalante et al. [49] | M | M |
| Adkin et al. [59] | M | M | Shenoy et al. [83] | L | M |
| Mochizuki et al. [84] | L | L | Palmer et al. [85] | M | M |
| Adkin et al. [41] | M | M | An et al. [86] | M | M |
| Sibley et al. [87] | M | M | Mezzina et al. [88] | M | M |
| Bogost et al. [89] | M | M | Peterson and Ferris [48] | H | H |
| Little and Woollcott [90] | M | M | Mihara et al. [64] | L | L |
| Mochizuki et al. [91] | L | M | Mihara et al. [65] | L | L |
| Quant et al. [92] | M | M | Koren et al. [50] | M | M |
| Duckrow et al. [63] | M | M | Lee et al. [51] | M | M |
| Jacobs et al. [93] | M | M | Mierau et al. [94] | M | M |
| Alexandre et al. [95] | L | L | Payne et al. [96] | H | H |
| Dietz et al. [60] | L | L | Shirazi and Huang [97] | M | M |
| Mochizuki et al. [42] | L | L | |||
| Study | Sample | Type of Perturbations | Behavioral, Kinematic Outcomes | PEP | Electrode Sites | Key Findings |
|---|---|---|---|---|---|---|
| Task position: Sitting | ||||||
| Ditz et al. [76] | 15 young | UNPRED medial/lateral perturbations via mechanical chair | - | P1, N1, P2 | Central, Parietal | N1 and P2 amplitude: UNPRED > rest No differences in P1 |
| Task position: Standing | ||||||
| Goel et al. [78] | 10 young | UNPRED forward/backward, low/high perturbations via movable platform | - | N1 | Frontal, Central | N1 amplitude: high > low for both N1 latency: high < low for both |
| Quant et al. [80] | 7 young | UNPRED backward perturbations via movable platform (TASK 1: immediate deceleration TASK 2: delayed deceleration) | - | P1, N1, P2, N2 | Central | All PEPs: TASK 1 ≈ TASK 2 |
| Payne and Ting [43] | 16 young | UNPRED backward perturbations (variable magnitude) via movable platform (planned/unplanned stepping, stepping/ non-stepping) | - | N1 | Central | ↑ N1 amplitude with ↑ perturbation magnitude, stepping > no-stepping, planned ≈ unplanned stepping |
| Payne and Ting [44] | 20 young | UNPRED backward perturbations (3 levels: easy, moderate, difficult) via movable platform | Beam walking (balance) | N1 | Central | ↑ N1 amplitude with ↑ perturbation intensity ↓ N1 latency with ↑ perturbation magnitude N1 amplitude −ve correlation with Beam Walking task performance |
| Adkin et al. [59] | 8 young | PRED and UNPRED perturbations forward perturbations via padded device | - | N1 | Central | N1 latency: PRED ≈ UNPRED N1 amplitude: PRED < UNPRED |
| Mochizuki et al. [84] | 15 young | PRED and UNPRED backward perturbations via load release | - | N1 | Frontal, Central | Pre-perturbation activity: PRED > UNPRED N1 characteristics: UNPRED > PRED |
| Adkin et al. [41] | 10 young | PRED and UNPRED forward perturbations at LOW (ground) and HIGH (3.2 m above ground) via hand-held bar | Balance confidence Fear of falling Perceived stability | N1 | Central | N1 amplitude: HIGH > LOW for UNPRED, HIGH ≈ LOW for PRED N1 latency: same for all conditions Changes in N1 +ve correlation with changes in all behavioral outcomes |
| Sibley et al. [87] | 10 young | UNPRED forward perturbations at LOW (ground) and HIGH (1.60 m above ground) via load release | Electrodermal responses | N1, P2 | Central | N1 amplitude: HIGH > LOW, N1 latency, P2 amplitude: HIGH ≈ LOW No correlation between N1 and electrodermal responses |
| Bogost et al. [89] | 15 young | UNPRED backward perturbations via movable platform with (DT) and without cognitive task (ST) | - | N1 | Prefrontal, Premotor, Central, Centro- parietal | Regions of interest: ST: prefrontal, premotor, primary motor and supplementary motor area. DT: all areas for ST+ frontal, temporal and occipital area. N1 amplitude: ST > DT |
| Little and Woollcott [90] | 14 young | UNPRED backward perturbations with cognitive task (DT) and cognitive task only (ST) | - | P1, N1 P2 | Prefrontal, Premotor, Central, Centro- parietal | Regions of interest: ST: prefrontal, premotor, primary motor and supplementary motor area DT: all areas from ST+ temporal and occipital area. N1 amplitude: ST > DT |
| Mochizuki et al. [91] | 26 young | PRED and UNPRED forward perturbations via lean release with (DT) and without cognitive task (ST) | - | N1 | Central | Pre-perturbation activity: PRED: DT < ST N1 amplitude: PRED: ST < DT, UNPRED: ST ≈ DT |
| Quant et al. [92] | 7 young | UNPRED forward/backward perturbations via moveable platform with (DT) and without (ST) cognitive task | - | N1 | Central | N1 amplitude: DT < ST N1 latency: ST ≈ DT |
| Duckrow et al. [63] | 8 young 32 old | UNPRED forward perturbations via movable platform | Subject height Physical performance battery | P1, N1, N2 | Central | P1 latency: Old > Young N1 amplitude: Old < Young N2 latency: ↑ in Old with ↓ SPPB P1 latency +ve correlation with height N1-N2 interval −ve correlation with with physical performance |
| Jacobs et al. [93] | 13 LBP 13 Healthy | UNPRED forward/backward perturbations via movable platform | Center of Mass displacement, Brief pain inventory, Fear avoidance Coping strategies | N1, P2 | Frontal, Central, Centro- parietal | P2 amplitude: LBP > Healthy No group differences in N1 amplitude P2 amplitude −ve correlation with all behavioral outcomes |
| Alexandre et al. [95] | 12 TBI 6 Healthy | UNPRED forward/backward perturbations via movable platform | Center of pressure displacement, Berg Balance scale | P1, N1, N2 | Central | N1 amplitude: TBI < Healthy N1 amplitude +ve correlation with Berg Balance Scale |
| Multiple task positions | ||||||
| Dietz et al. [60] | 10 young | UNPRED forward perturbations in stance and gait via treadmill | - | P1, N1 | Central | P1 latency: stance < gait N1 latency: stance < gait |
| Mochizuki et al. [42] | 8 young | UNPRED backward perturbations in stance via load release and in sitting via chair tilting | - | N1, P2 | Frontal, Central Centro- parietal | N1 characteristics at central (standing ≈ sitting) P2 amplitude: standing > sitting |
| Study | Sample | Type of Perturbations | Behavioral, Kinematic Outcomes | Frequency Band | Cortical Sites | Key Findings |
|---|---|---|---|---|---|---|
| Task position: Standing | ||||||
| Varghese et al. [77] | 14 Young | UNPRED forward perturbations via lean release | - | N1 | Fronto- central | Simultaneous ↑ in power (theta, delta, alpha and beta) during N1 |
| Varghese et al. [79] | 14 Young | UNPRED forward perturbations via lean release | - | N1 | N/A | Functional connectivity strength ↑ delta, theta, alpha and beta during N1 |
| Solis-Escalante et al. [81] | 10 Young | UNPRED backward perturbations via movable platform Phases: cue observation, response preparation, response execution (RE) | - | alpha, beta, theta | Supplementary motor, Sensorimotor, Prefrontal, Posterior parietal, Anterior cingulate cortex | ERSPs Cue observation: ↓ alpha + ↓ beta Response preparation: ↓ beta, ↓ theta, ↓ alpha and gamma Response execution: ↑ theta, alpha, beta. Stepping: ↓ beta in M1/S1 contralateral to support leg |
| Ghosn et al. [82] | 19 Young | UNPRED backward perturbations (easy-difficult) via movable platform | Beam walking task (balance) | beta | Central | ↑ beta power with ↑ perturbation magnitude Late phase beta power (150–250 ms post-perturbation) −ve correlation with balance performance |
| Solis-Escalante et al. [49] | 3 Stroke 6 Young | UNPRED forward/backward, medial/lateral perturbations via movable platform | - | theta | Central | ≈ pattern of changes in theta frequencies in both groups (i.e., ↑ theta after perturbation onset) |
| Shenoy et al. [83] | 18 TBI 18 Healthy | UNPRED anterior/posterior perturbations with low/high amplitude via movable platform | Center of pressure displacement, Berg Balance Scale | alpha beta theta | Frontal, Parietal, Temporal, Occipital | Regions-of-interest alpha connectivity: TBI < Healthy, beta connectivity: TBI ≈ Healthy in ROIs Theta band modularity −ve correlation with Berg Balance Scale, no correlation with center of pressure displacement |
| Palmer et al. [85] | 16 Old | UNPRED forward/backward, medial/lateral perturbations via movable platform | Mini Balance evaluation test (balance), Cognitive motor interference, Stepping threshold | beta | Primary motor, Sensory, Prefrontal | ↑ prefrontal, primary motor beta connectivity post-perturbation correlated with ↓ stepping threshold Beta power during late phase (150–250 ms) −ve correlation with MiniBEST. |
| Task position: Walking | ||||||
| An et al. [86] | 5 Young | UNPRED backward perturbations via split-belt treadmill Phases: Quiet standing, walking, recovery response. | - | alpha beta theta delta gamma | Sensorimotor, Posterior parietal | In sensorimotor cortex, ↑ theta power: walking > standing, ↑ alpha power: recovery < standing/walking In posterior-parietal cortex, ↑ theta power: walking > standing, ↑ alpha power: recovery > standing/ walking, ↑ beta power: recovery/walking < standing |
| Mezzina et al. [88] | 4 Young | UNPRED forward perturbations via split-belt treadmill | - | alpha beta theta | Frontal, Parietal | Cortical responsiveness (slope m) ↑ in m of all bands post perturbation. ↓ in m during recovery > walking and early balance loss phase |
| Multiple task positions | ||||||
| Peterson and Ferris [48] | 30 Young | UNPRED perturbations in standing or walking on a balance beam via waist pull Conditions: stand pull, walk pull | - | alpha beta theta and gamma | Occipital, Posterior parietal, Sensorimotor, Supplementary motor area | ↑ alpha, ↑ beta power: stand pull > walk pull in sensorimotor, posterior parietal and supplementary motor area ↓ gamma power: stand pull < walk pull in occipital and posterior parietal area |
| Study | Sample | Type of Perturbations | Behavioral, Kinematic Outcomes | Cortical Responses | Cortical Area | Key Findings |
|---|---|---|---|---|---|---|
| Task position: Standing | ||||||
| Mihara et al. [64] | 15 Young | UNPRED and PRED forward/backward, medial/lateral perturbations via movable platform | - | OxyHb, Deoxy Hb | Frontal, Parietal, Primary motor | ↑ OxyHb: PRED, UNPRED > pre-perturbation in frontal and parietal ↑ OxyHb: PRED > UNPRED in superior parietal & supplementary motor area |
| Mihara et al. [65] | 20 Stroke | UNPRED forward/backward perturbations via movable platform | Berg Balance Scale (balance) | OxyHb, Deoxy Hb | Prefrontal Premotor Parietal | ↑ OxyHb: post > pre-perturbation in prefrontal, and parietal of unaffected hemisphere ↑ OxyHb in supplementary motor area and prefrontal cortex +ve correlation with Berg Balance scale |
| Task position: Walking | ||||||
| Koren et al. [50] | 20 Young | UNPRED over ground walk perturbations via mechatronic system. 3 conditions: Unperturbed walk, perturbed walk and own shoes | - | OxyHb, Deoxy Hb | Prefrontal cortex | ↑ OxyHb: perturbed walk > unperturbed/shoe in prefrontal cortex |
| Lee et al. [51] | 10 Young | UNPRED repeated slips via split-belt treadmill | - | OxyHb, Deoxy Hb | Prefrontal cortex and sub regions | ↑ OxyHb: walking, recovery period > pre-perturbation in prefrontal cortex |
| Study | Sample | Type of Perturbations | Behavioral, Kinematic Outcomes | PEP | Electrode Sites | Key Findings |
|---|---|---|---|---|---|---|
| Task position: Standing | ||||||
| Mierau et al. [94] | 37 Young | Repeated UNPRED forward/backward, medial/lateral perturbations via movable platform (10 trials: T1-T10) | Postural sway Electromyographic responses | P1, N1 | Fronto-central Centro-parietal | N1 amplitude: T1 > T2 > T10 N1 latency: T1 ≈ T2 ≈ T10 P1 characteristics: T1 ≈ T2 ≈ T10 N1 amplitude +ve correlation with postural sway and electromyographic responses |
| Payne et al. [96] | 16 Young | Repeated UNPRED forward/backward perturbations via movable platform | Electromyographic responses, (early and late) | N1 | Central | ↓ N1 amplitude with ↑ number of trials N1 amplitudes +ve correlation with early but no correlation with late electromyographic responses |
| Study | Sample | Type of Perturbations | Behavioral Kinematic Outcomes | Frequency Band | Cortical Sites | Key Findings |
|---|---|---|---|---|---|---|
| Task position: Sitting | ||||||
| Shirazi and Huang [97] | 17 young | Repeated UNPRED perturbations in sitting during mid-leg or full-leg extension onset via servo meter | - | theta | Supplementary motor area, Anterior cingulate cortex | ↑ theta power: pre-> post-perturbation in Right supplementary motor area ↑ theta power: post > pre-perturbation in anterior cingulate and Left supplementary motor area ↓ theta power with repeated perturbations |
| Study | Sample | Type of Perturbations | Behavioral Kinematic Outcomes | Frequency Band | Cortical Sites | Key Findings |
|---|---|---|---|---|---|---|
| Task position: Walking | ||||||
| Lee et al. [51] | 10 young | Repeated UNPRED backward perturbations during walking via split-belt treadmill | - | OxyHb and Deoxy Hb | Prefrontal cortex and subregions | Trial 1–3: ≈ OxyHb in prefrontal sub-regions Trial 3–6: ↓ OxyHb in ventrolateral and frontopolar prefrontal cortex, ↑ OxyHb in orbitofrontal cortex. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purohit, R.; Bhatt, T. Mobile Brain Imaging to Examine Task-Related Cortical Correlates of Reactive Balance: A Systematic Review. Brain Sci. 2022, 12, 1487. https://doi.org/10.3390/brainsci12111487
Purohit R, Bhatt T. Mobile Brain Imaging to Examine Task-Related Cortical Correlates of Reactive Balance: A Systematic Review. Brain Sciences. 2022; 12(11):1487. https://doi.org/10.3390/brainsci12111487
Chicago/Turabian StylePurohit, Rudri, and Tanvi Bhatt. 2022. "Mobile Brain Imaging to Examine Task-Related Cortical Correlates of Reactive Balance: A Systematic Review" Brain Sciences 12, no. 11: 1487. https://doi.org/10.3390/brainsci12111487




