Neurophysiological Hallmarks of Axonal Degeneration in CIDP Patients: A Pilot Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Treatment
2.2. Clinical Parameters
2.3. Nerve Conduction Studies and Motor Unit Potential Parameters
2.4. Interference Pattern Analysis (IPA)
2.5. Statistical Analysis
3. Results
3.1. Clinical Evaluation
3.2. Analysis of Nerve Conduction Parameters and Electromyographic Activity
3.3. Clinical-Neurophysiological Correlation Analysis
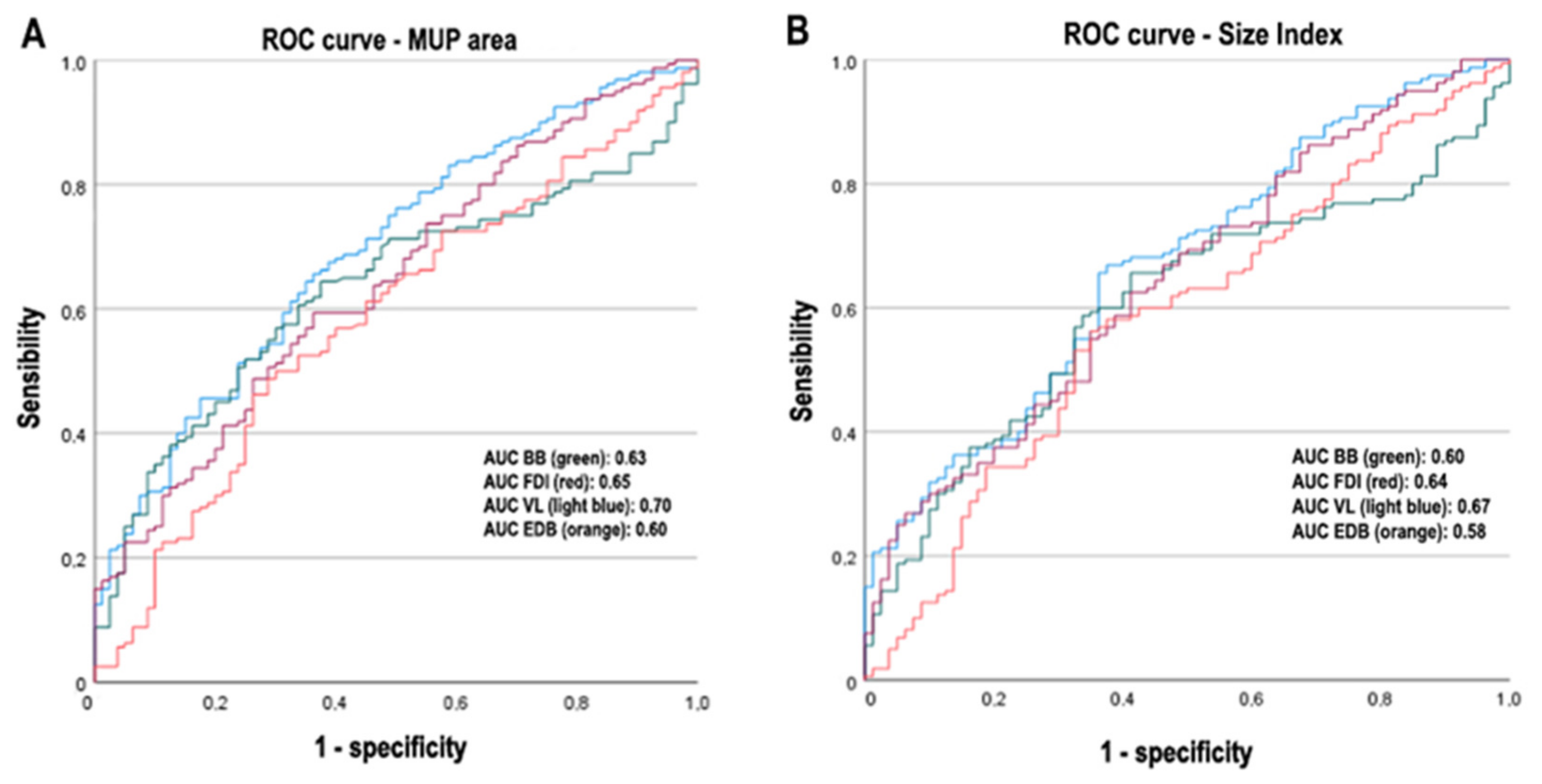
3.4. Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuwabara, S.; Misawa, S. Chronic Inflammatory Demyelinating Polyneuropathy. In Myelin; Springer Science & Business Media: Berlin, Germany, 2019; pp. 333–343. [Google Scholar]
- Lehmann, H.C.; Burke, D.; Kuwabara, S. Chronic inflammatory demyelinating polyneuropathy: Update on diagnosis, immunopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2019, 90, 981–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luigetti, M.; Romano, A.; Di Paolantonio, A.; Bisogni, G.; Rossi, S.; Conte, A.; Madia, F.; Sabatelli, M. Pathological Findings in Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Single-Center Experience. Brain Sci. 2020, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Koike, H.; Nishi, R.; Kawagashira, Y.; Iijima, M.; Katsuno, M.; Sobue, G. Clinicopathological characteristics of subtypes of chronic inflammatory demyelinating polyradiculoneuropathy. J. Neurol. Neurosurg. Psychiatry 2019, 90, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, P.Y.K.; van Doorn, P.A.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Allen, J.A.; Attarian, S.; Blomkwist-Markens, P.H.; Cornblath, D.R.; Eftimov, F.; et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force-Second revision. J. Peripher. Nerv. Syst. 2021, 26, 242–268. [Google Scholar] [CrossRef] [PubMed]
- Uncini, A.; Vallat, J.-M. Autoimmune nodo-paranodopathies of peripheral nerve: The concept is gaining ground. J. Neurol. Neurosurg. Psychiatry 2018, 89, 627–635. [Google Scholar] [CrossRef]
- Nagamatsu, M.; Terao, S.; Misu, K.; Li, M.; Hattori, N.; Ichimura, M.; Sakai, M.; Yamamoto, H.; Watanabe, H.; Riku, S.; et al. Axonal and perikaryal involvement in chronic inflammatory demyelinating polyneuropathy. J. Neurol. Neurosurg. Psychiatry 1999, 66, 727–733. [Google Scholar] [CrossRef]
- Moss, K.R.; Bopp, T.S.; Johnson, A.E.; Höke, A. New evidence for secondary axonal degeneration in demyelinating neuropathies. Neurosci. Lett. 2021, 744, 135595. [Google Scholar] [CrossRef]
- Grüter, T.; Motte, J.; Bulut, Y.; Kordes, A.; Athanasopoulos, D.; Fels, M.; Schneider-Gold, C.; Gold, R.; Fisse, A.L.; Pitarokoili, K. Axonal damage determines clinical disability in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): A prospective cohort study of different CIDP subtypes and disease stages. Eur. J. Neurol. 2022, 29, 583–592. [Google Scholar] [CrossRef]
- Pegat, A.; Boisseau, W.; Maisonobe, T.; Debs, R.; Lenglet, T.; Psimaras, D.; Azoulay-Cayla, A.; Fournier, E.; Viala, K. Motor chronic inflammatory demyelinating polyneuropathy (CIDP) in 17 patients: Clinical characteristics, electrophysiological study, and response to treatment. J. Peripher. Nerv. Syst. 2020, 25, 162–170. [Google Scholar] [CrossRef]
- Allen, J.A.; Merkies, I.S.J.; Lewis, R.A. Monitoring Clinical Course and Treatment Response in Chronic Inflammatory Demyelinating Polyneuropathy During Routine Care: A Review of Clinical and Laboratory Assessment Measures. JAMA Neurol. 2020, 77, 1159–1166. [Google Scholar] [CrossRef]
- Cirillo, G.; Todisco, V.; Tedeschi, G. Long-term neurophysiological and clinical response in patients with chronic inflammatory demyelinating polyradiculoneuropathy treated with subcutaneous immunoglobulin. Clin. Neurophysiol. 2018, 129, 967–973. [Google Scholar] [CrossRef]
- Cirillo, G.; Todisco, V.; Ricciardi, D.; Tedeschi, G. Clinical-neurophysiological correlations in chronic inflammatory demyelinating polyradiculoneuropathy patients treated with subcutaneous immunoglobulin. Muscle Nerve 2019, 60, 662–667. [Google Scholar] [CrossRef]
- Al-Zuhairy, A.; Jakobsen, J.; Krarup, C. Early axonal loss predicts long-term disability in chronic inflammatory demyelinating polyneuropathy. Clin. Neurophysiol. 2021, 132, 1000–1007. [Google Scholar] [CrossRef]
- Merkies, I.S.J.; Schmitz, P.I.M.; Van der Meché, F.G.A.; Samijn, J.P.A.; Van Doorn, P.A. Inflammatory Neuropathy Cause and Treatment (INCAT) group Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J. Neurol. Neurosurg. Psychiatry 2002, 72, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Markvardsen, L.H.; Sindrup, S.H.; Christiansen, I.; Olsen, N.K.; Jakobsen, J.; Andersen, H. Subcutaneous immunoglobulin as first-line therapy in treatment-naive patients with chronic inflammatory demyelinating polyneuropathy: Randomized controlled trial study. Eur. J. Neurol. 2017, 24, 412–418. [Google Scholar] [CrossRef]
- Merkies, I.S.J.; Schmitz, P.I.M.; van der Meche, F.G.A.; van Doorn, P.A. Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Neurology 2000, 54, 943–949. [Google Scholar] [CrossRef]
- van Nes, S.I.; Vanhoutte, E.K.; van Doorn, P.A.; Hermans, M.; Bakkers, M.; Kuitwaard, K.; Faber, C.G.; Merkies, I.S.J. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 2011, 76, 337–345. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- American Association of Electrodiagnostic Medicine. Guidelines in electrodiagnostic medicine. Muscle Nerve 1992, 15, 229–253. [Google Scholar] [CrossRef]
- Sonoo, M.; Stålberg, E. The ability of MUP parameters to discriminate between normal and neurogenic MUPs in concentric EMG: Analysis of the MUP “thickness” and the proposal of “size index”. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 291–303. [Google Scholar] [CrossRef]
- Sonoo, M.; Ogawa, G.; Hokkoku, K.; Stålberg, E. Updated size index valid for both neurogenic and myogenic changes. Muscle Nerve 2020, 62, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang-Frederiksen, A. The utility of interference pattern analysis. Muscle Nerve 2000, 23, 18–36. [Google Scholar] [CrossRef]
- Kurca, E.; Drobný, M. Four quantitative EMG methods and theirs individual parameter diagnostic value. Electromyogr. Clin. Neurophysiol. 2000, 40, 451–458. [Google Scholar] [PubMed]
- Abel, E.W.; Meng, H.; Forster, A.; Holder, D. Singularity characteristics of needle EMG IP signals. IEEE Trans. Biomed. Eng. 2006, 53, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. EMG-interference pattern analysis. J. Electromyogr. Kinesiol. 2001, 11, 231–246. [Google Scholar] [CrossRef]
- Nandedkar, S.D.; Barkhaus, P.E.; Stålberg, E.V. Motor unit number index (MUNIX): Principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle Nerve 2010, 42, 798–807. [Google Scholar] [CrossRef]
- Sghirlanzoni, A.; Solari, A.; Ciano, C.; Mariotti, C.; Fallica, E.; Pareyson, D. Chronic inflammatory demyelinating polyradiculoneuropathy: Long-term course and treatment of 60 patients. Neurol. Sci. 2000, 21, 31–37. [Google Scholar] [CrossRef]
- Iijima, M.; Yamamoto, M.; Hirayama, M.; Tanaka, F.; Katsuno, M.; Mori, K.; Koike, H.; Hattori, N.; Arimura, K.; Nakagawa, M.; et al. Clinical and electrophysiologic correlates of IVIg responsiveness in CIDP. Neurology 2005, 64, 1471–1475. [Google Scholar] [CrossRef]
- Al-Zuhairy, A.; Jakobsen, J.; Moldovan, M.; Krarup, C. Axonal loss at time of diagnosis as biomarker for long-term disability in CIDP. Muscle Nerve 2022, 1–8. [Google Scholar] [CrossRef]
- Dziadkowiak, E.; Waliszewska-Prosół, M.; Nowakowska-Kotas, M.; Budrewicz, S.; Koszewicz, Z.; Koszewicz, M. Pathophysiology of the Different Clinical Phenotypes of Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP). Int. J. Mol. Sci. 2021, 23, 179. [Google Scholar] [CrossRef]
- Fressinaud, C.; Dubas, F. Axon cytoskeleton ultrastructure in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2011, 44, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Paramanathan, S.; Tankisi, H.; Andersen, H.; Fuglsang-Frederiksen, A. Axonal loss in patients with inflammatory demyelinating polyneuropathy as determined by motor unit number estimation and MUNIX. Clin. Neurophysiol. 2016, 127, 898–904. [Google Scholar] [CrossRef]
- Okhovat, A.A.; Advani, S.; Ziaadini, B.; Panahi, A.; Salehizadeh, S.; Nafissi, S.; Haghi Ashtiani, B.; Rajabally, Y.A.; Fatehi, F. The value of MUNIX as an objective electrophysiological biomarker of disease progression in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2022, 65, 433–439. [Google Scholar] [CrossRef]
- Harbo, T.; Andersen, H.; Jakobsen, J. Length-dependent weakness and electrophysiological signs of secondary axonal loss in chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 2008, 38, 1036–1045. [Google Scholar] [CrossRef]
- Gilmore, K.J.; Kirk, E.A.; Doherty, T.J.; Kimpinski, K.; Rice, C.L. Abnormal motor unit firing rates in chronic inflammatory demyelinating polyneuropathy. J. Neurol. Sci. 2020, 414, 116859. [Google Scholar] [CrossRef]
- Al-Zuhairy, A.; Sindrup, S.H.; Andersen, H.; Jakobsen, J. A population-based study of long-term outcome in treated chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2020, 61, 316–324. [Google Scholar] [CrossRef]

| Parameters | CIDP Patients | HCs |
|---|---|---|
| Age (years) | 61.13 ± 3.25 | 59.7 ± 5.33 |
| Gender (M/F) | 10/6 | 8/8 |
| Disease duration (months) at diagnosis | 70.86 ± 12.96 | / |
| Delay of ScIg treatment from disease onset (months) | 27.06 ± 7 | / |
| ScIg dose (g/kg/week) | 0.2–0.4 | / |
| Mean dose ScIg (g/week) | 21.25 ± 2.23 | / |
| Clinical Parameters/Cut-Off | Baseline | IVIg Treatment | ScIg Treatment | p |
|---|---|---|---|---|
| MRCS/60 | 31.3 ± 1.0 | 51.3 ± 0.9 | 56.5 ± 0.6 | <0.01 |
| INCAT/20 | 9.5 ± 0.7 | 6.7 ± 0.8 | 5.6 ± 0.9 | <0.01 |
| R-ODS/48 | 26 ± 1.9 | 35.2 ± 4.6 | 42.7 ± 3.1 | <0.01 |
| ODSS/12 | 7.2 ± 0.6 | 5.3 ± 0.9 | 3.0 ± 0.5 | <0.01 |
| EQ-VAS/100 | 34.2 ± 3.1 | 68.9 ± 2.8 | 79.4 ± 4.9 | <0.01 |
| CIDP Patients | HCs | |||||||
|---|---|---|---|---|---|---|---|---|
| Nerve | MC | Ul | Fem | DP | MC | Ul | Fem | DP |
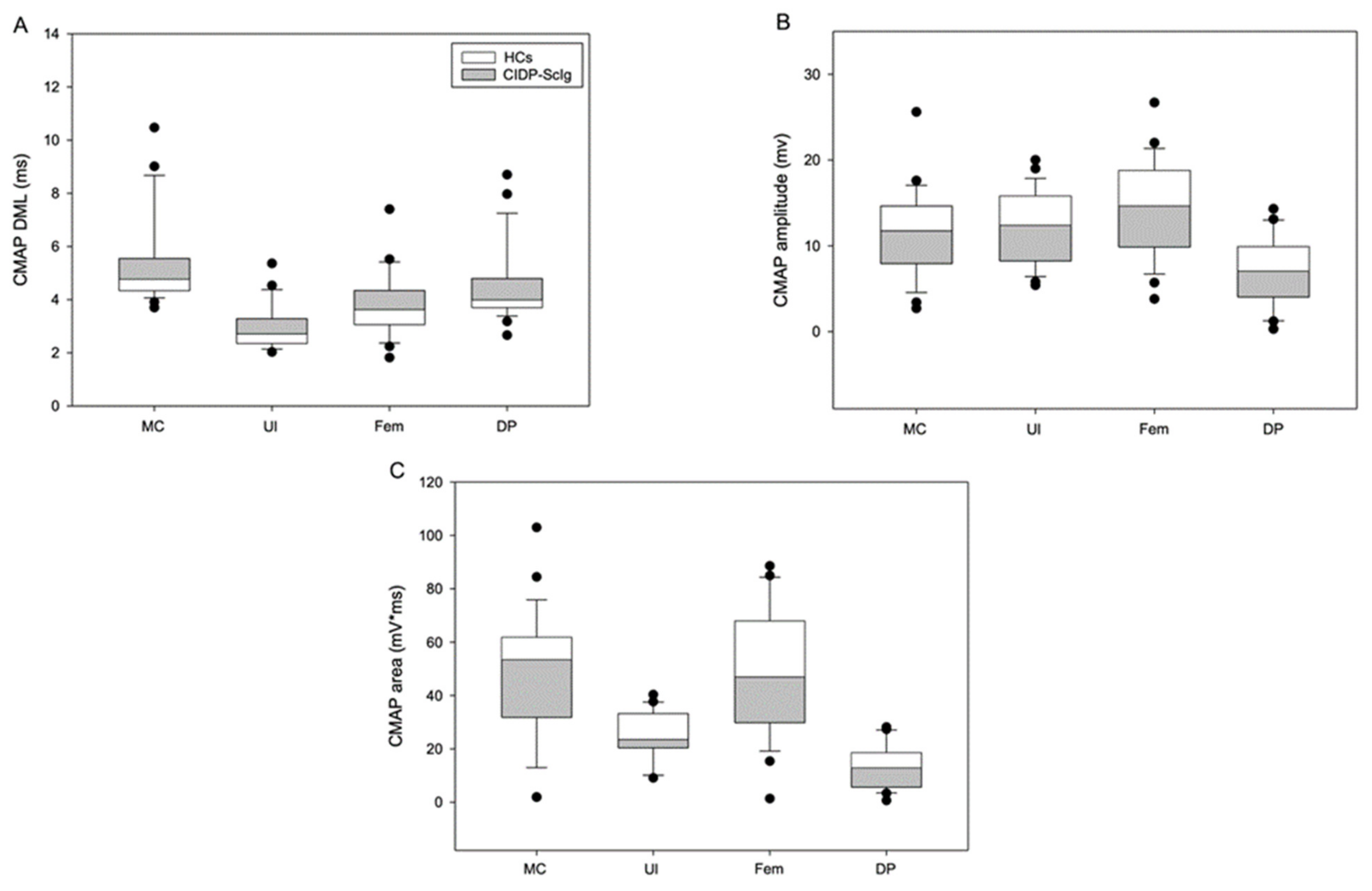
| DML (ms) | 5.8 ± 0.4 | 3.2 ± 0.2 | 4.1 ± 0.3 | 4.8 ± 0.4 | 4.3 ± 0.01 | 2.4 ± 0.01 | 3.1 ± 0.1 | 3.7 ± 0.2 |
| p | <0.05 | <0.01 | <0.05 | <0.01 | ||||
| CMAP Amplitude (mV) | 9.7 ± 1.0 | 10.2 ± 0.8 | 12.2 ± 1.3 | 5.6 ± 0.9 | 15.0 ± 1.6 | 16.2 ± 0.8 | 18.6 ± 1.6 | 9.6 ± 1.1 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | ||||
| CMAP Area (mV*ms) | 43.8 ± 5.2 | 21.2 ± 1.7 | 42.2 ± 4.7 | 11.2 ± 2.0 | 60.9 ± 7.6 | 32.5 ± 2.4 | 62.8 ± 9.1 | 18.8 ± 1.8 |
| p | <0.01 | <0.01 | <0.05 | <0.05 | ||||
| CIDP Patients | HCs | |||||||
|---|---|---|---|---|---|---|---|---|
| Muscle | BB | FDI | VL | EDB | BB | FDI | VL | EDB |
| Phases (n) | 3.1 ± 0.2 | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.7 ± 0.3 | 2.9 ± 0.1 | 3.3 ± 0.2 | 3.4 ± 0.1 | 3.3 ± 0.3 |
| p | 0.10 | 0.60 | 0.60 | 0.09 | ||||
| Amplitude (mV) | 0.8 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.3 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 1.4 ± 0.2 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | ||||
| Duration (ms) | 11.3 ± 0.2 | 10.7 ± 0.2 | 14.6 ± 0.3 | 11.4 ± 0.2 | 10.1 ± 0.2 | 9.6 ± 0.2 | 13.5 ± 0.2 | 10.2 ± 0.1 |
| p | <0.05 | <0.01 | <0.05 | <0.01 | ||||
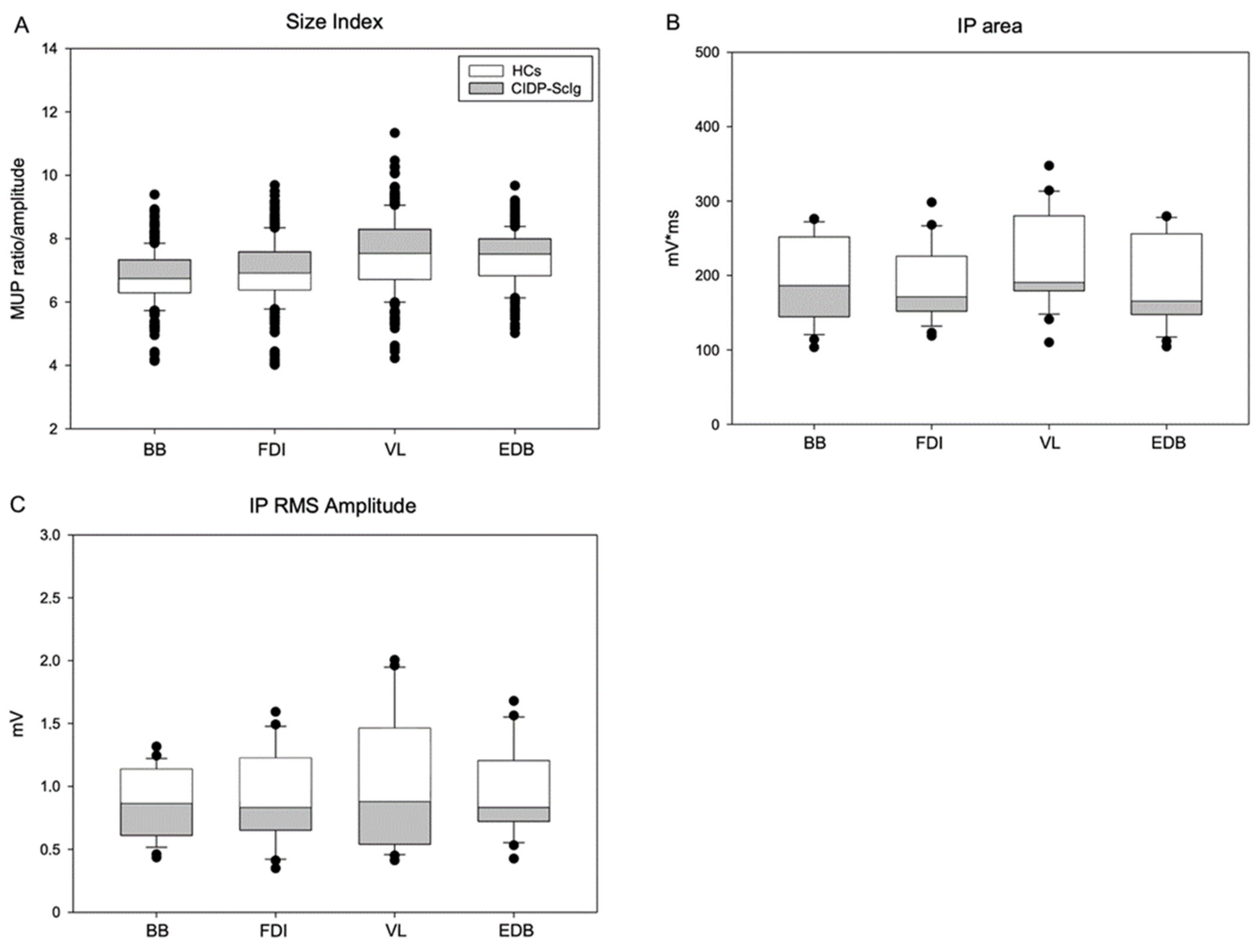
| MUP Area (μV*ms) | 1160 ± 87 | 1759 ± 138 | 2629 ± 188 | 2474 ± 139 | 736 ± 42 | 1022 ± 73 | 1498 ± 94 | 2053 ± 163 |
| p | <0.01 | <0.01 | <0.01 | <0.05 | ||||
| MUP Thickness | 1.4 ± 0.05 | 1.4 ± 0.05 | 1.9 ± 0.06 | 1.5 ± 0.036 | 1.4 ± 0.06 | 1.3 ± 0.05 | 1.8 ± 0.07 | 1.4 ± 0.05 |
| p | 0.89 | 0.16 | 0.28 | 0.25 | ||||
| Size Index | 7.0 ± 0.06 | 7.4 ± 0.06 | 8.1 ± 0.06 | 7.8 ± 0.05 | 6.1 ± 0.05 | 6.0 ± 0.07 | 6.3 ± 0.07 | 6.5 ± 0.07 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | ||||
| IP Area (mV*ms) | 169.5 ± 19.5 | 155.3 ± 15.5 | 178.9 ± 25.5 | 149.7 ± 22.8 | 254.6 ± 25.8 | 247.8 ± 23.0 | 294.6 ± 49.7 | 262.8 ± 27.8 |
| p | <0.05 | <0.01 | <0.05 | <0.01 | ||||
| IP RMS (mV) | 0.76 ± 0.1 | 0.71 ± 0.1 | 0.87 ± 0.1 | 0.74 ± 0.1 | 1.12 ± 0.1 | 1.33 ± 0.1 | 1.39 ± 0.2 | 1.43 ± 0.2 |
| p | <0.05 | <0.01 | <0.05 | <0.01 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciardi, D.; Amitrano, F.; Coccia, A.; Todisco, V.; Trojsi, F.; Tedeschi, G.; Cirillo, G. Neurophysiological Hallmarks of Axonal Degeneration in CIDP Patients: A Pilot Analysis. Brain Sci. 2022, 12, 1510. https://doi.org/10.3390/brainsci12111510
Ricciardi D, Amitrano F, Coccia A, Todisco V, Trojsi F, Tedeschi G, Cirillo G. Neurophysiological Hallmarks of Axonal Degeneration in CIDP Patients: A Pilot Analysis. Brain Sciences. 2022; 12(11):1510. https://doi.org/10.3390/brainsci12111510
Chicago/Turabian StyleRicciardi, Dario, Federica Amitrano, Armando Coccia, Vincenzo Todisco, Francesca Trojsi, Gioacchino Tedeschi, and Giovanni Cirillo. 2022. "Neurophysiological Hallmarks of Axonal Degeneration in CIDP Patients: A Pilot Analysis" Brain Sciences 12, no. 11: 1510. https://doi.org/10.3390/brainsci12111510





