Vigilant Attention, Cerebral Blood Flow and Grey Matter Volume Change after 36 h of Acute Sleep Deprivation in Healthy Male Adults: A Pilot Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
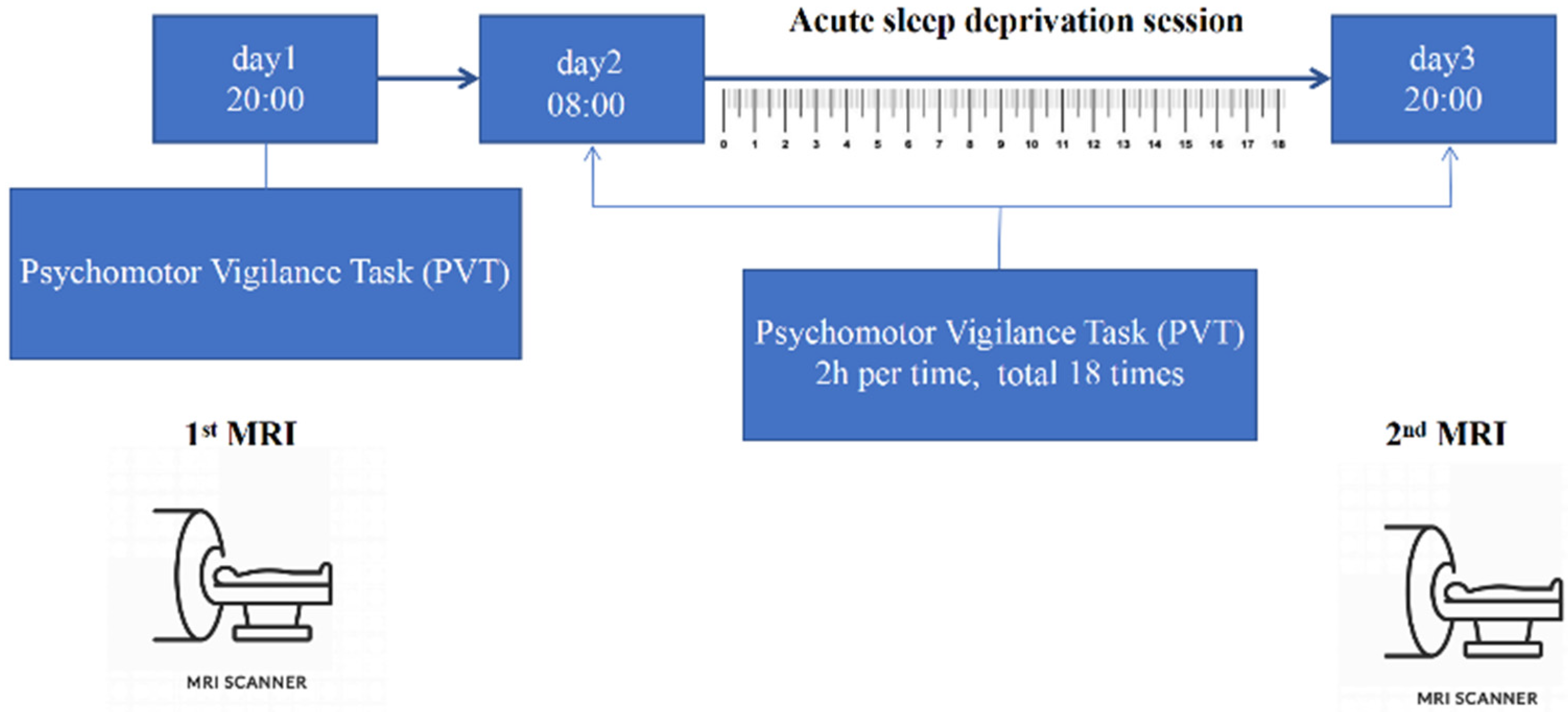
2.2. Experiment Paradigm
2.3. MRI Data Acquisition
2.4. Data Processing
2.5. Cognitive Measurement
2.6. Sample Size
2.7. Statistical Analysis
3. Results
3.1. PVT Change Performance
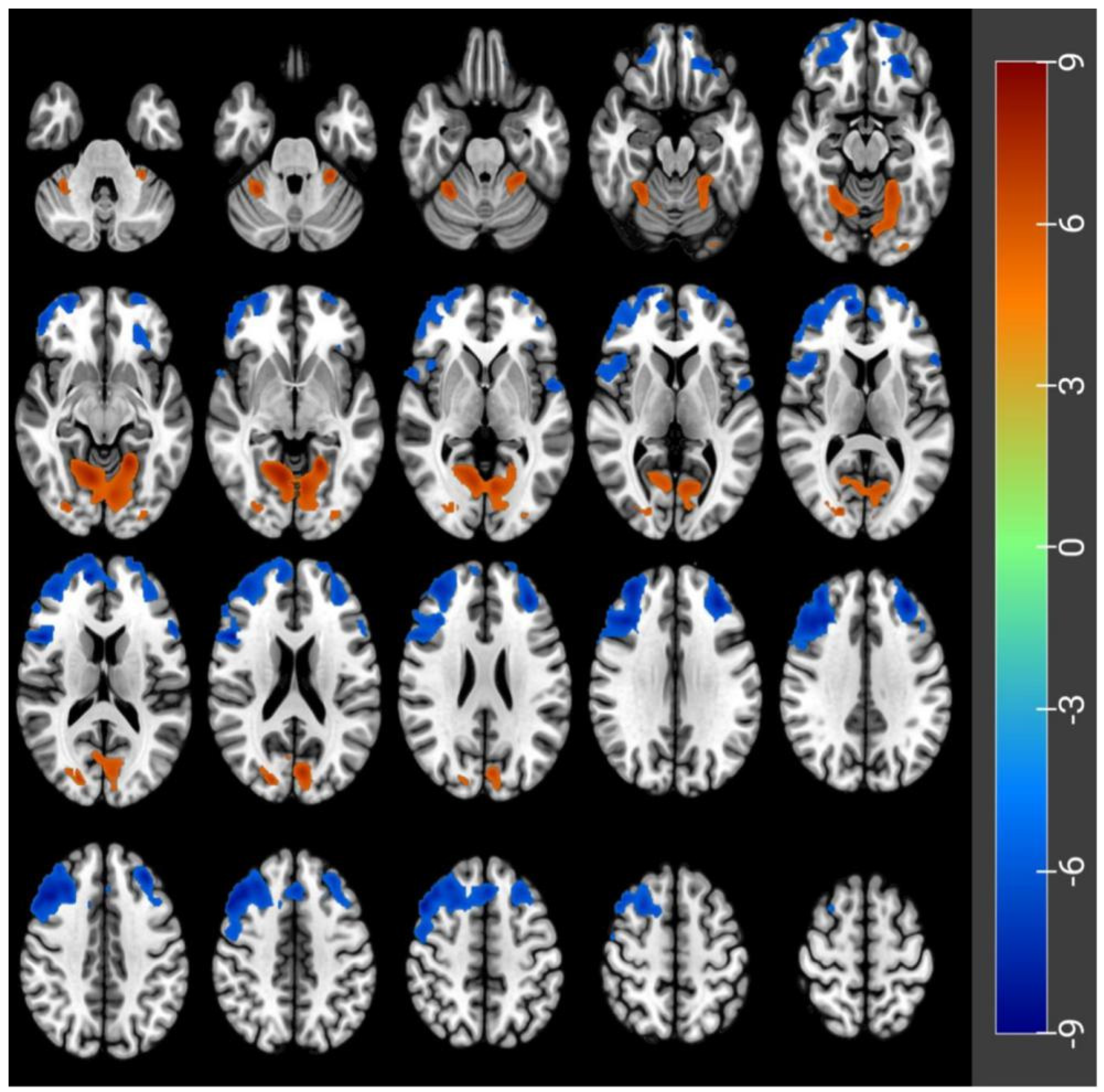
3.2. CBF Differences before and after Acute SD
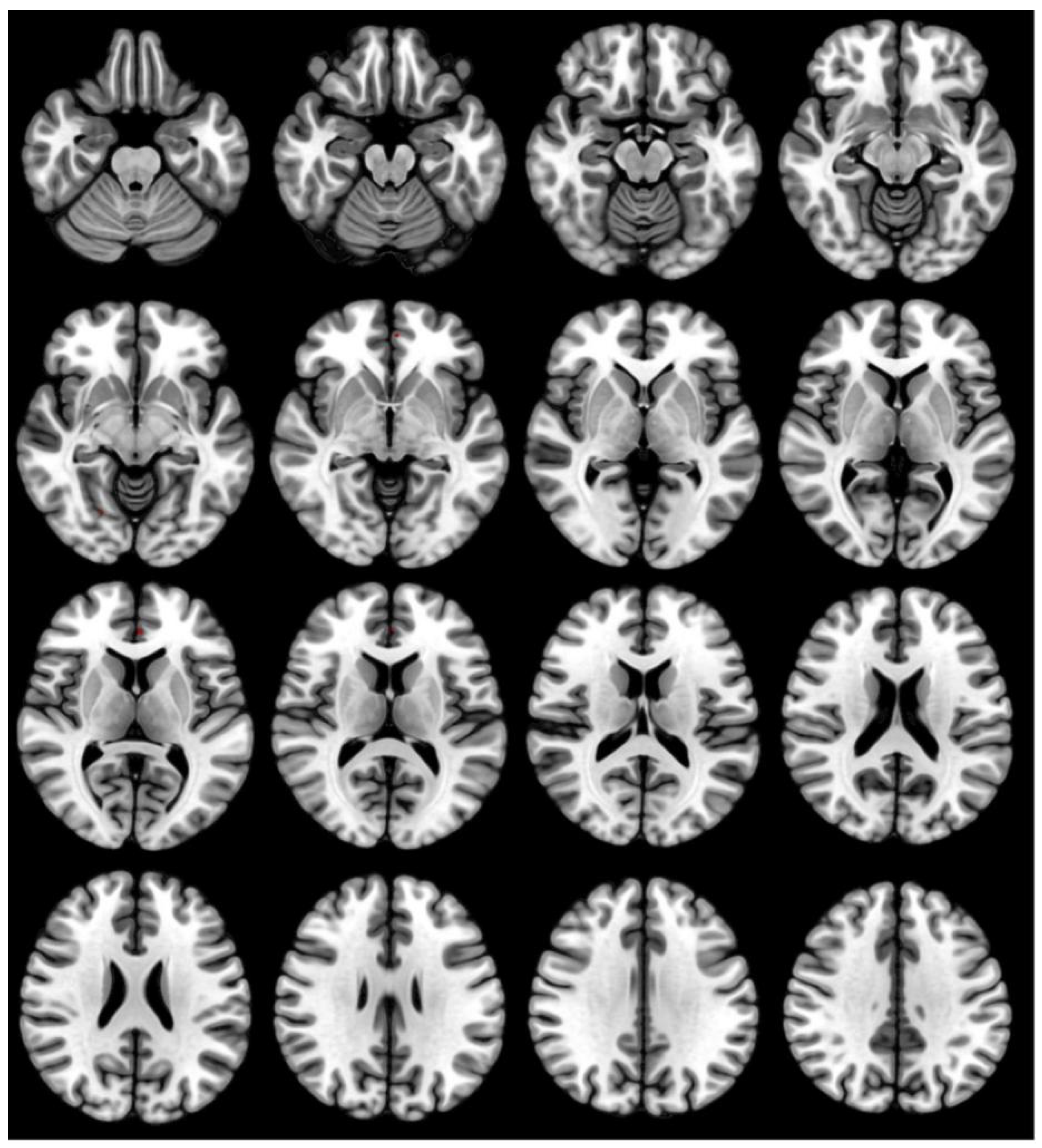
3.3. GMV Differences before and after Acute SD
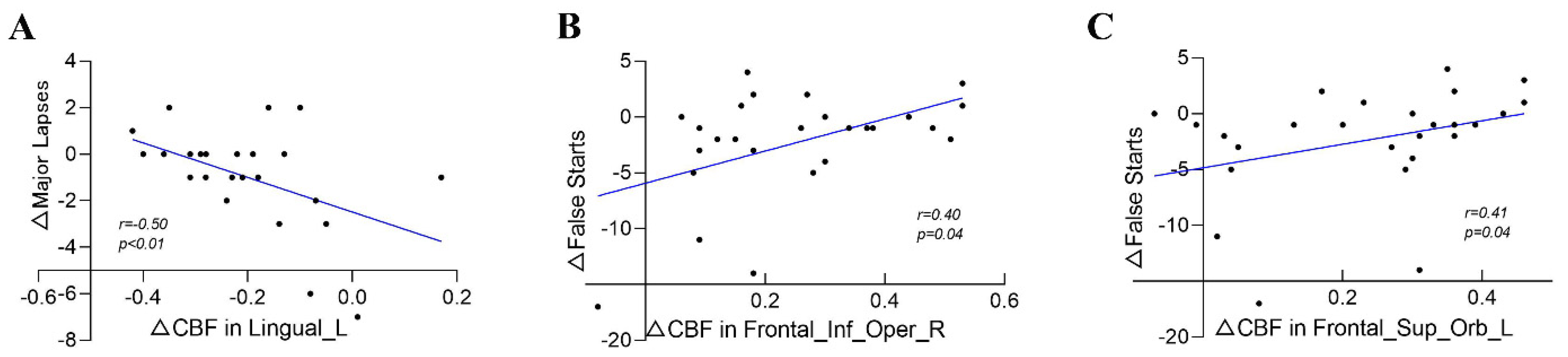
3.4. Correlation between PVT Changes and CBF Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Honma, A.; Revell, V.L.; Gunn, P.J.; Davies, S.K.; Middleton, B.; Raynaud, F.I.; Skene, D.J. Effect of acute total sleep deprivation on plasma melatonin, cortisol and metabolite rhythms in females. Eur. J. Neurosci 2020, 51, 366–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Fronczek-Poncelet, J.; Lange, D.; Hennecke, E.; Kroll, T.; Matusch, A.; Aeschbach, D.; Bauer, A.; Elmenhorst, E.M.; Elmenhorst, D. Impact of acute sleep deprivation on dynamic functional connectivity states. Hum. Brain Mapp. 2020, 41, 994–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, D.A.; Layton, M.E.; Riedy, S.M.; Van Dongen, H.P. Psychomotor Vigilance Impairment During Total Sleep Deprivation Is Exacerbated in Sleep-Onset Insomnia. Nat. Sci. Sleep 2019, 11, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restom, K.; Bangen, K.J.; Bondi, M.W.; Perthen, J.E.; Liu, T.T. Cerebral blood flow and BOLD responses to a memory encoding task: A comparison between healthy young and elderly adults. Neuroimage 2007, 37, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detre, J.A.; Leigh, J.S.; Williams, D.S.; Koretsky, A.P. Perfusion imaging. Magn. Reson. Med. 1992, 23, 37–45. [Google Scholar] [CrossRef]
- Killgore, W.D.; Schwab, Z.J.; Kipman, M.; DelDonno, S.R.; Weber, M. Voxel-based morphometric gray matter correlates of daytime sleepiness. Neurosci Lett. 2012, 518, 10–13. [Google Scholar] [CrossRef]
- Baillet, M.; Dilharreguy, B.; Pérès, K.; Dartigues, J.F.; Mayo, W.; Catheline, G. Activity/rest cycle and disturbances of structural backbone of cerebral networks in aging. Neuroimage 2017, 146, 814–820. [Google Scholar] [CrossRef]
- Dai, X.J.; Jiang, J.; Zhang, Z.; Nie, X.; Liu, B.X.; Pei, L.; Gong, H.; Hu, J.; Lu, G.; Zhan, Y. Plasticity and Susceptibility of Brain Morphometry Alterations to Insufficient Sleep. Front. Psychiatry 2018, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Rees, G. Brain network dynamics in high-functioning individuals with autism. Nat. Commun. 2017, 8, 16048. [Google Scholar] [CrossRef] [Green Version]
- Speer, S.P.H.; Smidts, A.; Boksem, M.A.S. Cognitive control increases honesty in cheaters but cheating in those who are honest. Proc. Natl. Acad. Sci. USA 2020, 117, 19080–19091. [Google Scholar] [CrossRef]
- van de Sande-Lee, S.; Pereira, F.R.; Cintra, D.E.; Fernandes, P.T.; Cardoso, A.R.; Garlipp, C.R.; Chaim, E.A.; Pareja, J.C.; Geloneze, B.; Li, L.M.; et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 2011, 60, 1699–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brickman, A.M.; Muraskin, J.; Zimmerman, M.E. Structural neuroimaging in Altheimer’s disease: Do white matter hyperintensities matter? Dialogues Clin. Neurosci 2009, 11, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhao, R.; Yang, X.; Deng, H.; Zhu, Y.; Chen, Y.; Yuan, K.; Xi, Y.; Yin, H.; Qin, W. Alteration of Brain Gray Matter Density After 24 h of Sleep Deprivation in Healthy Adults. Front. Neurosci 2020, 14, 754. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sheng, W.; Lu, F.; Long, Z.; Han, S.; Pang, Y.; Chen, Y.; Luo, W.; Yu, Y.; Nan, X.; et al. Altered resting-state cerebral blood flow and functional connectivity of striatum in bipolar disorder and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Reifman, J.; Kumar, K.; Khitrov, M.Y.; Liu, J.; Ramakrishnan, S. PC-PVT 2.0: An updated platform for psychomotor vigilance task testing, analysis, prediction, and visualization. J. Neurosci Methods 2018, 304, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Beck, T.W. The importance of a priori sample size estimation in strength and conditioning research. J. Strength Cond. Res. 2013, 27, 2323–2337. [Google Scholar] [CrossRef]
- Meess, A. Platelet transfusion in chemotherapy patients: Comparison of the effect of intravenous infusion pumps versus gravity transfusion. Br. J. Biomed Sci. 2015, 72, 111–114. [Google Scholar] [CrossRef]
- Arnal, P.J.; Sauvet, F.; Leger, D.; van Beers, P.; Bayon, V.; Bougard, C.; Rabat, A.; Millet, G.Y.; Chennaoui, M. Benefits of Sleep Extension on Sustained Attention and Sleep Pressure Before and During Total Sleep Deprivation and Recovery. Sleep 2015, 38, 1935–1943. [Google Scholar] [CrossRef]
- Drummond, S.P.; Bischoff-Grethe, A.; Dinges, D.F.; Ayalon, L.; Mednick, S.C.; Meloy, M.J. The neural basis of the psychomotor vigilance task. Sleep 2005, 28, 1059–1068. [Google Scholar]
- Jung, C.M.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P., Jr. Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J. Sleep Res. 2011, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Louca, M.; Short, M.A. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep 2014, 37, 1799–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada-Quintero, H.F.; Bolkhovsky, J.B.; Qin, M.; Chon, K.H. Human Performance Deterioration Due to Prolonged Wakefulness Can Be Accurately Detected Using Time-Varying Spectral Analysis of Electrodermal Activity. Hum. Factors 2018, 60, 1035–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poudel, G.R.; Innes, C.R.; Jones, R.D. Cerebral perfusion differences between drowsy and nondrowsy individuals after acute sleep restriction. Sleep 2012, 35, 1085–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muto, V.; Jaspar, M.; Meyer, C.; Kussé, C.; Chellappa, S.L.; Degueldre, C.; Balteau, E.; Shaffii-Le Bourdiec, A.; Luxen, A.; Middleton, B.; et al. Local modulation of human brain responses by circadian rhythmicity and sleep debt. Science 2016, 353, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Victoria, L.W.; Pyles, J.A.; Tarr, M.J. The relative contributions of visual and semantic information in the neural representation of object categories. Brain Behav. 2019, 9, e01373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palejwala, A.H.; Dadario, N.B.; Young, I.M.; O’Connor, K.; Briggs, R.G.; Conner, A.K.; O’Donoghue, D.L.; Sughrue, M.E. Anatomy and White Matter Connections of the Lingual Gyrus and Cuneus. World Neurosurg. 2021, 151, e426–e437. [Google Scholar] [CrossRef]
- Sullan, M.J.; Drummond, S.P.A.; Granholm, E. Sleep deprivation and compensatory cognitive effort on a visual information processing task. Sleep 2021, 44, zsaa177. [Google Scholar] [CrossRef]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef]
- Li, P.; Bing, D.; Wang, S.; Chen, J.; Du, Z.; Sun, Y.; Qi, F.; Zhang, Y.; Chu, H. Sleep Deprivation Modifies Noise-Induced Cochlear Injury Related to the Stress Hormone and Autophagy in Female Mice. Front. Neurosci. 2019, 13, 1297. [Google Scholar] [CrossRef]
- Kong, D.; Soon, C.S.; Chee, M.W. Reduced visual processing capacity in sleep deprived persons. Neuroimage 2011, 55, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Gillin, J.C.; Buchsbaum, M.S.; Chen, P.; Keator, D.B.; Khosla Wu, N.; Darnall, L.A.; Fallon, J.H.; Bunney, W.E. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology 2006, 31, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Cullen, T.; Thomas, G.; Wadley, A.J.; Myers, T. The effects of a single night of complete and partial sleep deprivation on physical and cognitive performance: A Bayesian analysis. J. Sports Sci. 2019, 37, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Javaheripour, N.; Shahdipour, N.; Noori, K.; Zarei, M.; Camilleri, J.A.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B.; Eickhoff, C.R.; Rosenzweig, I.; et al. Functional brain alterations in acute sleep deprivation: An activation likelihood estimation meta-analysis. Sleep Med. Rev. 2019, 46, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, R.; Höller, Y.; Sebastianelli, L.; Versace, V.; Saltuari, L.; Brigo, F.; Lochner, P.; Trinka, E. Cortical morphometric changes after spinal cord injury. Brain Res. Bull. 2018, 137, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Hinard, V.; Mikhail, C.; Pradervand, S.; Curie, T.; Houtkooper, R.H.; Auwerx, J.; Franken, P.; Tafti, M. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J. Neurosci. 2012, 32, 12506–12517. [Google Scholar] [CrossRef] [Green Version]
- Riemann, D.; Voderholzer, U.; Spiegelhalder, K.; Hornyak, M.; Buysse, D.J.; Nissen, C.; Hennig, J.; Perlis, M.L.; van Elst, L.T.; Feige, B. Chronic insomnia and MRI-measured hippocampal volumes: A pilot study. Sleep 2007, 30, 955–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, E.Y.; Kim, H.; Suh, S.; Hong, S.B. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: Magnetic resonance imaging morphometry. Sleep 2014, 37, 1189–1198. [Google Scholar] [CrossRef] [Green Version]
- Mondello, S.; Kobeissy, F.; Mechref, Y.; Zhao, J.; Talih, F.R.; Cosentino, F.; Antelmi, E.; Moresco, M.; Plazzi, G.; Ferri, R. Novel biomarker signatures for idiopathic REM sleep behavior disorder: A proteomic and system biology approach. Neurology 2018, 91, e1710–e1715. [Google Scholar] [CrossRef]
- Chen, J.J.; Jann, K.; Wang, D.J. Characterizing Resting-State Brain Function Using Arterial Spin Labeling. Brain Connect 2015, 5, 527–542. [Google Scholar] [CrossRef]
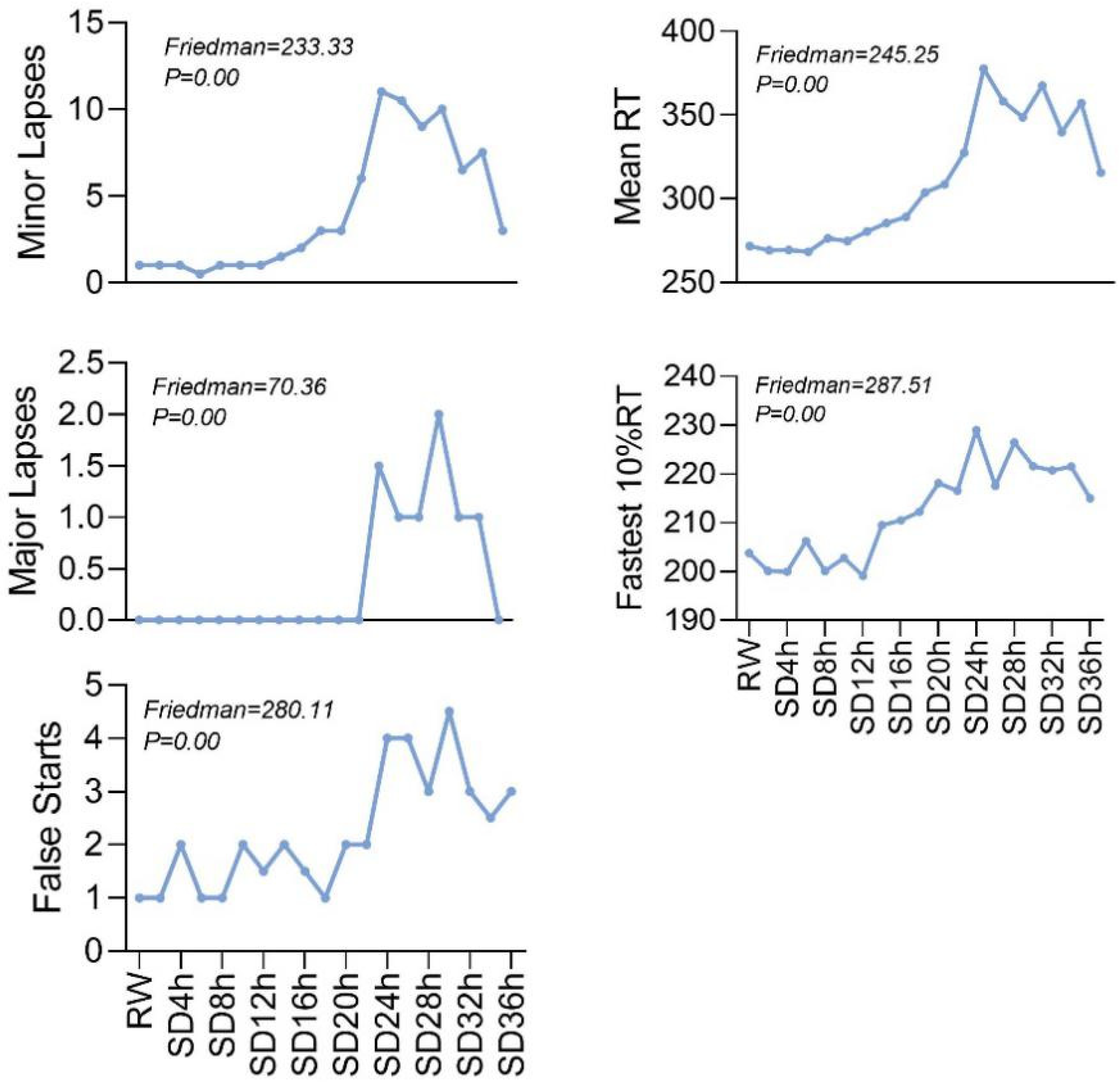
| Periods | Minor Lapses | Major Lapses | False Starts | Mean RT | Fastest 10% RT |
|---|---|---|---|---|---|
| RW | 1 (0, 2) | 0 (0, 1) | 1 (0, 3) | 271.77 (243.29, 286.73) | 203.75 (187.75, 217.42) |
| SD2h | 1 (0, 2) | 0 (0, 0) | 1 (0, 3) | 269.14 (248.20, 289.85) | 200.06 (179.40, 211.73) |
| SD4h | 1 (0, 2) | 0 (0, 0) | 2 (0, 3) | 269.43 (257.78, 285.34) | 199.94 (183.78, 211.90) |
| SD6h | 0.5 (0, 2) | 0 (0, 0) | 1 (0, 3) | 268.27 (255.67, 298.42) | 206.21 (192.60, 214.10) |
| SD8h | 1 (0, 2) | 0 (0, 0) | 1 (0, 4) | 276.34 (257.18, 296.77) | 200.09 (192.90, 217.70) |
| SD10h | 1 (0, 3) | 0 (0, 0) | 2 (0, 6) | 274.60 (264.77, 298.31) | 202.77 (183.67, 209.67) |
| SD12h | 1 (0, 2) | 0 (0, 0) | 1.5 (0, 4) | 280.30 (262.20, 296.73) | 199.11 (191.30, 218.00) |
| SD14h | 1.5 (1, 3) | 0 (0, 0) | 2 (1, 3) | 285.50 (261.49, 312.90) | 209.47 (200.33, 222.60) |
| SD16h | 2 (1, 4) | 0 (0, 0) | 1.5 (0, 3) | 289.02 (269.29, 320.59) | 210.45 (195.78, 227.70) |
| SD18h | 3 (1, 7) | 0 (0, 1) | 1 (0, 3) | 303.61 (277.70, 346.80) | 212.20 (202.33, 231.33) |
| SD20h | 3 (1, 6) | 0 (0, 1) | 2 (0, 4) | 308.67 (288.99, 328.13) | 218.06 (206.70, 224.36) |
| SD22h | 6 (2, 8) | 0 (0, 1) | 2 (0, 4) | 327.42 (301.67, 359.56) | 216.55 (204.90, 236.90) |
| SD24h | 11 (5, 16) | 1.5 (0, 3) | 4 (1, 6) | 377.71 (320.55, 438.73) | 228.96 (218.44, 237.50) |
| SD26h | 10.5 (4, 18) | 1 (0, 5) | 4 (1, 7) | 358.15 (326.30, 468.54) | 217.54 (210.67, 229.13) |
| SD28h | 9 (2, 13) | 1 (0, 4) | 3 (1, 7) | 348.57 (298.82, 413.51) | 226.41 (205.90, 241.40) |
| SD30h | 10 (5, 15) | 2 (0, 5) | 4.5 (2, 8) | 367.55 (331.16, 420.60) | 221.50 (203.50, 232.78) |
| SD32h | 6.5 (4, 16) | 1 (0, 4) | 3 (1, 5) | 339.68 (310.89, 423.39) | 220.72 (205.44, 231.40) |
| SD34h | 7.5 (5, 12) | 1 (0, 3) | 2.5 (1, 6) | 357.11 (312.21, 397.39) | 221.43 (207.00, 233.33) |
| SD36h | 3 (1, 10) | 0 (0, 2) | 3 (1, 5) | 315.53 (286.93, 359.24) | 214.93 (201.20, 225.20) |
| Friedman | 233.33 | 70.36 | 280.11 | 245.25 | 287.51 |
| P | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Peak Location | Side | Cluster Size | MNI Coordinates | Peak t-Score | p-Value Correctedby FWE | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Frontal_Mid_Orb | L | 372 | −26 | 40 | −18 | −7.2 | <0.05 |
| Frontal_Sup_Orb | L | 2006 | −12 | 62 | −18 | −9 | <0.05 |
| Frontal_Inf_Oper | R | 7735 | 48 | 14 | 18 | −8.56 | <0.05 |
| Frontal_Inf_Tri | L | 121 | −56 | 22 | 14 | −7.19 | <0.05 |
| Rolandic_Oper | L | 116 | −54 | 2 | 2 | −7.07 | <0.05 |
| Lingual | L | 3910 | −20 | −52 | −8 | 8.29 | <0.05 |
| Occipital_Sup | R | 346 | 20 | −84 | 18 | 7.06 | <0.05 |
| False Starts | Minor Lapses | Major Lapses | Mean RT | Fastest 10% RT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | p | R | p | R | p | r | p | r | p | |
| Frontal_Mid_Orb_L | 0.25 | 0.22 | 0.26 | 0.19 | 0.30 | 0.14 | 0.29 | 0.15 | 0.10 | 0.63 |
| Frontal_Inf_Oper_R | 0.40 | 0.04 | 0.23 | 0.25 | 0.35 | 0.08 | 0.31 | 0.12 | 0.03 | 0.89 |
| Frontal_Sup_Orb_L | 0.41 | 0.04 | 0.32 | 0.11 | 0.21 | 0.31 | 0.34 | 0.09 | 0.16 | 0.45 |
| Frontal_Inf_Tri_L | 0.35 | 0.08 | 0.32 | 0.11 | 0.35 | 0.08 | 0.39 | 0.05 | 0.16 | 0.44 |
| Rolandic_Oper_L | 0.35 | 0.08 | 0.32 | 0.11 | 0.35 | 0.08 | 0.39 | 0.05 | 0.16 | 0.44 |
| Lingual_L | −0.38 | 0.05 | −0.26 | 0.20 | −0.50 | 0.01 | −0.39 | 0.05 | −0.09 | 0.67 |
| Occipital_Sup_R | −0.39 | 0.05 | −0.26 | 0.19 | −0.27 | 0.19 | −0.16 | 0.43 | 0.05 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.-R.; Xu, F.; Zhang, J.; Cao, Q.-F.; Wang, Y.-H.; Zhang, P.; Shao, Y.-C.; Wu, S.-P.; Weng, X.-C. Vigilant Attention, Cerebral Blood Flow and Grey Matter Volume Change after 36 h of Acute Sleep Deprivation in Healthy Male Adults: A Pilot Study. Brain Sci. 2022, 12, 1534. https://doi.org/10.3390/brainsci12111534
Zeng H-R, Xu F, Zhang J, Cao Q-F, Wang Y-H, Zhang P, Shao Y-C, Wu S-P, Weng X-C. Vigilant Attention, Cerebral Blood Flow and Grey Matter Volume Change after 36 h of Acute Sleep Deprivation in Healthy Male Adults: A Pilot Study. Brain Sciences. 2022; 12(11):1534. https://doi.org/10.3390/brainsci12111534
Chicago/Turabian StyleZeng, Han-Rui, Fan Xu, Jing Zhang, Qiong-Fang Cao, Yu-Han Wang, Peng Zhang, Yong-Cong Shao, Shao-Ping Wu, and Xie-Chuan Weng. 2022. "Vigilant Attention, Cerebral Blood Flow and Grey Matter Volume Change after 36 h of Acute Sleep Deprivation in Healthy Male Adults: A Pilot Study" Brain Sciences 12, no. 11: 1534. https://doi.org/10.3390/brainsci12111534






