Posterior Fossa Approaches Using the Leksell Vantage Frame with a Virtual Planning Approach in a Series of 10 Patients—Feasibility, Accuracy, and Pitfalls
Abstract
:1. Introduction
2. Materials and Methods
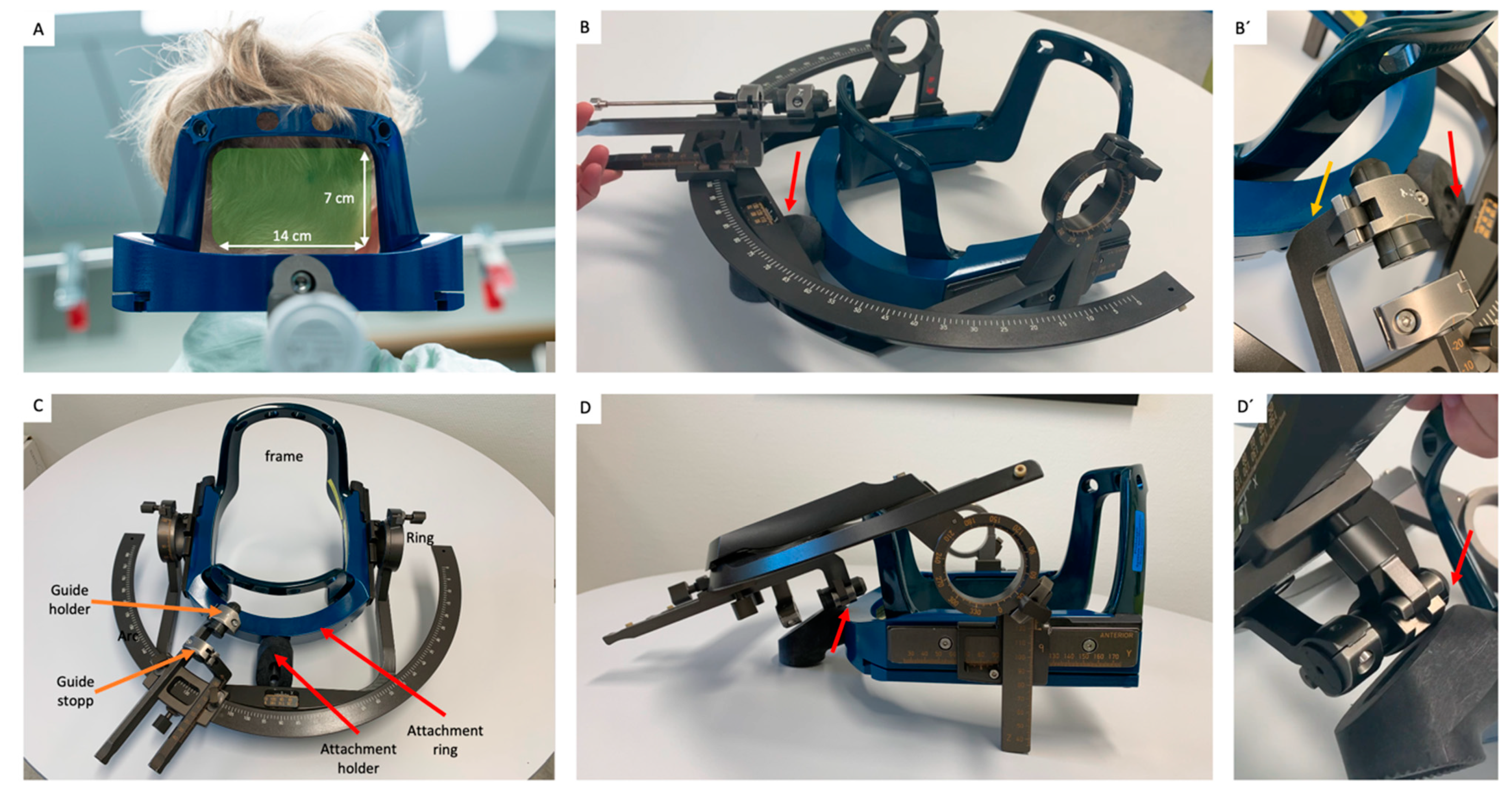
2.1. Basic Considerations
2.2. Determination of Available Ring and Arc Angles
2.3. Patients
2.4. Virtual Frame-Placement
- 1.
- One-time preparation:
- The Vantage frame was fixed to a skull phantom and its CT fiducial box attached;
- A CT scan (0.7 mm slice thickness, Somatom Force, Siemens Healthineers, Munich, Germany) was obtained of the above ensemble (P-CT);
- The object segmentation tool was used to segment the frame and its fiducial box.
- 2.
- Patient-specific planning:
- The trajectory planning tool was used to plan a trajectory to the surgical target on preoperatively acquired, patient-specific MR images;
- The fusion tool was used to co-register the P-CT with its segmented frame to patient-specific images (Figure 3A,B);
- The fusion tool was used, when necessary, to adjust frame height (in coronal view), yaw (in axial view) and pitch (in sagittal view) in relation to the patient-specific images (Figure 3E,F);
- Coordinates were re-checked (Figure 3G,H) and the process repeated until all coordinates and trajectory were feasible;
- The trajectory planning tool was used to display the spatial relationship between the frame and the patient-specific 3D surface render. Frontal, posterior, and lateral views were saved and printed (Figure 3E`,E``,F`);
- The location of the planned target and entry point as well as anterior and posterior posts from skin landmarks was measured and recorded.
2.5. Actual Frame-Placement
2.6. Actual Surgical Planning
2.7. Surgical Procedure
2.8. Evaluation of Accuracy between Virtual and Actual Frame Placement
2.9. Ethics
3. Results
3.1. Patients
3.2. Histological Results
3.3. Accuracy of Frame-Placement
3.4. Surgical Complications
4. Discussion
4.1. Feasibility and Accuracy of Frame Placement
4.2. Frame Placement–Essentials
4.2.1. Increasing the Ring Angle
4.2.2. Limitations to Frame Placement
4.3. Adverse Events
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furtak, J.; Śledzińska, P.; Bebyn, M.G.; Szylberg, T.; Krajewski, S.; Birski, M.; Harat, M. Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions. Brain Sci. 2021, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.J.; Leber, M.J.; Gossett, L.; Lulu, B.A.; Hamilton, A.J. Stereotactic Biopsy of Intracranial Brain Lesions. Stereotact. Funct. Neurosurg. 1998, 71, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Gonçalves-Ferreira, A.J.; Herculano-Carvalho, M.; Pimentel, J. Stereotactic biopsies of focal brainstem lesions. Surg. Neurol. 2003, 60, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Samadani, U.; Judy, K.D. Stereotactic Brainstem Biopsy Is Indicated for the Diagnosis of a Vast Array of Brainstem Pathology. Stereotact. Funct. Neurosurg. 2003, 81, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Willeit, P.; Simon, T.; Ruge, M.I. Diagnostic Value and Safety of Stereotactic Biopsy for Brainstem Tumors: A Systematic Review and Meta-analysis of 1480 Cases. Neurosurgery 2013, 72, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Olvera, J.L.; Aguado-Carrillo, G.; Vintimilla-Sarmiento, J.D.; Parra-Romero, G.; Guartazaca-Guerrero, M.S.; Carrillo-Ruiz, J.D. Concordance and diagnostic yield of stereotactic biopsies for posterior fossa: Technique and experience in a reference hospital. Cir Cir 2022, 90, 433–438. [Google Scholar] [PubMed]
- Abernathey, C.D.; Camacho, A.; Kelly, P.J. Stereotaxic suboccipital transcerebellar biopsy of pontine mass lesions. J. Neurosurg. 1989, 70, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathisen, J.R.; Giunta, F.; Marini, G.; Backlund, E.-O. Transcerebellar biopsy in the posterior fossa: 12 years experience. Surg. Neurol. 1987, 28, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Gleason, C.A.; Wise, B.L.; Feinstein, B. Stereotactic localization (with computerized tomographic scanning), biopsy, and radiofrequency treatment of deep brain lesions. Neurosurgery 1978, 2, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, B.L.; Steinberg, G.K.; Adler, J.R. Posterior fossa stereotaxic biopsy using the Brown-Roberts-Wells stereotaxic system: Technical note. J. Neurosurg. 1989, 70, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Spiegelmann, R.; Friedman, W.A. Stereotactic suboccipital transcerebellar biopsy under local anesthesia using the Cosman-Roberts-Wells frame: Technical note. J. Neurosurg. 1991, 75, 486–488. [Google Scholar] [CrossRef]
- Nakagawa, J.M.; Trippel, M.; Doostkam, S.; Mader, I.; Coenen, V.A.; Reinacher, P.C. The stereotactic suboccipitaltranscerebellar approach to lesions of the brainstem and the cerebellum. Clin. Neurol. Neurosurg. 2018, 166, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, S.; Nakano, H.; Kawamata, T.; Taira, T. Novel Use of the Leksell Gamma Frame for Stereotactic Biopsy of Posterior Fossa Lesions. World Neurosurg. 2017, 107, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, J.F.; Camporesi, S.; Franzin, A.; Barzaghi, L.R.; Picozzi, P.; Mortini, P. Inverted positioning of Leksell Frame G for very low posterior fossa and brain stem lesions biopsies. J. Neurosurg. Sci. 2019, 63, 194–199. [Google Scholar] [CrossRef]
- Neal, J.H.; Van Norman, A.S. Transcerebellar biopsy of posterior fossa lesions using the Leksell gamma model stereotactic frame. Neurosurgery 1993, 32, 473–474; discussion 474–475. [Google Scholar] [CrossRef] [PubMed]




| Right X | Y | Z | Ring | Arc | Left X | Y | Z | Ring | Arc |
|---|---|---|---|---|---|---|---|---|---|
| 90–100 | 60–70 | 130–140 | 169–180 163–180 | 68–112 55–128 | 100–110 | 60–75 | 100–115 | 340–353 340–357 | 69–105 64–122 |
| 90–100 | 60–70 | 141–150 | 163–171 158–178 | 68–105 55–128 | 100–110 | 60–75 | 116–130 | 344–2 344–6 | 69–105 64–122 |
| 101–110 | 60–70 | 130–140 | 169–180 163–180 | 62–103 51–123 | 111–120 | 60–75 | 100–115 | 340–353 340–357 | 77–112 69–124 |
| 101–110 | 60–70 | 141–150 | 163–171 158–178 | 62–98 51–123 | 111–120 | 60–75 | 116–130 | 344–2 344–6 | 77–112 69–124 |
| 111–120 | 60–70 | 130–140 | 169–180 163–180 | 57–96 47–116 | 121–130 | 60–75 | 100–115 | 340–353 340–357 | 84–117 77–130 |
| 111–120 | 60–70 | 141–150 | 163–171 158–178 | 57–87 47–116 | 121–130 | 60–75 | 116–130 | 344–2 344–6 | 84–117 77–130 |
| 90–100 | 71–80 | 130–140 | 171–178 166–178 | 73–107 68–118 | 100–110 | 76–90 | 100–115 | 341–354 341–357 | 73–104 68–117 |
| 90–100 | 71–80 | 141–150 | 166–172 160–176 | 73–100 68–118 | 100–110 | 76–90 | 116–130 | 347–359 347–3 | 73–104 68–117 |
| 101–110 | 71–80 | 130–140 | 171–178 166–178 | 67–99 63–113 | 111–120 | 76–90 | 100–115 | 341–354 341–357 | 79–108 75–122 |
| 101–110 | 71–80 | 141–150 | 166–172 160–176 | 67–95 63–113 | 111–120 | 76–90 | 116–130 | 347–359 347–3 | 79–108 75–122 |
| 111–120 | 71–80 | 130–140 | 171–178 166–178 | 63–92 56–109 | 121–130 | 76–90 | 100–115 | 341–354 341–357 | 85–119 80–125 |
| 111–120 | 71–80 | 141–150 | 166–172 160–176 | 63–90 56–109 | 121–130 | 76–90 | 116–130 | 347–359 347–3 | 85–119 80–125 |
| No | Age | Sex | Size (cm3) | Appr. | Location | Histolopathology |
|---|---|---|---|---|---|---|
| 1 | 26 | F | 21 | Right | Pons and Medulla right | Astrocytoma Grade 3 |
| 2 | 45 | M | 1.9 | Right | Mesencephalon/ Tegmentum | Lymphoma |
| 3 | 7 | M | 0.17 | Right | Cerebellum right | Ganglioglioma Grade 1 |
| 4 | 39 | M | 3.62 | Right | Pons and Medulla left | Oligodendroglioma Grade 2 |
| 5 | 66 | M | 1.3 | Left | Cerebellum left | Metastatic melanoma |
| 6 | 2 | F | 14.4 | Right | Medulla left | Pilocytic astrocytoma Grade 1 |
| 7 | 37 | F | 1.2 | Right | Cerebellum (Vermis) | Medulloblastoma Grade 4 |
| 8 | 83 | M | 0.7 | Left | Cerebellum left | Hemangioblastoma |
| 9 | 10 | M | 0.42 | Right | Cerebellum right | Pilocytic astrocytoma Grade 1 |
| 10 | 3 | F | 9.3 | Left | Pons | High-grade glioma |
| Pat | Virt. X | Y | Z | Ring | Arc | Act. X | Y | Z | Ring | Arc | Dif. X | Y | Z | Ring | Arc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 99.5 | 80.5 | 137.5 | 171 | 68 | 93 | 74 | 154.5 | 168 | 78 | 6.5 | 5.5 | 17 | 3 | 10 |
| 2 | 99.5 | 80 | 133 | 179 | 66 | 100.5 | 80.5 | 143 | 177.5 | 71 | 1 | 0.5 | 10 | 1.5 | 5 |
| 3 | 90.5 | 65.5 | 135 | 172.5 | 73.5 | 92.5 | 71 | 145 | 177 | 82 | 2 | 5.5 | 10 | 5.5 | 2.5 |
| 4 | 115.5 | 69.5 | 128 | 165 | 145 | 116 | 71 | 140.5 | 153 | 140 | 0.5 | 1.5 | 13 | 12 | 5 |
| 5 | 127 | 70 | 108.5 | 347 | 82 | 121.5 | 66.5 | 120.5 | 350 | 92.5 | 6 | 3.5 | 12.5 | 3 | 10.5 |
| 6 | 102 | 75 | 142 | 167 | 110 | 96.5 | 71 | 151.2 | 170 | 104 | 5.5 | 4 | 11 | 3 | 6 |
| 7 | 110 | 70 | 130 | 177 | 64 | 108 | 76.5 | 141 | 178 176 | 64 | 2 | 6.5 | 11 | 1 | 12.5 |
| 8 | 95.5 | 68.5 | 111 | 341.5 | 74.5 | 93.5 | 64 | 105 | 343 | 75 | 2 | 4.5 | 6 | 1.5 | 0.5 |
| 9 | 77 | 46.5 | 133 | 174 | 99.5 | 70.5 | 61 | 129.5 | 172 | 95 | 6.5 | 14.5 | 4 | 2 | 4.5 |
| 10 | 107.5 | 90 | 126.5 | 177.5 | 103 | 106.5 | 105.5 | --- 103.0 | 184 357 | 106 83 | 1 | 15 | 23.5 | 6.5 | 3 |
| 3.3 | 6.1 | 11.6 | 3.9 | 5.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, M.T.; Terrapon, A.P.R.; Hoyningen, A.; Kim, C.-H.O.; Lauber, A.; Bozinov, O. Posterior Fossa Approaches Using the Leksell Vantage Frame with a Virtual Planning Approach in a Series of 10 Patients—Feasibility, Accuracy, and Pitfalls. Brain Sci. 2022, 12, 1608. https://doi.org/10.3390/brainsci12121608
Krüger MT, Terrapon APR, Hoyningen A, Kim C-HO, Lauber A, Bozinov O. Posterior Fossa Approaches Using the Leksell Vantage Frame with a Virtual Planning Approach in a Series of 10 Patients—Feasibility, Accuracy, and Pitfalls. Brain Sciences. 2022; 12(12):1608. https://doi.org/10.3390/brainsci12121608
Chicago/Turabian StyleKrüger, Marie T., Alexis P. R. Terrapon, Alexander Hoyningen, Chan-Hi Olaf Kim, Arno Lauber, and Oliver Bozinov. 2022. "Posterior Fossa Approaches Using the Leksell Vantage Frame with a Virtual Planning Approach in a Series of 10 Patients—Feasibility, Accuracy, and Pitfalls" Brain Sciences 12, no. 12: 1608. https://doi.org/10.3390/brainsci12121608
APA StyleKrüger, M. T., Terrapon, A. P. R., Hoyningen, A., Kim, C.-H. O., Lauber, A., & Bozinov, O. (2022). Posterior Fossa Approaches Using the Leksell Vantage Frame with a Virtual Planning Approach in a Series of 10 Patients—Feasibility, Accuracy, and Pitfalls. Brain Sciences, 12(12), 1608. https://doi.org/10.3390/brainsci12121608




