Wakeful Rest Benefits Recall, but Not Recognition, of Incidentally Encoded Memory Stimuli in Younger and Older Adults
Abstract
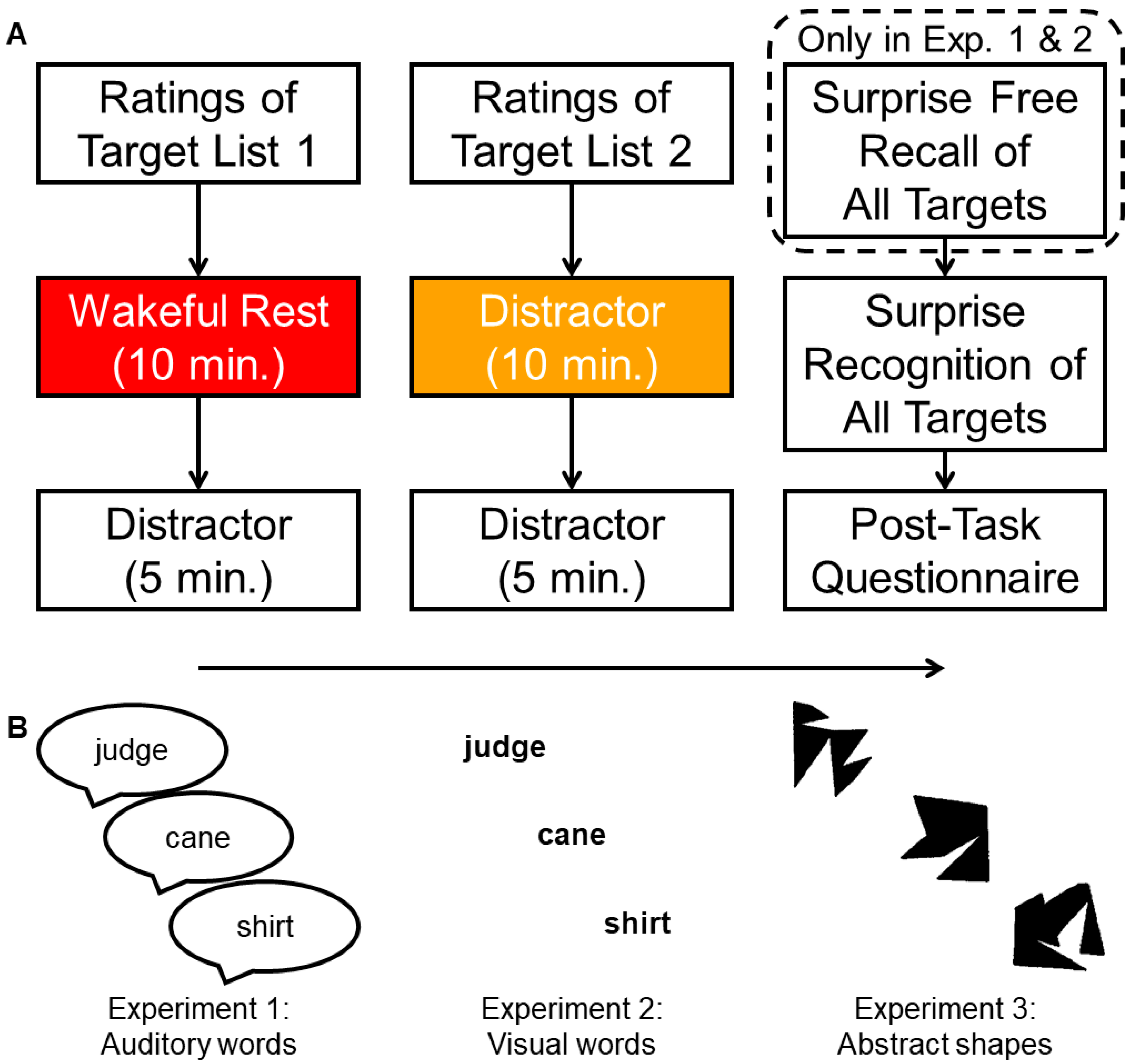
:1. Introduction
2. Experiment 1
2.1. Method
2.1.1. Participants
2.1.2. Design
2.1.3. Materials
2.1.4. Procedure
2.1.5. Statistical Analysis
2.2. Results
2.2.1. Questionnaire
2.2.2. Recall
2.2.3. Recognition
2.3. Discussion
3. Experiment 2
3.1. Method
3.1.1. Participants
3.1.2. Design
3.1.3. Materials
3.1.4. Procedure
3.1.5. Statistical Analysis
3.2. Results
3.2.1. Questionnaire
3.2.2. Recall
3.2.3. Recognition
3.3. Discussion
4. Experiment 3
4.1. Method
4.1.1. Participants
4.1.2. Design
4.1.3. Materials
4.1.4. Procedure
4.1.5. Statistical Analysis
4.2. Results
4.2.1. Questionnaire
4.2.2. Recognition
4.3. Discussion
5. General Discussion
5.1. Consistent Wakeful Rest Effects for Younger and Older Adults
5.2. Benefit of Wakeful Rest on Recall for Incidentally Encoded Verbal Stimuli
5.3. Failure to Observe Wakeful Rest Effects in Recognition Memory
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGaugh, J.L. Memory—A Century of Consolidation. Science 2000, 287, 248–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGaugh, J.L. Consolidating Memories. Annu. Rev. Psychol. 2015, 66, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Dudai, Y. The Neurobiology of Consolidations, Or, How Stable Is the Engram? Annu. Rev. Psychol. 2004, 55, 51–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankland, P.W.; Bontempi, B. The Organization of Recent and Remote Memories. Nat. Rev. Neurosci. 2005, 6, 119–130. [Google Scholar] [CrossRef]
- Wixted, J.T. The Psychology and Neuroscience of Forgetting. Annu. Rev. Psychol. 2004, 55, 235–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wixted, J.T.; Cai, D.J. Memory Consolidation. In The Oxford Handbook of Cognitive Neuroscience, Volume 1: Core Topics; Oxford University Press: Oxford, UK, 2013; pp. 436–455. [Google Scholar]
- Nadel, L.; Moscovitch, M. Memory Consolidation, Retrograde Amnesia and the Hippocampal Complex. Curr. Opin. Neurobiol. 1997, 7, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Moscovitch, M.; Nadel, L.; Winocur, G.; Gilboa, A.; Rosenbaum, R.S. The Cognitive Neuroscience of Remote Episodic, Semantic and Spatial Memory. Curr. Opin. Neurobiol. 2006, 16, 179–190. [Google Scholar] [CrossRef]
- Yonelinas, A.P.; Ranganath, C.; Ekstrom, A.; Wiltgen, B. A Contextual Binding Theory of Episodic Memory: Systems Consolidation Reconsidered. Nat. Rev. Neurosci. 2019, 20, 364–375. [Google Scholar] [CrossRef]
- Squire, L.R.; Alvarez, P. Reterograde Amnesia and Memory Consolidation: A Neurobiological Persepctive. Curr. Opin. Neurobiol. 1995, 5, 169–177. [Google Scholar] [CrossRef]
- Squire, L.R.; Haist, F.; Shimamura, A.P. The Neurology of Memory: Quantitative Assessment of Retrograde Amnesia in Two Groups of Amnesic Patients. J. Neurosci. 1989, 9, 828–839. [Google Scholar] [CrossRef]
- Clark, R.E.; Broadbent, N.J.; Zola-Morgan, S.M.; Squire, L.R. Anterograde Amnesia and Temporally Graded Retrograde Amnesia for a Nonspatial Memory Task after Lesions of Hippocampus and Subiculum. J. Neurosci. 2002, 22, 4663–4669. [Google Scholar] [CrossRef] [PubMed]
- McClelland, J.L.; McNaughton, B.L.; O’Reilly, R.C. Why There Are Complementary Learning Systems in the Hippocampus and Neocortex: Insights From the Successes and Failures of Connectionist Models of Learning and Memory. Psychol. Rev. 1995, 102, 419–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stickgold, R. Sleep-Dependent Memory Consolidation. Nature 2005, 437, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gais, S.; Born, J. Low Acetylcholine during Slow-Wave Sleep Is Critical for Declarative Memory Consolidation. Proc. Natl. Acad. Sci. USA 2004, 101, 2140–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasch, B.H.; Born, J.; Gais, S. Combined Blockade of Cholinergic Receptors Shifts the Brain from Stimulus Encoding to Memory Consolidation. J. Cogn. Neurosci. 2006, 18, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Wilson, M.A. Coordinated Memory Replay in the Visual Cortex and Hippocampus during Sleep. Nat. Neurosci. 2007, 10, 100–107. [Google Scholar] [CrossRef]
- Tambini, A.; Ketz, N.; Davachi, L. Enhanced Brain Correlations during Rest Are Related to Memory for Recent Experiences. Neuron 2010, 65, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Wamsley, E.J. Memory Consolidation during Waking Rest. Trends Cogn. Sci. 2019, 23, 171–173. [Google Scholar] [CrossRef]
- Wamsley, E.J. Offline Memory Consolidation during Waking Rest. Nat. Rev. Psychol. 2022, 1, 441–453. [Google Scholar] [CrossRef]
- Dewar, M.T.; Alber, J.; Butler, C.; Cowan, N.; Della Sala, S. Brief Wakeful Resting Boosts New Memories over the Long Term. Psychol. Sci. 2012, 23, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Alber, J.; Della Sala, S.; Dewar, M.T. Minimizing Interference With Early Consolidation Boosts 7-Day Retention in Amnesic Patients. Neuropsychology 2014, 28, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, K.; Tishler, W.; Manceor, S.; Hamilton, K.; Gaulden, A.; Parr, E.; Wamsley, E.J. Resting State EEG Correlates of Memory Consolidation. Neurobiol. Learn. Mem. 2016, 130, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Della Sala, S.; Dewar, M.T. Autobiographical Thinking Interferes with Episodic Memory Consolidation. PLoS ONE 2014, 9, e93915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewar, M.T.; Alber, J.; Cowan, N.; Della Sala, S. Boosting Long-Term Memory via Wakeful Rest: Intentional Rehearsal Is Not Necessary, Consolidation Is Sufficient. PLoS ONE 2014, 9, e109542. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T. Wakeful Rest Alleviates Interference-Based Forgetting. Memory 2015, 23, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Baker, K.C.; Culbreth, J.L.; Tracy, O.; Arora, M.; Liu, T.; Morris, S.; Collins, M.B.; Wamsley, E.J. “Sleep-Dependent” Memory Consolidation? Brief Periods of Post-Training Rest and Sleep Provide an Equivalent Benefit for Both Declarative and Procedural Memory. Learn. Mem. 2021, 28, 195–203. [Google Scholar] [CrossRef]
- Craig, M.; Dewar, M.T.; Della Sala, S.; Wolbers, T. Rest Boosts the Long-Term Retention of Spatial Associative and Temporal Order Information. Hippocampus 2015, 25, 1017–1027. [Google Scholar] [CrossRef] [Green Version]
- Craig, M.; Wolbers, T.; Harris, M.A.; Hauff, P.; Della Sala, S.; Dewar, M.T. Comparable Rest-Related Promotion of Spatial Memory Consolidation in Younger and Older Adults. Neurobiol. Aging 2016, 48, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Craig, M.; Dewar, M.T.; Harris, M.A.; Della Sala, S.; Wolbers, T. Wakeful Rest Promotes the Integration of Spatial Memories into Accurate Cognitive Maps. Hippocampus 2015, 193, 185–193. [Google Scholar] [CrossRef]
- Craig, M.; Dewar, M.T. Rest-Related Consolidation Protects the Fine Detail of New Memories. Sci. Rep. 2018, 8, 6857. [Google Scholar] [CrossRef]
- Humiston, G.B.; Wamsley, E.J. A Brief Period of Eyes-Closed Rest Enhances Motor Skill Consolidation. Neurobiol. Learn. Mem. 2018, 155, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Sachse, P. Factors Modulating the Effects of Waking Rest on Memory. Cogn. Process 2020, 21, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.; Takashima, A.; Krewinkel, S.; van Kooten, M.; Fu, L.; Medendorp, W.P.; Kessels, R.P.C.; Daselaar, S.M. Non-Interfering Effects of Active Post-Encoding Tasks on Episodic Memory Consolidation in Humans. Front. Behav. Neurosci. 2017, 11, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.; Daselaar, S.M.; Kessels, R.P.C.; Takashima, A. Promotion and Suppression of Autobiographical Thinking Differentially Affect Episodic Memory Consolidation. PLoS ONE 2018, 13, e0201780. [Google Scholar] [CrossRef] [PubMed]
- King, O.; Nicosia, J. The Effects of Wakeful Rest on Memory Consolidation in an Online Memory Study. Front. Psychol. 2022, 13, 932592. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.; Riedlsperger, B.; Maran, T.; Sachse, P. The Effect of Post-Learning Wakeful Rest on the Retention of Second Language Learning Material over the Long Term. Curr. Psychol. 2017, 39, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Humiston, G.B.; Tucker, M.A.; Summer, T.; Wamsley, E.J. Resting States and Memory Consolidation: A Preregistered Replication and Meta-Analysis. Sci. Rep. 2019, 9, 19345. [Google Scholar] [CrossRef] [Green Version]
- Richter, J.; Seffen, A.; Benedict, T.; Gast, A. No Evidence of Consolidation of Evaluative Conditioning during Waking Rest and Sleep. Cogn. Emot. 2021, 35, 844–858. [Google Scholar] [CrossRef]
- Craik, F.I.M.; Byrd, M. Aging and Cognitive Deficits. In Aging and Cognitive Processes; Craik, F.I.M., Trehub, S., Eds.; Springer US: Boston, MA, USA, 1982; pp. 191–211. ISBN 978-1-4684-4178-9. [Google Scholar]
- Craik, F.I.M.; Jennings, J.M. Human Memory. In The Handbook of Aging and Cognition; Hardcover; Craik, F.I.M., Salthouse, T.A., Eds.; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 1992; pp. 51–110. ISBN 0-8058-0713-6. [Google Scholar]
- Balota, D.A.; Dolan, P.O.; Duchek, J.M. Memory Changes in Healthy Young and Older Adults. In The Oxford Handbook of Memory; Tulving, E., Craik, F.I.M., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 395–409. ISBN 0-19-512265-8. [Google Scholar]
- Martini, M.; Martini, C.; Bernegger, C.; Sachse, P. Post-Encoding Wakeful Resting Supports the Retention of New Verbal Memories in Children Aged 13–14 Years. Br. J. Dev. Psychol. 2018, 37, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.; Zamarian, L.; Sachse, P.; Martini, C.; Delazer, M. Wakeful Resting and Memory Retention: A Study with Healthy Older and Younger Adults. Cogn. Process 2018, 20, 125–131. [Google Scholar] [CrossRef]
- Sacripante, R.; McIntosh, R.D.; Della Sala, S. Benefit of Wakeful Resting on Gist and Peripheral Memory Retrieval in Healthy Younger and Older Adults. Neurosci. Lett. 2019, 705, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, S.; Takashima, A.; Fu, L.; Kessels, R.P.C. Mindwandering Propensity Modulates Episodic Memory Consolidation. Aging Clin. Exp. Res. 2019, 31, 1601–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicosia, J.; Balota, D.A. Targeted Memory Reactivation and Consolidation-like Processes during Mind-Wandering in Younger and Older Adults. J. Exp. Psychol. Learn. Mem. Cogn. 2022, in press. [CrossRef]
- Giambra, L.M. Task-Unrelated-Thought Frequency as a Function of Age: A Laboratory Study. Psychol. Aging 1989, 4, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.D.; Balota, D.A. Mind-Wandering in Younger and Older Adults: Converging Evidence from the Sustained Attention to Response Task and Reading for Comprehension. Psychol. Aging 2012, 27, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Jordaõ, M.; Ferreira-Santos, F.; Pinho, M.S.; St Jacques, P.L. Meta-Analysis of Aging Effects in Mind Wandering: Methodological and Sociodemographic Factors. Psychol. Aging 2019, 34, 531–544. [Google Scholar] [CrossRef]
- Martini, M.; Martini, C.; Maran, T.; Sachse, P. Effects of Post-Encoding Wakeful Rest and Study Time on Long-Term Memory Performance. J. Cogn. Psychol. 2018, 30, 558–569. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.; Marhenke, R.; Martini, C.; Rossi, S.; Sachse, P. Individual Differences in Working Memory Capacity Moderate Effects of Post - Learning Activity on Memory Consolidation over the Long Term. Sci. Rep. 2020, 10, 17976. [Google Scholar] [CrossRef]
- McCabe, D.P.; Roediger, H.L.; McDaniel, M.A.; Balota, D.A.; Hambrick, D.Z. The Relationship between Working Memory Capacity and Executive Functioning: Evidence for a Common Executive Attention Construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef] [Green Version]
- Roediger, H.L.; Karpicke, J.D. Test-Enhanced Learning: Taking Memory Tests Imporves Long-Term Retention. Psychol. Sci. 2006, 17, 249–255. [Google Scholar] [CrossRef]
- Balota, D.A.; Neely, J.H. Test-Expectancy and Word-Frequency Effects in Recall and Recognition. J. Exp. Psychol. Hum. Learn. Mem. 1980, 6, 576–587. [Google Scholar] [CrossRef]
- Hartley, T.; Houghton, G. A Linguistically Constrained Model of Short-Term Memory for Nonwords. J. Mem. Lang. 1996, 35, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Multhaup, K.S.; Balota, D.A.; Cowan, N. Implications of Aging, Lexicality, and Item Length for Mechanisms Underlying Memory Span. Psychon. Bull. Rev. 1996, 3, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R.; Brown, T.; Fuld, P.; Peck, A.; Schechter, R.; Schimmel, H. Validation of a Short Orientation-Memory-Concentration Test of Cognitive Impairment. Am. J. Psychiatry 1983, 140, 734–739. [Google Scholar] [PubMed]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and Neuropsychological Assessment of Alzheimer’s Disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Balota, D.A.; Yap, M.J.; Cortese, M.J.; Hutchison, K.A.; Kessler, B.; Loftis, B.; Neely, J.H.; Nelson, D.L.; Simpson, G.B.; Treiman, R. The English Lexicon Project. Behav. Res. Methods 2007, 39, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Salthouse, T.A. Speed of Behavior and Its Implications for Cognition. In Handbook of the Psychology of Aging; Birren, J.E., Schaie, K.W., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1985; pp. 400–426. [Google Scholar]
- Cerella, J. Aging and Information-Processing Rate. In Handbook of the Psychology of Aging; Birren, J.S., Schaie, K.W., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 201–221. [Google Scholar]
- Faust, M.E.; Balota, D.A.; Spieler, D.H.; Ferraro, F.R. Individual Differences in Information-Processing Rate and Amount: Implications for Group Differences in Response Latency. Psychol. Bull. 1999, 125, 777–799. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Penney, C.G. Modality Effects and the Structure of Short-Term Verbal Memory. Mem. Cognit. 1989, 17, 398–422. [Google Scholar] [CrossRef]
- Ginns, P. Meta-Analysis of the Modality Effect. Learn. Instr. 2005, 15, 313–331. [Google Scholar] [CrossRef]
- Lindner, K.; Blosser, G.; Cunigan, K. Visual versus Auditory Learning and Memory Recall Performance on Short-Term versus Long-Term Tests. Mod. Psychol. Stud. 2009, 15, 6. [Google Scholar]
- Vanderplas, J.M.; Garvin, E.A. The Association Value of Random Shapes. J. Exp. Psychol. 1951, 57, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.J.; Bogg, T.; Walton, K.E.; Wood, D.; Harms, P.D.; Lodi-Smith, J.; Edmonds, G.W.; Roberts, B.W. Not All Conscientiousness Scales Change Alike: A Multimethod, Multisample Study of Age Differences in the Facets of Conscientiousness. J. Pers. Soc. Psychol. 2009, 96, 446–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicosia, J.; Balota, D.A. Dispositional Factors Account for Age Differences in Self-Reported Mind-Wandering. Psychol. Aging 2021, 36, 421–432. [Google Scholar] [CrossRef]
- Perfect, T.J.; Dasgupta, Z.R.R. What Underlies the Deficit in Reported Recollective Experience in Old Age? Mem. Cogn. 1997, 25, 849–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertzog, C.; McGuire, C.L.; Lineweaver, T.T. Aging, Attributions, Perceived Control, and Strategy Use in a Free Recall Task. Aging, Neuropsychol. Cogn. 1998, 5, 85–106. [Google Scholar] [CrossRef]
- Naveh-Benjamin, M.; Brav, T.K.; Levy, O. The Associative Memory Deficit of Older Adults: The Role of Strategy Utilization. Psychol. Aging 2007, 22, 202–208. [Google Scholar] [CrossRef]
- Kirchhoff, B.A.; Anderson, B.A.; Barch, D.M.; Jacoby, L.L. Cognitive and Neural Effects of Semantic Encoding Strategy Training in Older Adults. Cereb. Cortex 2012, 22, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Hasher, L.; Zacks, R.T. Working Memory, Comprehension, and Aging: A Review and a New View. In The Psychology of Learning and Motivation; Bower, G., Ed.; Academic Press: New York, NY, USA, 1988; Volume 22, pp. 193–225. [Google Scholar]
- Berntsen, D. Involuntary Autobiographical Memories. Appl. Cogn. Psychol. 1996, 10, 435–454. [Google Scholar] [CrossRef]
- Neely, J.H.; Balota, D.A. Test-Expectancy and Semantic-Organization Effects in Recall and Recognition. Mem. Cognit. 1981, 9, 283–300. [Google Scholar] [CrossRef]
- Craik, F.I.M. A Functional Account of Age Differences in Memory. In Human Memory and Cognitive Capabilities: Mechanisms and Performances; Klix, F., Hagendorf, H., Eds.; Elsevier Science Publishers B.V.: North-Holland, The Netherlands, 1986; pp. 409–422. ISBN 0444700714. [Google Scholar]
- Salthouse, T.A. Methodological Assumptions in Cognitive Aging Research. In Handbook of Aging and Cognition; Lawrence Erlbaum Associates, Inc.: Hillsdale, NJ, USA, 2000; pp. 467–498. [Google Scholar]



| Experiment 1 | Experiment 2 | Experiment 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Younger, n = 24 | Older, n = 16 | p Value | η2 | Younger, n = 24 | Older, n = 16 | p Value | η2 | Younger, n = 32 | Older, n = 32 | p Value | η2 | |
| Age | <0.001 | 0.93 | <0.001 | 0.95 | <0.001 | 0.94 | ||||||
| Mean (SD) | 20.9 (1.4) | 64.2 (9.7) | 20.7 (1.7) | 66.0 (8.3) | 20.0 (1.2) | 63.7 (7.9) | ||||||
| Sex | >0.9 | 0.7 | >0.9 | |||||||||
| Female | 17 (71%) | 12 (75%) | 19 (79%) | 11 (69%) | 20 (62%) | 20 (67%) | ||||||
| Male | 7 (29%) | 4 (25%) | 5 (21%) | 5 (31%) | 12 (38%) | 10 (33%) | ||||||
| Education | 0.3 | 0.03 | <0.001 | 0.36 | <0.001 | 0.29 | ||||||
| Mean (SD) | 14.6 (1.4) | 15.3 (2.6) | 13.9 (1.3) | 16.6 (2.4) | 13.4 (1.2) | 15.9 (2.7) | ||||||
| Race | 0.007 | 0.4 | 0.029 | |||||||||
| White | 13 (54%) | 12 (75%) | 15 (62%) | 12 (75%) | 20 (62%) | 24 (80%) | ||||||
| Black or African American | 0 (0%) | 3 (19%) | 3 (12%) | 2 (12%) | 3 (9.4%) | 5 (17%) | ||||||
| Asian | 10 (42%) | 0 (0%) | 4 (17%) | 1 (6.2%) | 8 (25%) | 0 (0%) | ||||||
| More than one race | 1 (4.2%) | 0 (0%) | 0 (0%) | 1 (6.2%) | 0 (0%) | 1 (3.3%) | ||||||
| Prefer not to respond | 0 (0%) | 1 (6.2%) | 2 (8.3%) | 0 (0%) | 1 (3.1%) | 0 (0%) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millar, P.R.; Balota, D.A. Wakeful Rest Benefits Recall, but Not Recognition, of Incidentally Encoded Memory Stimuli in Younger and Older Adults. Brain Sci. 2022, 12, 1609. https://doi.org/10.3390/brainsci12121609
Millar PR, Balota DA. Wakeful Rest Benefits Recall, but Not Recognition, of Incidentally Encoded Memory Stimuli in Younger and Older Adults. Brain Sciences. 2022; 12(12):1609. https://doi.org/10.3390/brainsci12121609
Chicago/Turabian StyleMillar, Peter R., and David A. Balota. 2022. "Wakeful Rest Benefits Recall, but Not Recognition, of Incidentally Encoded Memory Stimuli in Younger and Older Adults" Brain Sciences 12, no. 12: 1609. https://doi.org/10.3390/brainsci12121609
APA StyleMillar, P. R., & Balota, D. A. (2022). Wakeful Rest Benefits Recall, but Not Recognition, of Incidentally Encoded Memory Stimuli in Younger and Older Adults. Brain Sciences, 12(12), 1609. https://doi.org/10.3390/brainsci12121609





