Negative Impacts of Sleep–Wake Rhythm Disturbances on Attention in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
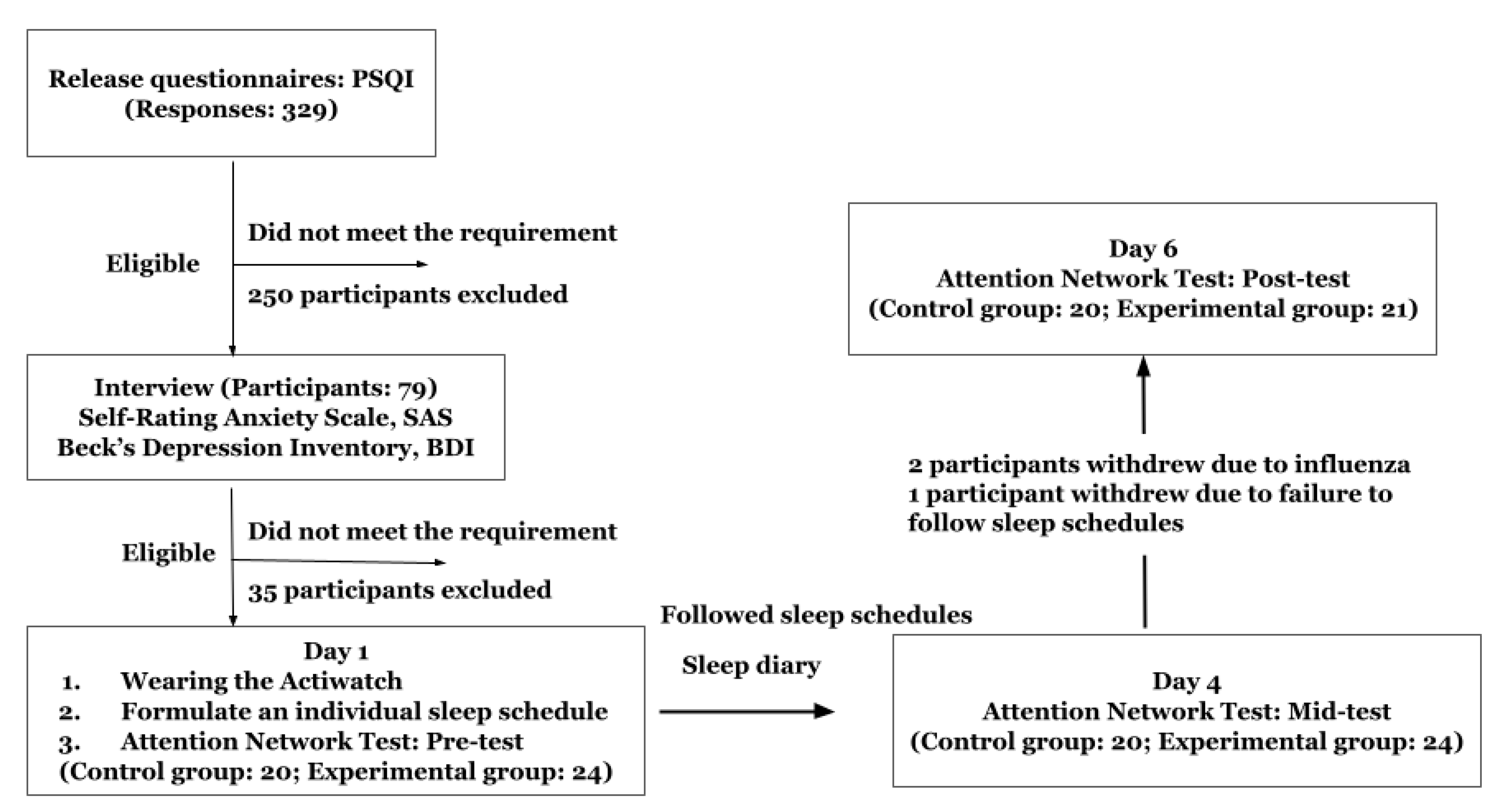
2.1. Participants
2.2. Study Design
2.3. Apparatus, Materials, and Procedure
2.3.1. Measurement of Sleep Parameters
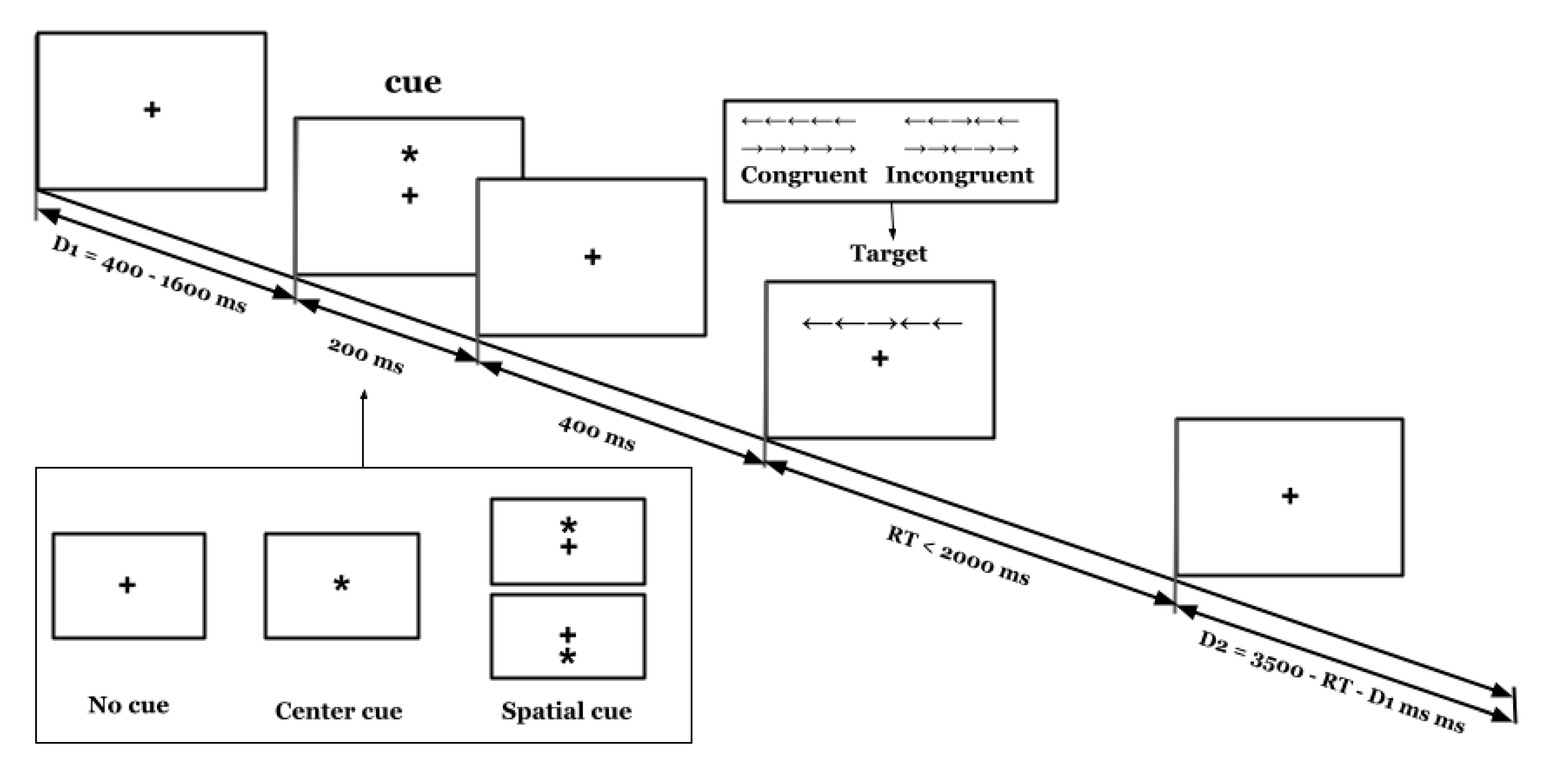
2.3.2. Measurement of Attention
2.3.3. Procedures
2.3.4. Statistical Analysis
3. Results
3.1. Characteristics of Participants
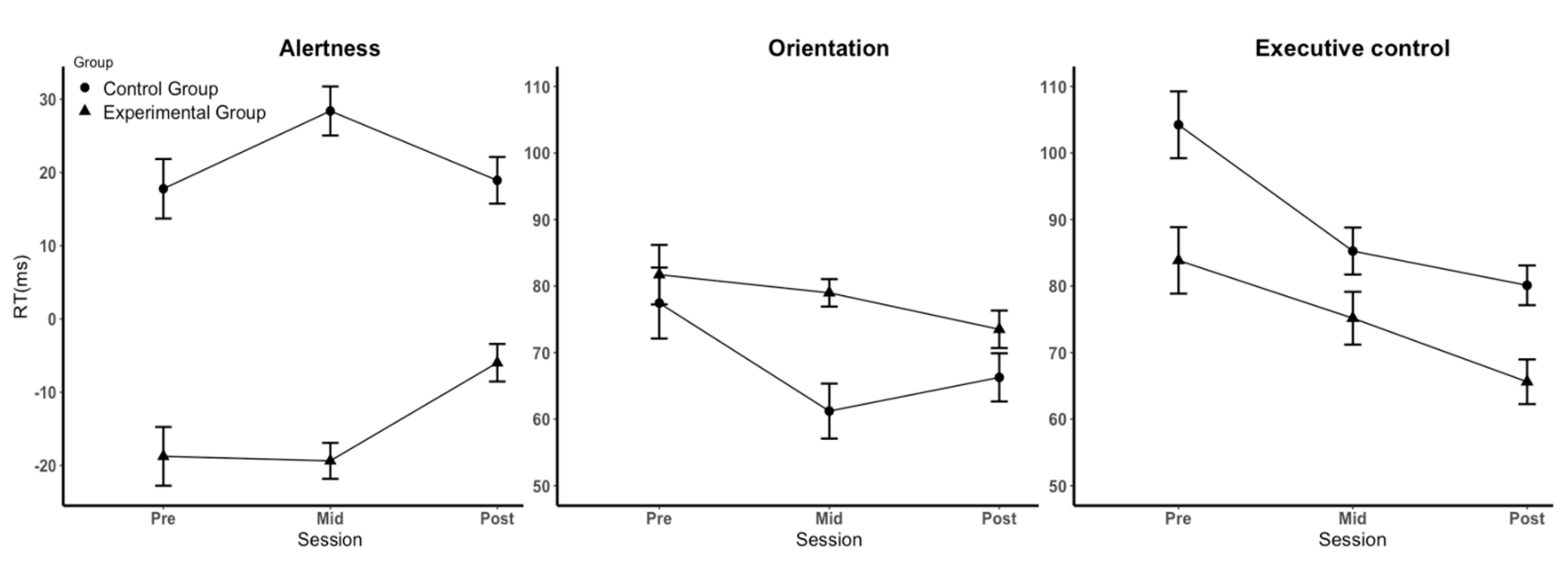
3.2. Effects of Sleep–Wake Rhythm Disturbances on Attention
4. Discussion
4.1. Effects of Sleep–Wake Rhythms on the Three Subfunctions of Attention
4.2. Control of Irrelevant Variables
4.3. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSQI | Pittsburgh Sleep Quality Index |
| BDI | Beck’s Depression Inventory |
| SAS | Self-rating Anxiety Scale |
| ANT | Attention Network Test |
| CBT-i | Cognitive Behavioral Therapy for insomnia |
| RT | Response Time |
| CRT | Cathode Ray Tube |
| SD | Standard Deviation |
| M | Mean |
| ANT-I | Attention Network Test-Interaction |
| ADHD | Attention-Deficit/Hyperactivity Disorder |
References
- Walker, M.P. Why We Sleep: Unlocking the Power of Sleep and Dreams, First Scribner Hardcover Edition; Scribner-Simon & Schuster, Inc.: New York, NY, USA, 2017; pp. 107–164. [Google Scholar]
- Chinese Sleep Research Society, CSRS. 2017 White Paper on Sleep Status of Chinese Youth—World Sleep Day. In Proceedings of the Chinese Sleep Research Society, Shenzhen, China, 20 March 2017. [Google Scholar]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Stothard, E.R.; McHill, A.W.; Depner, C.M.; Birks, B.R.; Moehlman, T.M.; Ritchie, H.K.; Guzzetti, J.R.; Chinoy, E.D.; LeBourgeois, M.K.; Axelsson, J.; et al. Circadian Entrainment to the Natural Light-Dark Cycle across Seasons and the Weekend. Curr. Biol. 2017, 27, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Abbott, S.M.; Reid, K.J.; Zee, P.C. Circadian Rhythm Sleep-Wake Disorders. Psychiatr. Clin. N. Am. 2015, 38, 805–823. [Google Scholar] [CrossRef]
- Morgenthaler, T.I.; Lee-Chiong, T.; Alessi, C.; Friedman, L.; Aurora, R.N.; Boehlecke, B.; Brown, T.; Chesson, A.L., Jr.; Kapur, V.; Maganti, R.; et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 2007, 30, 1445–1459. [Google Scholar] [CrossRef] [Green Version]
- Louca, M.; Short, M.A. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep 2014, 37, 1799–1807. [Google Scholar] [CrossRef] [Green Version]
- Patrick, Y.; Lee, A.; Raha, O.; Pillai, K.; Gupta, S.; Sethi, S.; Mukeshimana, F.; Gerard, L.; Moghal, M.U.; Saleh, S.N.; et al. Effects of sleep deprivation on cognitive and physical performance in university students. Sleep Biol. Rhythm. 2017, 15, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Owens, J.A.; Weiss, M.R. Insufficient sleep in adolescents: Causes and consequences. Minerva Pediatr. 2017, 69, 326–336. [Google Scholar] [CrossRef]
- Phillips, A.J.K.; Clerx, W.M.; O’Brien, C.S.; Sano, A.; Barger, L.K.; Picard, R.W.; Lockley, S.W.; Klerman, E.B.; Czeisler, C.A. Irregular sleep/wake patterns are associated with poorer academic performance and delayed circadian and sleep/wake timing. Sci. Rep. 2017, 7, 3216. [Google Scholar] [CrossRef]
- Alfonsi, V.; Scarpelli, S.; D’Atri, A.; Stella, G.; De Gennaro, L. Later School Start Time: The Impact of Sleep on Academic Performance and Health in the Adolescent Population. Int. J. Environ. Res. Public Health 2020, 17, 2574. [Google Scholar] [CrossRef] [Green Version]
- Curcio, G.; Ferrara, M.; De Gennaro, L. Sleep loss, learning capacity and academic performance. Sleep Med. Rev. 2006, 10, 323–337. [Google Scholar] [CrossRef]
- Galambos, N.L.; Dalton, A.L.; Maggs, J.L. Losing Sleep Over It: Daily Variation in Sleep Quantity and Quality in Canadian Students’ First Semester of University. J. Res. Adolesc. 2009, 19, 741–761. [Google Scholar] [CrossRef]
- Sun, W.; Ling, J.; Zhu, X.; Lee, T.M.; Li, S.X. Associations of weekday-to-weekend sleep differences with academic performance and health-related outcomes in school-age children and youths. Sleep Med. Rev. 2019, 46, 27–53. [Google Scholar] [CrossRef]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Dewald-Kaufmann, J.F.; Oort, F.J.; Bögels, S.M.; Meijer, A.M. Why Sleep Matters: Differences in Daytime Functioning between Adolescents with Low and High Chronic Sleep Reduction and Short and Long Sleep Durations. J. Cogn. Behav. Psychother. 2013, 13, 171–182. [Google Scholar]
- Chuah, L.Y.; Chee, M.W. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. J. Neurosci. 2008, 28, 11369–11377. [Google Scholar] [CrossRef] [Green Version]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar]
- Peters, J.D.; Biggs, S.N.; Bauer, K.M.; Lushington, K.; Kennedy, D.; Martin, J.; Dorrian, J. The sensitivity of a PDA-based psychomotor vigilance task to sleep restriction in 10-year-old girls. J. Sleep Res. 2009, 18, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Jugovac, D.; Cavallero, C. Twenty-four hours of total sleep deprivation selectively impairs attentional networks. Exp. Psychol. 2012, 59, 115–123. [Google Scholar] [CrossRef]
- Gradisar, M.; Crowley, S.J. Delayed sleep phase disorder in youth. Curr. Opin. Psychiatry 2013, 26, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Valdez, P. Focus: Attention science: Circadian rhythms in attention. Yale J. Biol. Med. 2019, 92, 81. [Google Scholar]
- Bratzke, D.; Steinborn, M.B.; Rolke, B.; Ulrich, R. Effects of sleep loss and circadian rhythm on executive inhibitory control in the Stroop and Simon tasks. Chronobiol. Int. 2012, 29, 55–61. [Google Scholar] [CrossRef]
- Rossa, K.R.; Smith, S.S.; Allan, A.C.; Sullivan, K.A. The effects of sleep restriction on executive inhibitory control and affect in young adults. J. Adolesc. Health 2014, 55, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Rabat, A.; Gomez-Merino, D.; Roca-Paixao, L.; Bougard, C.; Van Beers, P.; Dispersyn, G.; Guillard, M.; Bourrilhon, C.; Drogou, C.; Arnal, P.J.; et al. Differential Kinetics in Alteration and Recovery of Cognitive Processes from a Chronic Sleep Restriction in Young Healthy Men. Front. Behav. Neurosci. 2016, 10, 95. [Google Scholar] [CrossRef]
- Liu, X.-C.; Tang, M.-Q.; Hu, L.; Wang, A.; Hu, H.; Zhao, G. Reliability & validity study of PSQI. Chin. J. Psychiatry 1996, 29, 103–107. [Google Scholar]
- Sack, R.L.; Auckley, D.; Auger, R.R.; Carskadon, M.A.; Wright, K.P., Jr.; Vitiello, M.V.; Zhdanova, I.V. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 2007, 30, 1484–1501. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M.I. Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef]
- Sadeh, A. The role and validity of actigraphy in sleep medicine: An update. Sleep Med. Rev. 2011, 15, 259–267. [Google Scholar] [CrossRef]
- Costa, A.; Hernandez, M.; Sebastian-Galles, N. Bilingualism aids conflict resolution: Evidence from the ANT task. Cognition 2008, 106, 59–86. [Google Scholar] [CrossRef]
- Roehrs, T.; Timms, V.; Zwyghuizen-Doorenbos, A.; Roth, T. Sleep extension in sleepy and alert normals. Sleep 1989, 12, 449–457. [Google Scholar] [CrossRef]
- Auger, R.R.; Burgess, H.J.; Emens, J.S.; Deriy, L.V.; Thomas, S.M.; Sharkey, K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015, 11, 1199–1236. [Google Scholar] [CrossRef]
- Pigeon, W.R. Treatment of adult insomnia with cognitive-behavioral therapy. J. Clin. Psychol. 2010, 66, 1148–1160. [Google Scholar] [CrossRef]
- Reed, D.L.; Sacco, W.P. Measuring Sleep Efficiency: What Should the Denominator Be? J. Clin. Sleep Med. 2016, 12, 263–266. [Google Scholar] [CrossRef]
- Wright, K.P., Jr.; Hull, J.T.; Czeisler, C.A. Relationship between alertness, performance, and body temperature in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R1370–R1377. [Google Scholar] [CrossRef]
- Belenky, G.; Wesensten, N.J.; Thorne, D.R.; Thomas, M.L.; Sing, H.C.; Redmond, D.P.; Russo, M.B.; Balkin, T.J. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J. Sleep Res. 2003, 12, 1–12. [Google Scholar] [CrossRef]
- Waldon, J.; Vriend, J.; Davidson, F.; Corkum, P. Sleep and attention in children with ADHD and typically developing peers. J. Atten. Disord. 2018, 22, 933–941. [Google Scholar]
- Kuula, L.; Pesonen, A.K.; Heinonen, K.; Kajantie, E.; Eriksson, J.G.; Andersson, S.; Lano, A.; Lahti, J.; Wolke, D.; Raikkonen, K. Naturally occurring circadian rhythm and sleep duration are related to executive functions in early adulthood. J. Sleep Res. 2018, 27, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Banks, S.; Dinges, D.F. Behavioral and physiological consequences of sleep restriction. J. Clin. Sleep Med. 2007, 3, 519–528. [Google Scholar]
- Ishigami, Y.; Klein, R.M. Repeated measurement of the components of attention using two versions of the Attention Network Test (ANT): Stability, isolability, robustness, and reliability. J. Neurosci. Methods 2010, 190, 117–128. [Google Scholar] [CrossRef]
- Tonetti, L.; Conca, A.; Giupponi, G.; Filardi, M.; Natale, V. Circadian activity rhythm in adult attention-deficit hyperactivity disorder. J. Psychiatr. Res. 2018, 103, 1–4. [Google Scholar] [CrossRef]
| Experimental Group (n = 21) | Control Group (n = 20) | 95% Confidence Interval | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower | Upper | |
| Age | 20.6 | 1.7 | 20.2 | 1.1 | −0.5 | 1.3 |
| Sleep duration (h) | 7.5 | 1.0 | 7.6 | 0.5 | −0.3 | 0.2 |
| Sleep efficiency | 91% | 0.1 | 92% | 0.1 | −0.03 | 0.02 |
| BDI | 6.8 | 5.9 | 4.5 | 5.4 | −1.3 | 6.0 |
| SAS | 42.0 | 4.8 | 35.9 | 4.2 | 2.7 | 9.6 |
| Condition | Group d | Pretest (M ± SD) | Midtest (M ± SD) | Posttest (M ± SD) |
|---|---|---|---|---|
| No cue | 1 | 638 ± 150 | 558 ± 36 | 553 ± 53 |
| 2 | 654 ± 60 | 600 ± 50 | 571 ± 42 | |
| Central cue | 1 | 657 ± 133 | 577 ± 38 | 558 ± 48 |
| 2 | 636 ± 63 | 571 ± 42 | 552 ± 45 | |
| Valid cue | 1 | 575 ± 143 | 498 ± 37 | 485 ± 54 |
| 2 | 559 ± 57 | 510 ± 43 | 486 ± 42 | |
| Incongruent stimulus | 1 | 665 ± 140 | 581 ± 42 | 564 ± 56 |
| 2 | 666 ± 64 | 600 ± 47 | 574 ± 44 | |
| Congruent stimulus | 1 | 581 ± 143 | 505 ± 34 | 499 ± 48 |
| 2 | 561 ± 53 | 514 ± 38 | 494 ± 39 | |
| Alertness a | 1 | −19 ± 26 | −19 ± 16 | −6 ± 16 |
| 2 | 18 ± 26 | 28 ± 21 | 19 ± 20 | |
| Orientation b | 1 | 82 ± 29 | 79 ± 13 | 74 ± 18 |
| 2 | 77 ± 34 | 61 ± 26 | 66 ± 23 | |
| Executive control c | 1 | 84 ± 32 | 75 ± 25 | 66 ± 21 |
| 2 | 104 ± 32 | 85 ± 23 | 80 ± 19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Fu, S.; Jiang, F.; Nan, W. Negative Impacts of Sleep–Wake Rhythm Disturbances on Attention in Young Adults. Brain Sci. 2022, 12, 1643. https://doi.org/10.3390/brainsci12121643
Li Z, Fu S, Jiang F, Nan W. Negative Impacts of Sleep–Wake Rhythm Disturbances on Attention in Young Adults. Brain Sciences. 2022; 12(12):1643. https://doi.org/10.3390/brainsci12121643
Chicago/Turabian StyleLi, Zijun, Shimin Fu, Fan Jiang, and Weizhi Nan. 2022. "Negative Impacts of Sleep–Wake Rhythm Disturbances on Attention in Young Adults" Brain Sciences 12, no. 12: 1643. https://doi.org/10.3390/brainsci12121643





