Clock Genes Profiles as Diagnostic Tool in (Childhood) ADHD—A Pilot Study
Abstract
:1. Introduction
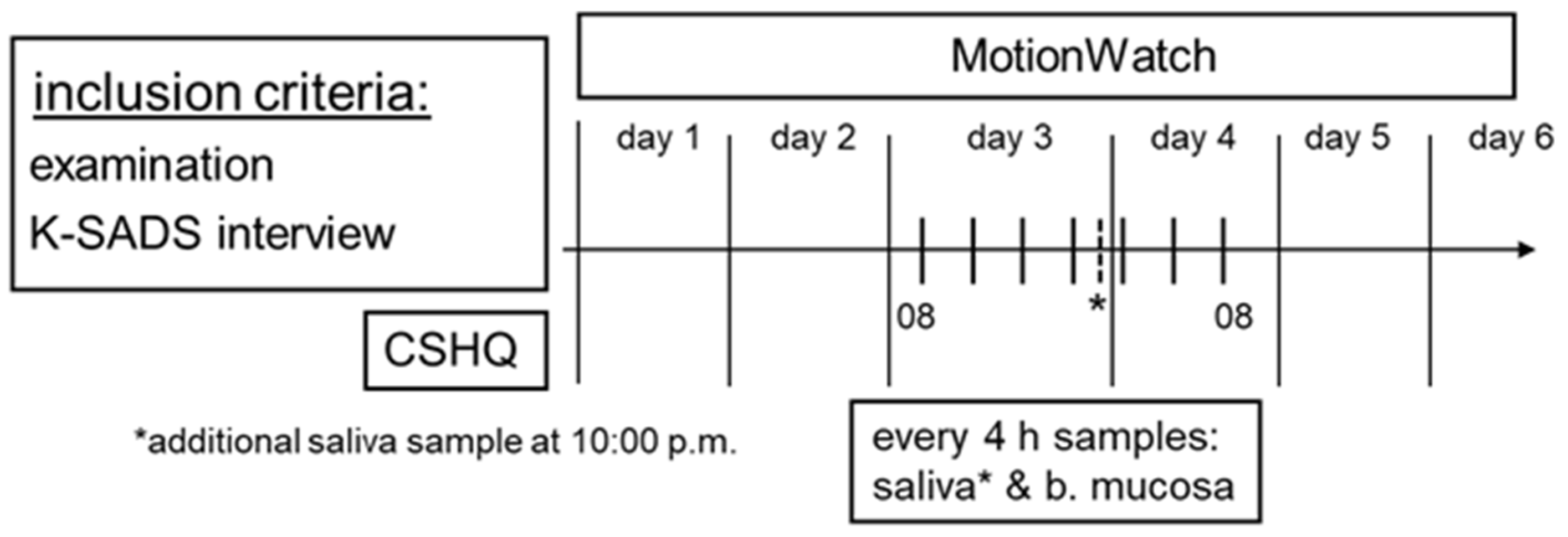
2. Materials and Methods
2.1. Subjects and Ethics
2.2. Questionnaire
2.3. Actigraphy
2.4. Melatonin and Cortisol Analysis
2.5. Clock Gene Expression Analysis
2.6. Statistical Analysis
3. Results
3.1. Questionnaire
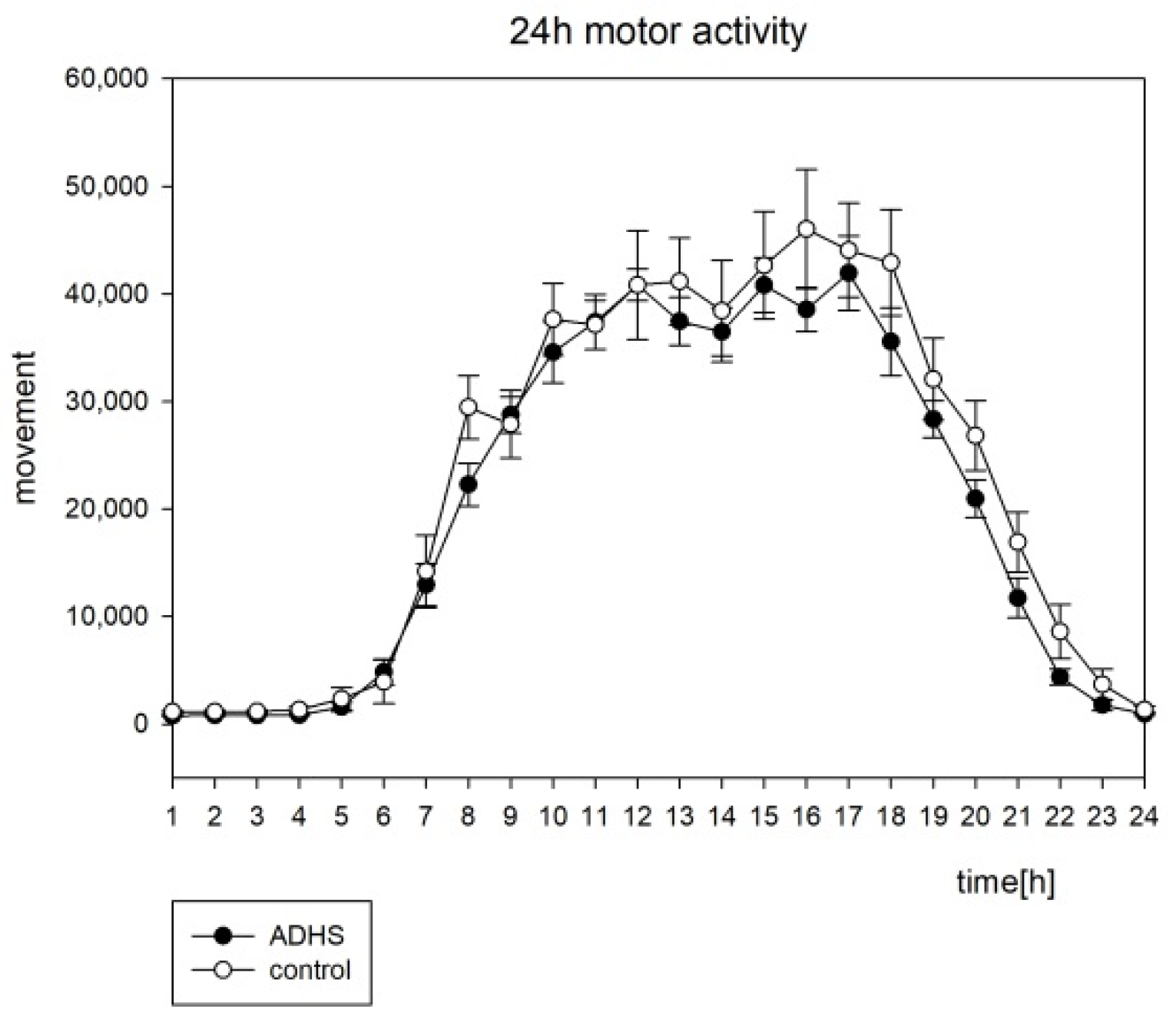
3.2. Actigraphy
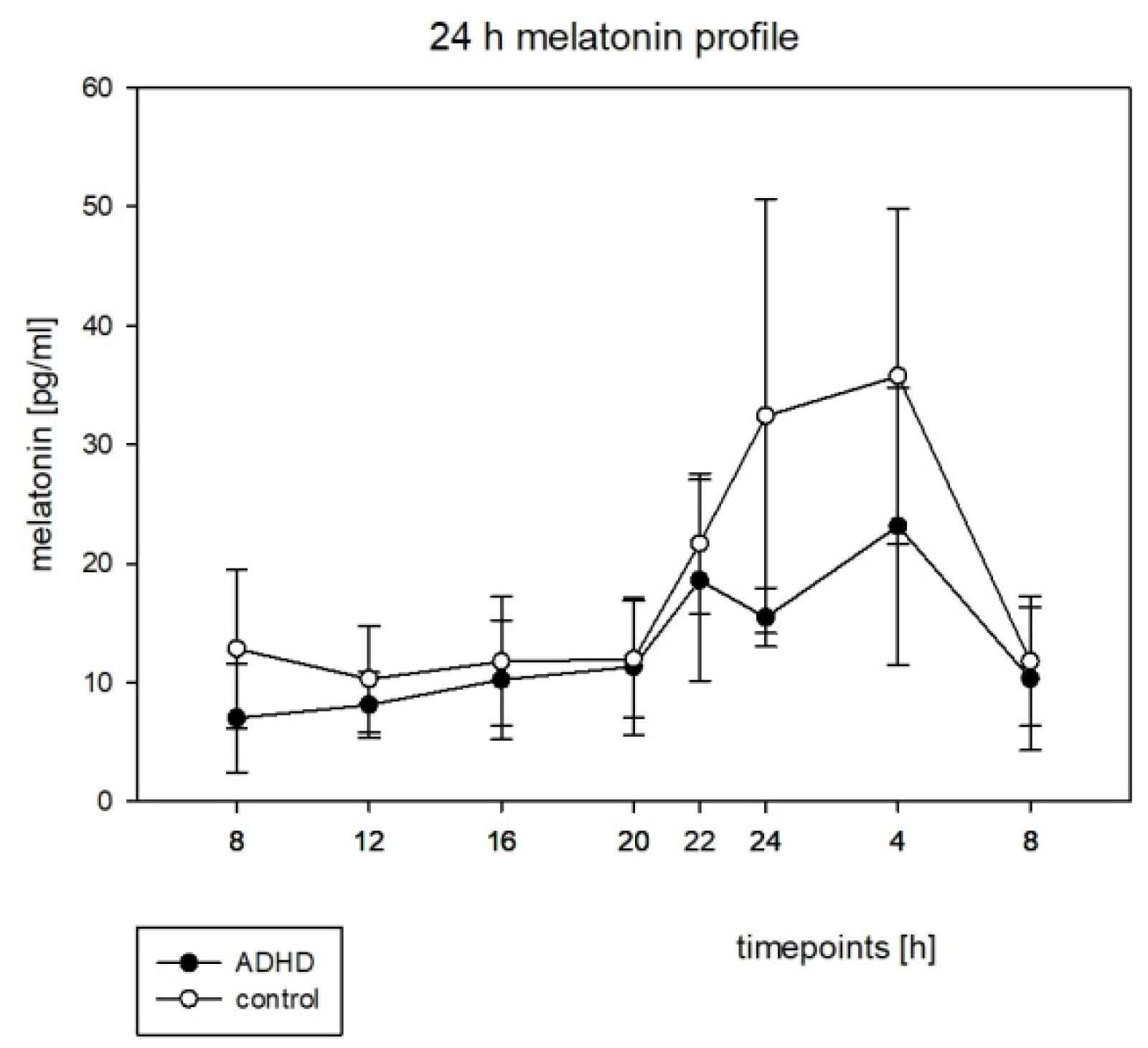
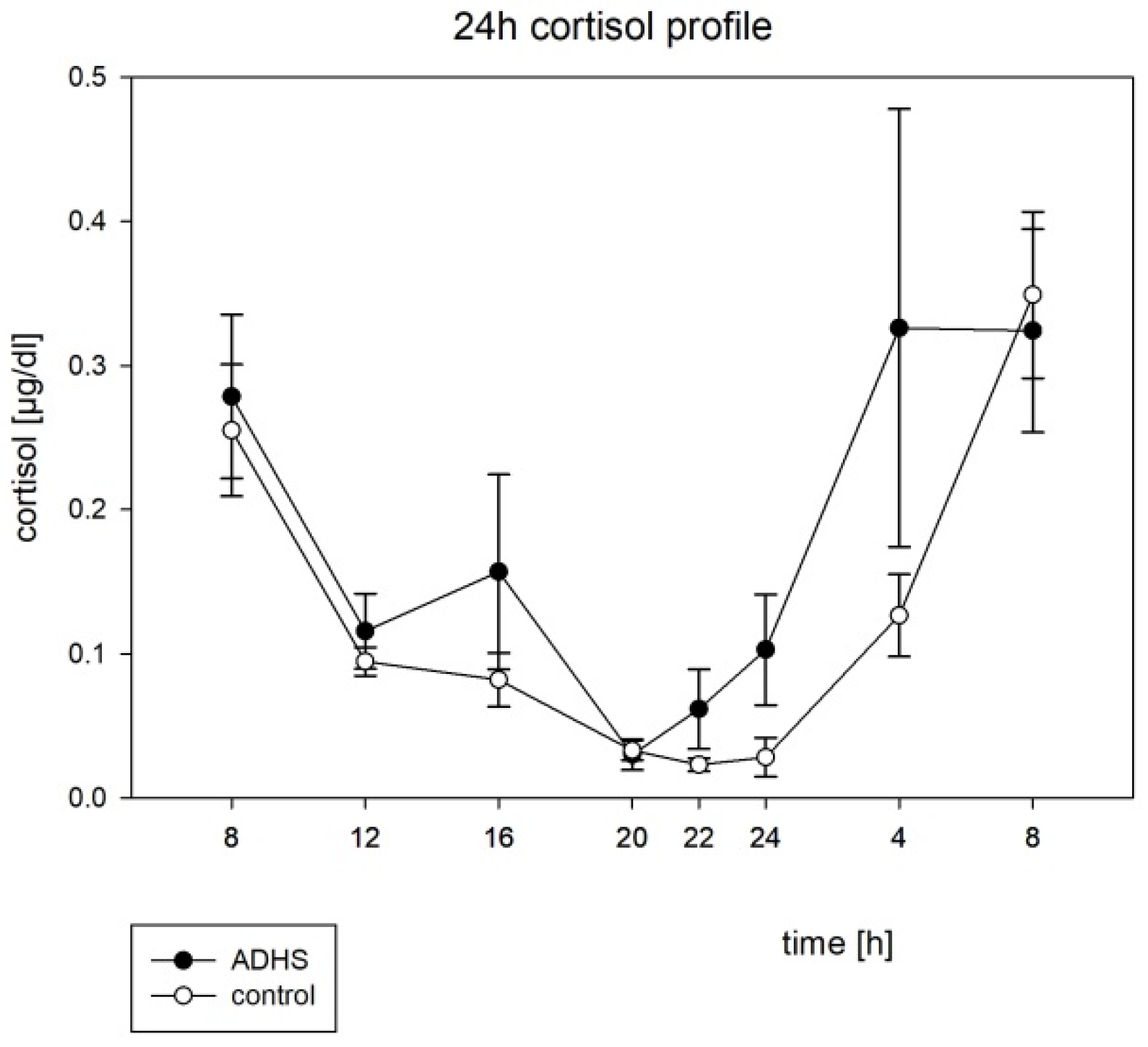
3.3. Hormone Rhythms
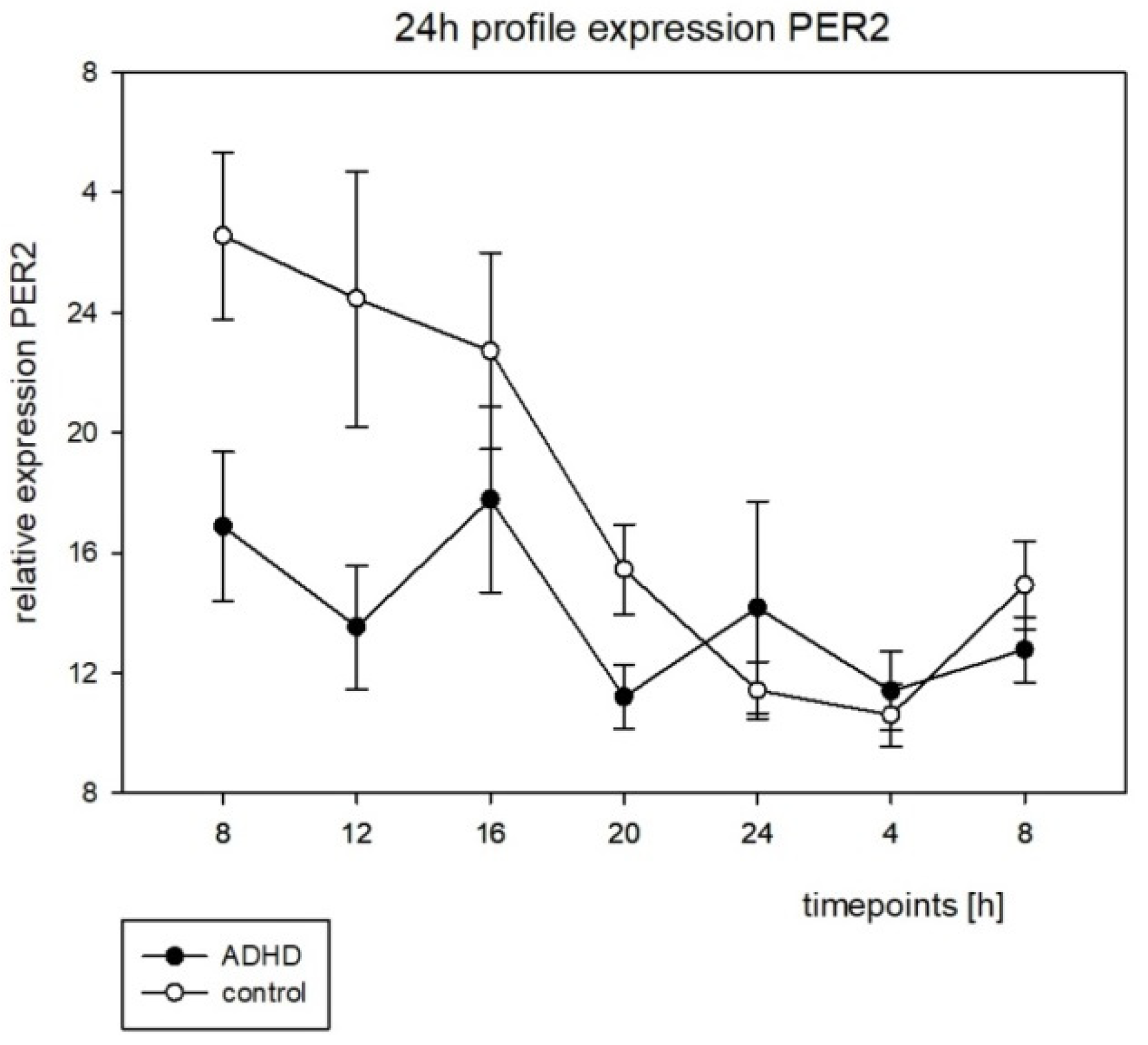
3.4. Clock Gene Expression Rhythms
4. Discussion
4.1. Questionnaire
4.2. Actigraphy
4.3. Melatonin and Cortisol
4.4. Clock Gene Expression Rhythms
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polanczyk, G.; De Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The Worldwide Prevalence of ADHD: A Systematic Review and Metaregression Analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, J.; De Graaf, R.; Kessler, R.; Alonso, J.; Angermeyer, M.; Demyttenaere, K.; de Girolamo, G.; Haro, J.M.; Karam, E.G.; Lara, C.; et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br. J. Psychiatry 2007, 190, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Ruzicka, D.L.; Giordani, B.J.; Weatherly, R.A.; Dillon, J.E.; Hodges, E.K.; Marcus, C.L.; Guire, K.E. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics 2006, 117, e769–e778. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Kooij, J.J.S.; Oosterlaan, J.; Sergeant, J.A.; Buitelaar, J.K.; Van Someren, E.J.W. Hyperactive night and day? Actigraphy studies in adult ADHD: A baseline comparison and the effect of methylphenidate. Sleep 2007, 30, 433–442. [Google Scholar] [CrossRef]
- Silvestri, R.; Gagliano, A.; Aricò, I.; Calarese, T.; Cedro, C.; Bruni, O.; Condurso, R.; Germanò, E.; Gervasi, G.; Siracusano, R.; et al. Sleep disorders in children with Attention-Deficit/Hyperactivity Disorder (ADHD) recorded overnight by video-polysomnography. Sleep Med. 2009, 10, 1132–1138. [Google Scholar] [CrossRef]
- Van Der Heijden, K.B.; Smits, M.G.; Gunning, W.B. Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J. Sleep Res. 2006, 15, 55–62. [Google Scholar] [CrossRef]
- Díaz-Román, A.; Hita-Yáñez, E.; Buela-Casal, G. Sleep Characteristics in Children with Attention Deficit Hyperactivity Disorder: Systematic Review and Meta-Analyses. J. Clin. Sleep Med. 2016, 12, 747–756. [Google Scholar] [CrossRef]
- Coogan, A.N.; McGowan, N.M. A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. ADHD Atten. Deficit Hyperact. Disord. 2017, 9, 129–147. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Armando, M.; Mazzone, L.; Ciliberto, M.; Sciannamea, M.; Figueroa, C.; Janiri, L.; Quested, D.; Vicari, S. The use of actigraphy in the monitoring of methylphenidate versus placebo in ADHD: A meta-analysis. ADHD Atten. Deficit Hyperact. Disorders 2014, 6, 49–58. [Google Scholar] [CrossRef]
- Owens, J.A.; Maxim, R.; Nobile, C.; McGuinn, M.; Msall, M. Parental and self-report of sleep in children with attention-deficit/hyperactivity disorder. Arch. Pediatr. Adolesc. Med. 2000, 154, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Rybak, Y.E.; McNeely, H.E.; Mackenzie, B.E.; Jain, U.R.; Levitan, R.D. Seasonality and circadian preference in adult attention-deficit/hyperactivity disorder: Clinical and neuropsychological correlates. Compr. Psychiatry 2007, 48, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, K.B.; Smits, M.G.; Van Someren, E.J.; Gunning, W.B. Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: A circadian rhythm sleep disorder. Chronobiol. Int. 2005, 22, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, M.M.; Kooij, J.S.; Boonstra, A.M.; Gordijn, M.C.; Van Someren, E.J. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol. Psychiatry 2010, 67, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Helfrich-Förster, C.; Oster, H. The role of the circadian clock system in physiology. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 227–239. [Google Scholar] [CrossRef]
- Neumann, A.-M.; Schmidt, C.X.; Brockmann, R.M.; Oster, H. Circadian regulation of endocrine systems. Auton. Neurosci. 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Von Schantz, M. Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J. Biol. Rhythm. 2005, 20, 279–290. [Google Scholar] [CrossRef]
- Imeraj, L.; Antrop, I.; Roeyers, H.; Swanson, J.; Deschepper, E.; Bal, S.; Deboutte, D. Time-of-day effects in arousal: Disrupted diurnal cortisol profiles in children with ADHD. J. Child Psychol. Psychiatry 2012, 53, 782–789. [Google Scholar] [CrossRef]
- Isaksson, J.; Allen, M.; Nilsson, K.W.; Lindblad, F. Polymorphisms in the FK506 binding protein 5 gene are associated with attention deficit hyperactivity disorder and diurnal cortisol levels. Acta Paediatr. 2015, 104, 910–915. [Google Scholar] [CrossRef]
- Nováková, M.; Paclt, I.; Ptáček, R.; Kuželová, H.; Hájek, I.; Sumová, A. Salivary melatonin rhythm as a marker of the circadian system in healthy children and those with attention-deficit/hyperactivity disorder. Chronobiol. Int. 2011, 28, 630–637. [Google Scholar] [CrossRef]
- Baird, A.L.; Coogan, A.N.; Siddiqui, A.; Donev, R.; Thome, J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol. Psychiatry 2012, 17, 988–995. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Kissling, C.; Retz, W.; Wiemann, S.; Coogan, A.; Clement, R.M.; Hünnerkopf, R.; Conner, A.C.; Freitag, C.M.; Rösler, M.; Thome, J. A polymorphism at the 3′-untranslated region of the CLOCK gene is associated with adult attention-deficit hyperactivity disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Breen, G.; Chen, C.-K.; Huang, Y.-S.; Wu, Y.-Y.; Asherson, P. Association study between a polymorphism at the 3′-untranslated region of CLOCK gene and attention deficit hyperactivity disorder. Behav. Brain Funct. 2010, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, S.; Liu, T.; Zhang, Y.; Li, H.; Li, X.; Tao, W.; Shi, Y. The potential role of clock genes in children attention-deficit/hyperactivity disorder. Sleep Med. 2020, 71, 18–27. [Google Scholar] [CrossRef]
- BfArM. ICD-10-GM Version 2021, 18th ed.; Verlag W. Kohlhammer GmbH: Stuttgart, Germany, 2020.
- Kaufman, J.; Birmaher, B.; Brent, D.A.; Ryan, N.D.; Rao, U. K-SADS-PL. J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 1208. [Google Scholar] [CrossRef]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef]
- Ishii, R.; Obara, H.; Nagamitsu, S.; Matsuoka, M.; Suda, M.; Yuge, K.; Inoue, T.; Sakuta, R.; Oka, Y.; Kakuma, T.; et al. The Japanese version of the children’s sleep habits questionnaire (CSHQ-J): A validation study and influencing factors. Brain Dev. 2022, 44, 595–604. [Google Scholar] [CrossRef]
- Palacio-Ortiz, J.D.; Gomez-Cano, S.; Aguirre-Acevedo, D.C. Sleep problems and profiles in attention deficit hyperactivity disorder assessed by the Children Sleep Habits Questionnaire-Abbreviated in Colombia. Salud Ment. 2018, 41, 261–269. [Google Scholar] [CrossRef]
- Schlarb, A.A.; Schwerdtle, B.; Hautzinger, M. Validation and psychometric properties of the German version of the Children’s Sleep Habits Questionnaire (CSHQ-DE). Somnologie 2010, 14, 260–266. [Google Scholar] [CrossRef]
- Zaidman-Zait, A. The contribution of maternal executive functions and active coping to dyadic affective dynamics: Children with autism spectrum disorder and their mothers. Autism 2020, 24, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Goodlin-Jones, B.L.; Sitnick, S.L.; Tang, K.; Liu, J.; Anders, T.F. The Children’s Sleep Habits Questionnaire in toddlers and preschool children. J. Dev. Behav. Pediatr. 2008, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Görtz-Dorten, A.; Döpfner, M. Aufmerksamkeitsdefizit-/Hyperaktivitätsstörungen von Kindern und Jugendlichen im Elternurteil. [Attention deficit/hyperactive disorders in children and adolescents as assessed by parents]. Z. Kinder-Jugendpsychiatrie Psychother. 2009, 37, 183–194. [Google Scholar] [CrossRef]
- Witting, W.; Kwa, I.H.; Eikelenboom, P.; Mirmiran, M.; Swaab, D. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol. Psychiatry 1990, 27, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W.; Swaab, D.; Colenda, C.C.; Cohen, W.; McCall, W.; Rosenquist, P. Bright light therapy: Improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol. Int. 1999, 16, 505–518. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing (Version 4.0.3). 2020. Available online: https://www.R-project.org/ (accessed on 10 February 2014).
- Bingham, C.; Arbogast, B.; Guillaume, G.C.; Lee, J.K.; Halberg, F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 1982, 9, 397–439. [Google Scholar]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver & Boyd: Edinburgh, UK, 1925. [Google Scholar]
- Dane, A.V.; Schachar, R.J.; Tannock, R. Does actigraphy differentiate ADHD subtypes in a clinical research setting? J. Am. Acad. Child Adolesc. Psychiatry 2000, 39, 752–760. [Google Scholar] [CrossRef]
- Imeraj, L.; Antrop, I.; Roeyers, H.; Deschepper, E.; Bal, S.; Deboutte, D. Diurnal variations in arousal: A naturalistic heart rate study in children with ADHD. Eur. Child Adolesc. Psychiatry 2011, 20, 381–392. [Google Scholar] [CrossRef]
- Faedda, G.L.; Ohashi, K.; Hernandez, M.; McGreenery, C.E.; Grant, M.C.; Baroni, A.; Polcari, A.; Teicher, M.H. Actigraph measures discriminate pediatric bipolar disorder from attention-deficit/hyperactivity disorder and typically developing controls. J. Child Psychol. Psychiatry 2016, 57, 706–716. [Google Scholar] [CrossRef]
- Ironside, S.; Davidson, F.; Corkum, P. Circadian motor activity affected by stimulant medication in children with attention-deficit/hyperactivity disorder. J. Sleep Res. 2010, 19, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Molina-Carballo, A.; Naranjo-Gómez, A.; Uberos, J.; Justicia-Martínez, F.; Ruiz-Ramos, M.-J.; Cubero-Millán, I.; Contreras-Chova, F.; Augustin-Morales, M.-D.; Khaldy, H.; Muñoz-Hoyos, A. Methylphenidate effects on blood serotonin and melatonin levels may help to synchronise biological rhythms in children with ADHD. J. Psychiatr. Res. 2013, 47, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Millán, I.; Molina-Carballo, A.; Machado-Casas, I.; Fernández-López, L.; Martínez-Serrano, S.; Tortosa-Pinto, P.; Ruiz-López, A.; Luna-Del-Castillo, J.-D.; Uberos, J.; Muñoz-Hoyos, A. Methylphenidate ameliorates depressive comorbidity in ADHD children without any modification on differences in serum melatonin concentration between ADHD subtypes. Int. J. Mol. Sci. 2014, 15, 17115–17129. [Google Scholar] [CrossRef] [PubMed]
- Paclt, I.; Ptacek, R.; Kuzelova, H.; Cermáková, N.; Trefilová, A.; Kollárová, P.; Cihal, L. Circadian rhythms of saliva melatonin in ADHD, anxious and normal children. Neuroendocrinol. Lett. 2011, 32, 790–798. [Google Scholar]
- Hirvikoski, T.; Lindholm, T.; Nordenström, A.; Nordström, A.-L.; Lajic, S. High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Horm. Behav. 2009, 55, 418–424. [Google Scholar] [CrossRef]
- Mogavero, F.; Jager, A.; Glennon, J.C. Clock genes, ADHD and aggression. Neurosci. Biobehav. Rev. 2018, 91, 51–68. [Google Scholar] [CrossRef]
- Nie, K.; Wang, K.; Huang, D.-F.; Huang, Y.-B.; Yin, W.; Ren, D.-L.; Wang, H.; Hu, B. Effects of circadian clock protein Per1b on zebrafish visual functions. Chronobiol. Int. 2018, 35, 160–168. [Google Scholar] [CrossRef]
- Wittenbrink, N.; Ananthasubramaniam, B.; Münch, M.; Koller, B.; Maier, B.; Weschke, C.; Bes, F.; De Zeeuw, J.; Nowozin, C.; Wahnschaffe, A.; et al. High-accuracy determination of internal circadian time from a single blood sample. J. Clin. Investig. 2018, 128, 3826–3839. [Google Scholar] [CrossRef]
- Algahim, M.F.; Yang, P.B.; Wilcox, V.T.; Burau, K.D.; Swann, A.C.; Dafny, N. Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2009, 92, 93–99. [Google Scholar] [CrossRef]
- Antle, M.C.; Van Diepen, H.C.; Deboer, T.; Pedram, P.; Pereira, R.R.; Meijer, J.H. Methylphenidate modifies the motion of the circadian clock. Neuropsychopharmacology 2012, 37, 2446–2455. [Google Scholar] [CrossRef]
- O’Keeffe, S.M.; Thome, J.; Coogan, A.N. The noradrenaline reuptake inhibitor atomoxetine phase-shifts the circadian clock in mice. Neuroscience 2012, 201, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Coogan, A.N.; Schenk, M.; Palm, D.; Uzoni, A.; Grube, J.; Tsang, A.; Kolbe, I.; McGowan, N.; Wandschneider, R.; Colla, M.; et al. Impact of adult attention deficit hyperactivity disorder and medication status on sleep/wake behavior and molecular circadian rhythms. Neuropsychopharmacology 2019, 44, 1198–1206. [Google Scholar] [CrossRef] [PubMed]


| Group | N | IQ (Mean ± SD) | Age (Mean ± SD) [Months] | Height (Mean ± SD) [cm] | Weight (Mean ± SD) [kg] |
|---|---|---|---|---|---|
| ADHD | 12 | 95.42 ± 7.48 | 117 ± 14.76 | 144.13 ± 9.86 | 40.48 ± 13.25 |
| control | 11 | 103.73 ± 8.36 | 115.36 ± 15.01 | 145.00 ± 11.00 | 36.15 ± 15.01 |
| Control (Mean ± SD) | ADHD (Mean ± SD) | Z; p | |
|---|---|---|---|
| Sum score | 43.0 ± 3.57 | 40.18 ± 5.98 | 1.55; 0.12 |
| Subscores | |||
| Bedtime resistance | 7.09 ± 2.17 | 6.75 ± 1.06 | 0.14; 0.89 |
| Sleep onset delay | 1.18 ± 0.41 | 1.58 ± 0.79 | 1.33; 0.18 |
| Sleep duration | 3.36 ± 0.67 | 4.50 ± 2.07 | 1.16; 0.25 |
| Sleep anxiety | 4.64 ± 1.80 | 4.58 ± 1.00 | 0.72; 0.47 |
| Parasomias | 8.36 ± 1.29 | 8.17 ± 1.992 | 0.25; 0.80 |
| Sleep-disordered breathing | 3.18 ± 0.60 | 3.17 ± 1.19 | 0.76; 0.45 |
| Daytime sleepiness | 11.18 ± 2.86 | 12.33 ± 2.87 | 0.93; 0.35 |
| Night wakings | 3.45 ± 0.69 | 4.00 ± 1.50 | 0.86; 0.39 |
| % Rhythm | p-Value | |
|---|---|---|
| ADHD | 90.17 | <0.0001 * |
| control | 86.64 | <0.0001 * |
| ADHD (Mean ± SD) | Control (Mean ± SD) | Z; p | |
|---|---|---|---|
| Inter-daily stability (IS) | 0.617 ± 0.04 | 0.601 ± 0.11 | 0; 1 |
| Intra-daily variability (IV) | 0.571 ± 0.09 | 0.623 ± 0.10 | 1.2; 0.23 |
| M10 | 37,608 ± 5182 | 40,812 ± 11,655 | 1.42; 0.16 |
| L5 | 794 ± 220 | 1074 ± 445 | 1.42; 0.16 |
| Amplitude | 22,758 ± 3294 | 24,239 ± 7373 | 0. 74; 0.46 |
| Acrophase | −210.33 ± 10.18 | −212.73 ± 13.81 | 1.39; 0.165 |
| % Rhythm | p-Value | |
|---|---|---|
| Melatonin | ||
| ADHD | 36.22 | 0.0007 * |
| Control | 53.50 | 0.0302 * |
| Cortisol | ||
| ADHD | 49.63 | 0.0046 * |
| Control | 66.31 | 0.0003 * |
| ADHD | Control | p-Value | |
|---|---|---|---|
| Mesor | 0.121730139 | 0.16991 | 0.272 |
| Amplitude | 0.123209832 | 0.134743 | 0.918 |
| Acrophase | −2.136710329 | −1.77627 | 0.002 * |
| ADHD | Control | p-Value | |
|---|---|---|---|
| Mesor | 17.75686207 | 12.66987 | 0.544 |
| Amplitude | 12.831147 | 6.280137 | 0.696 |
| Acrophase | −0.490405853 | −0.30878 | 0.196 |
| % Rhythm | p-Value | |
|---|---|---|
| BMAL1 | ||
| ADHD | 37.15 | 0.277 |
| Control | 38.85 | 0.004 * |
| PER2 | ||
| ADHD | 38.48 | 0.580 |
| Control | 48.22 | 0.007 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dück, A.; Reis, O.; Wagner, H.; Wunsch, K.; Häßler, F.; Kölch, M.; Astiz, M.; Thome, J.; Berger, C.; Oster, H. Clock Genes Profiles as Diagnostic Tool in (Childhood) ADHD—A Pilot Study. Brain Sci. 2022, 12, 1198. https://doi.org/10.3390/brainsci12091198
Dück A, Reis O, Wagner H, Wunsch K, Häßler F, Kölch M, Astiz M, Thome J, Berger C, Oster H. Clock Genes Profiles as Diagnostic Tool in (Childhood) ADHD—A Pilot Study. Brain Sciences. 2022; 12(9):1198. https://doi.org/10.3390/brainsci12091198
Chicago/Turabian StyleDück, Alexander, Olaf Reis, Henrike Wagner, Katja Wunsch, Frank Häßler, Michael Kölch, Mariana Astiz, Johannes Thome, Christoph Berger, and Henrik Oster. 2022. "Clock Genes Profiles as Diagnostic Tool in (Childhood) ADHD—A Pilot Study" Brain Sciences 12, no. 9: 1198. https://doi.org/10.3390/brainsci12091198
APA StyleDück, A., Reis, O., Wagner, H., Wunsch, K., Häßler, F., Kölch, M., Astiz, M., Thome, J., Berger, C., & Oster, H. (2022). Clock Genes Profiles as Diagnostic Tool in (Childhood) ADHD—A Pilot Study. Brain Sciences, 12(9), 1198. https://doi.org/10.3390/brainsci12091198





