Cognition and Neuropsychological Changes at Altitude—A Systematic Review of Literature
Abstract
:1. Introduction
1.1. Current State of Research
1.2. Critical View upon the State of Research
2. Materials and Methods
2.1. Literature Search on High-Altitude Effects on Cognitive Performance
2.1.1. Search Strategy and Study Selection
2.1.2. Inclusion and Exclusion Criteria
2.2. Data Extraction and Management
2.3. Quality Assessment via STAR Data Reporting Guidelines for Clinical High-Altitude Research
3. Results
3.1. Quantitative Description of the Research Methods
3.2. Qualitative Review of the Collected Studies
3.3. Consideration and Further Classification of the Reviewed Neuropsychological Tests
3.3.1. Classification According to the Cognitive Domains Studied
- Orientation
- Attentional capacity, processing speed and working memory
- Concentration/Focused attention
- Processing speed
- Perception
- Memory
- Verbal functions and language skills
- Construction and motor performance
- Concept formatting and reasoning
- Executive functions
- Further and mixed domains
| Cognitive Domain | Neuropsychological Tests | Outcome and Altitude
| First Authors | |
|---|---|---|---|---|
| Orientation | ||||
| Spatiotemp-oral integration | Time Wall Estimation Task | Not affected at 3842 m (LT–HHC)
| De Bels (2019) [76] | |
| Time Perception Task | Impaired at 4350 (FT–P), underestimation of durations
| Davranche (2016) [24] | ||
| Confidence judgement | Feeling of Knowing | Impaired at 6500 m and 7100 (FT–A)
| Nelson (1990) [54] | |
| Attentional capacity, processing speed and working memory | ||||
| Short term memory | Code (Digit Symbol) Substitution –delayed recall | Not affected at 5260 m (FT–P)
| Subudhi (2014) [34] | |
| Corsi Block Forwards | Impaired 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | ||
| Digit Span Forward | Not affected at 3700 m (FT–NA)
| Zhang (2013) [60] | ||
Not affected at 3800 m (FT–NA)
| Phillips (1963) [57] | |||
Impaired at 5100 m (FT–A)
| Harris (2009) [25] | |||
Not affected at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | |||
Not affected at 7200 m (FT–A)
| Malle (2016) [39] | |||
Impaired at 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | |||
Impaired at 7620 m (LT–HHC)
| Asmaro (2013) [27] | |||
| Auditory Digit Span | Impaired at 4280 m (FT–P)
| Shi (2016) [45] | ||
| Visual Digit Span | Not affected at 4280 m (FT–P)
| Shi (2016) [45] | ||
| Memory Search Task | Impaired at 4330 m (FT–A)
| Kramer (1993) [28] | ||
| Picture Recall Test | Not affected at 4280 m (FT–P)
| Shi (2016) [45] | ||
| Picture Recognition Test | Impaired at 4280 m (FT–P)
| Shi (2016) [45] | ||
| Pocket Calculator Cognitive Motor Task | Not affected at 5400 m (FT–A)
| Bonnon (1999) [47] | ||
| Sentence Repetition | Not affected at 3109 m/3810 m (FT–NA)
| Weigle (2007) [59] | ||
| Verbal Free Recall | Impaired at 4500 m/5040 m (FT–A)
| Pelamatti (2003)[55] | ||
| Word Span Forward | Not affected at 3800 m (FT–NA)
| Phillips (1963) [57] | ||
Not affected at 3800 m (FT–NA)
| Phillips (1966) [58] | |||
| Working memory | CogState: Working Memory Task Accuracy | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | |
| Corsi Blocks Backwards | Impaired at 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | ||
| Digit Span Test Backwards | Not affected at 3700 m (FT–NA)
| Zhang (2013) [60] | ||
Not affected at 5100 m (FT–A)
| Harris (2009) [25] | |||
Not affected at 7200 m (FT–A)
| Malle (2016) [39] | |||
Impaired at 5334 m/7620 m (LT–HHC)
| Asmaro (2013) [27] | |||
Impaired at 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | |||
| Memory Interference Task (AB/AC paradigm) | Improved at 4000 m (LT–BHG)
| Loprinzi (2019) [63] | ||
| Operational Span Protocol with VWMC | Not affected at 3000 m (LT–NHC)
| Parker (2017) [68] | ||
| Random Number Generation | Impaired at 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | ||
| Running Memory Continuous Performance Test | Impaired at 4300 m (LT–NHC)
| Seo (2015) [72] | ||
Not affected at 4300 m (LT–NHC)
| Seo (2017) [73] | |||
| Sequence of Numbers and Letters | Impaired at 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | ||
| Sternberg’s Memory Search | Not affected at 5260 m (FT–P)
| Subudhi (2014) [34] | ||
| Concentration/Focused attention | ||||
| Attention | Attention Switching Task | Not affected at 5050 m (FT–P)
| Pun and Guadagni (2018) [31] | |
| Code (Letter Number) Substitution Task | Impaired at 4330 m (FT–A), insensitive to practice compared to CG
| Kramer (1993) [28] | ||
| CogState: Monitoring Task Reaction Time | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | ||
| CogState: Monitoring Task Accuracy | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | ||
| CogState: Working Memory Task Reaction Time | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | ||
| Colorado Perceptual Speed Test | Not affected at 5300 m (FT–A)
| Karinen (2017) [40] | ||
| Continuous Performance Test | Not affected at 5500 m (LT–BHG)
| Altbäcker (2019)[61] | ||
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
| Digit Symbol Substitution Test | Impaired at 3269 m—session 3 (FT–A)
| Falla (2021) [49] | ||
Impaired at 3700 m (FT–NA)
| Zhang (2013) [60] | |||
Not affected at 3800 m (FT–P)
| Frost (2021) [43] | |||
Not affected at 4554 m (FT–A)
| Bjursten (2010) [46] | |||
Improved at 5100 m (FT–A)
| Harris (2009) [25] | |||
Impaired at 5260 m (FT–P)
| Subudhi (2014) [34] | |||
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
| Divided Attention Steering Simulator | Not affected at 2590 m (FT–P)
| Latshang (2013) [38] | ||
| Eriksen Flanker | Not affected at 5160 m (FT–A)
| Lefferts (2019) [51] | ||
Not affected at 4500 m (LT–NHC)
| Williams (2019) [75] | |||
| Frankfurt Attention Inventory-2 | Impaired at 5339 m (FT–A)
| Limmer (2018) [36] | ||
Impaired at 5800 m (LT–NHC)
| ||||
| Letter Cancellation Test | Impaired at 3500 m/5300 m (FT–A)
| Griva (2017) [50] | ||
| Paced Auditory Serial Addition Test | Impaired at 4280 m (FT–P)
| Shi (2016) [45] | ||
Improved at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | |||
Not affected at 7200 m (FT–A)
| Malle (2016) [39] | |||
| Paced Visual Serial Addition Test | Impaired at 4280 m (FT–P)
| Shi (2016) [45] | ||
| Rapid Visual Processing Test | Not affected at 5050 m (FT–P)
| Pun and Guadagni (2018) [31] | ||
| Ruff 2 and 7 Selective Attention Test | Not affected at 6265 m (FT–A)
| Merz (2013) [53] | ||
| Selective Auditory Attention Task | Not affected at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | ||
| Simple Reaction Time Test Span dSRT | Impaired at 5260 m (FT–P)
| Roach (2014) [33] | ||
| Symbol Digit Modalities test | Impaired at 3500 m/5300 m (FT–A)
| Griva (2017) [50] | ||
| Trail Making Test A | Not affected at 2590 m (FT–P)
| Latshang (2013) [38] | ||
Impaired at 3500 m/5300 m (FT–A)
| Griva (2017) [50] | |||
Not affected at 5050 m (FT–P)
| Pun and Hartmann (2018) [32] | |||
Not affected at 5500 m (FT–A)
| Issa (2016) [23] | |||
Impaired at 5334 m/7620 m (LT–HHC)
| Asmaro (2013) [27] | |||
| Response Inhibition | Go/No-Go Test | Not affected at 4300 m (LT–NHC)
| Seo (2015) [72] | |
Not affected at 5260 m (FT–P)
| Subudhi (2014) [34] | |||
| Processing speed | ||||
| Reaction time | CogState: Simple Reaction Time | Improved at 5100 m (FT–A)
| Harris (2009) [25] | |
| Deary–Liewald Reaction Time Task | Not affected at 4500 m (LT–NHC)
| Williams (2019) [75] | ||
| Perceptual Vigilance task | Not affected at 3842 m (LT–HHC)
| De Bels (2019) [76] | ||
| Procedural Reaction Time | Impaired at 5260 m (FT–P)
| Subudhi (2014) [34] | ||
| Psychomotor Vigilance Test | Not affected at 2590 m (FT–P)
| Latshang (2013) [38] | ||
Not affected at 3269 m (FT–A) but higher impairment for poor sleepers
| Falla (2021) [49] | |||
Impaired at 3800 m (FT–P)
| Frost (2021) [43] | |||
Impaired at 5050 m (FT–P)
| Pun and Hartmann (2018) [32] | |||
| Reaction Time Task | Not affected at 5050 m (FT–P)
| Pun and Guadagni (2018) [31] | ||
| Simple Reaction Time Test | Not affected at 3700 m (FT–NA)
| Zhang (2013) [60] | ||
Impaired at 5260 m (FT–P)
| Subudhi (2014) [34] | |||
| Perception | ||||
| Visual perception | Line Bisection Test | Not affected at 6265 m (FT–A)
| Merz (2013) [53] | |
| Reading of Briefly Displayed Letters | Improved at 3450 m (FT–P)
| Schlaepfer (1992) [37] | ||
Improved at 3450 m (LT–BHG)
| ||||
| Visuospatial analytic ability | Abstract Matching | Not affected at 3800 m (FT–P)
| Frost (2021) [43] | |
| Line Orientation Task | Not affected at 3800 m (FT–P)
| Frost (2021) [43] | ||
Not affected at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | |||
| Pattern Comparison Task | Impaired at 4330 m (FT–A)
| Kramer (1993) [28] | ||
| Spatial Discrimination | Not affected at 5260 m (FT–P)
| Subudhi (2014) [34] | ||
| Memory | ||||
| Long term memory | FACTRETRIEVAL2 Test Battery | Not affected at 5400 m/6500 m/7100 m (FT–A)
| Nelson (1990) [54] | |
| Verbal memory | Rey’s Auditory–Verbal Learning Test | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | |
Impaired at 5300 m (FT–A)
| Griva (2017) [50] | |||
| Selective Reminding Test | Not affected at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | ||
| Verbal memory test | Not affected at 4554 m (FT–A)
| Bjursten (2010) [46] | ||
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
| Visual memory | CogState: Learning Task Accuracy | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | |
| Match to sample | Impaired at 5260 m (FT–P)
| Subudhi (2014) [34] | ||
| Visual Memory Test | Not affected at 4554 m (FT–A)
| Bjursten (2010) [46] | ||
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
| Visual Object Learning Task | Not affected at 3800 m (FT–P)
| Frost (2021) [43] | ||
| Verbal functions and language skills | ||||
| Speech production and syntax comprehension | Boston Naming Test | Impaired at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | |
| Robinson’s Rhymes (and Numbers) tests | Impaired at 4340 m (FT–NA)
| Phillips (1963) [57] | ||
| Construction and motor performance | ||||
| Copying | Benton Visual Retention Test | Not affected at 3700 m (FT–NA)
| Zhang (2013) [60] | |
| Assembling and building | Block Design | Impaired at 3500 m/5300 m (FT–A)
| Griva (2017) [50] | |
| Psychomotor ability | Computer-based Psychomotor Speed Task | Impaired at 3000 m (LT–NHC)
| Pighin (2012) [70] | |
| Finger Tapping | Not affected at 4330 m (FT–A)
| Kramer (1993) [28] | ||
Not affected at 4554 m (FT–A)
| Bjursten (2010) [46] | |||
Not affected at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | |||
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
| Motor Praxis Task | Not affected at 3800 m (FT–P)
| Frost (2021) [43] | ||
| Pegboard Psychomotor Test | Impaired at 5300 m (FT–A)
| Griva (2017) [50] | ||
Not affected at 6265 m (FT–A)
| Merz (2013) [53] | |||
Impaired at 5500 m (LT–HHC)
| Abraini (1998) [42] | |||
| Pursuit Aiming Test | Impaired at 3700 m (FT–NA)
| Zhang (2013) [60] | ||
| Santa Ana Manual Dexterity Test | Impaired at 3700 m (FT–NA)
| Zhang (2013) [60] | ||
| Surgical Skills | Impaired at 3000 m (LT–NHC)
| Parker (2017) [68] | ||
| Visual Motor Reaction Time | Impaired at 3109 m/3810 m (FT–NA)
| Weigle (2007) [59] | ||
| Ocular motor performance | King–Devick Test | Impaired at 7101 m (LT–BHG)
| Stepanek (2013) [65] | |
| Saccadic Eye Movement | Not affected at 6265 m (FT–A)
| Merz (2013) [53] | ||
| Concept formatting and Reasoning | ||||
| Concept formation | Category Search Task | Impaired at 4330 m (FT–A)
| Kramer (1993) [28] | |
| Mental Efficacy, reasoning | Gorham’s Proverbs | Not affected at 5273 m/6348 m (FT–A)
| Petiet (1988) [56] | |
| Number Ordination—Rey’s test | Impaired at 5500 m (LT–HHC)
| Abraini (1998) [42] | ||
| Robinson’s (Rhymes and) Numbers tests | Improved at 4340 m (FT–NA)
| Phillips (1963) [57] | ||
| Verbal Reasoning Test | Not affected at 3109 m/3810 m (FT–NA)
| Weigle (2007) [59] | ||
| Arithmetic Reasoning Problems | Modified Math Processing Task | Not affected at 3842 m (LT–HHC)
| De Bels (2019) [76] | |
| Executive functions | ||||
| Executive function | Attention Shifting Test | Not affected at 4554 m (FT–A)
| Bjursten (2010) [46] | |
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
| Category Fluency Tasks | Not affected at 3000 and 4500 m (LT–HHC)
| Pavlicek (2005) [78] | ||
| Controlled Oral Word Association | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | ||
Impaired at 3500 m/5300 m (FT–A)
| Griva (2017) [50] | |||
| Choice Reaction Test (Schuhfried) | Impaired at 3500 m and 5500 m (LT–NHC)
| Pramsohler (2017) [71] | ||
| CogState: Choice Reaction Time | Not affected at 5100 m (FT–A)
| Harris (2009) [25] | ||
| Four-Choice Reaction Time | Impaired at 4000 m/5565 m (FT–A)
| Dykiert (2010) [48] | ||
| MATB–Multiple Attribute Task Battery | Impaired at 2440 m (LT–BHG)
| Kourtidou-Papadeli (2008) [41] | ||
| N-Back Number Task | Not affected at 2590 m (FT–P)
| Latshang (2013) [38] | ||
Not affected at 3800 m (FT–P)
| Frost (2021) [43] | |||
Impaired at 5160 m (FT–A), improved reaction time
| Lefferts (2019) [51] | |||
Impaired at 4500 m (LT–NHC)
| Williams (2019) [75] | |||
| Number Comparison Test | Not affected at 5300 m (FT–A)
| Karinen (2017) [40] | ||
| One Touch Stockings of Cambridge Task | Not affected at 5050 m (FT–P)
| Pun and Guadagni (2018) [31] | ||
| Pro-Point and Anti-Point Tasks | Not affected at 4330 m (FT–P)
| Gibbons (2020) [44] | ||
| Rapid Cognitive Assessment Tool | Not affected at 5500 m (FT–A)
| Issa (2016) [23] | ||
| Ruff Figural Fluency Test | Not affected at 6265 m (FT–A)
| Merz (2013) [53] | ||
| Simon Task | Impaired at 4350 (FT–P)
| Davranche (2016) [24] | ||
| Stroop Test | Impaired at 3109 m, not affected at 3810 m (FT–NA)
| Weigle (2007) [59] | ||
Impaired at 3500 m/5300 m (FT–A)
| Griva (2017) [50] | |||
Improved at 4240 m (FT–A)
| Lefferts (2020) [52] | |||
Not affected at 4554 m (FT–A)
| Bjursten (2010) [46] | |||
Not affected at 5500 m (FT–A)
| Issa (2016) [23] | |||
Impaired at 3500 m (LT–BHG)
| Chroboczek (2021) [62] | |||
Impaired 4500 m (LT–NHC)
| De Aquino Lemos (2012) [66] | |||
Not affected at 2000 m/3500 m/5000 m (LT–BHG), improved reaction time
| Ochi (2018) [64] | |||
Impaired at 5500 m (LT–NHC)
| Turner (2015) [74] | |||
Impaired at 7620 m (LT–HHC)
| Asmaro (2013) [27] | |||
| Number-Size Stroop Variant Task | Not affected at 5500 m (LT–BHG)
| Altbäcker (2019) [61] | ||
| Trail Making Test B | Impaired at 3500 m and 5300 m (FT–A)
| Griva (2017) [50] | ||
Not affected at 5050 m (FT–P)
| Pun and Hartmann (2018) [32] | |||
Improved at 5100 m (FT–A)
| Harris (2009) [25] | |||
Not affected at 5500 m (FT–A)
| Issa (2016) [23] | |||
Impaired at 5334 m/7620 m (LT–HHC)
| Asmaro (2013) [27] | |||
| Verbal Letter Fluency | Not affected at 3000/4500 m (LT–HHC)
| Pavlicek (2005) [78] | ||
| Visual Choice Reaction Time | Impaired at 4330 m (FT–A)
| Kramer (1993) [28] | ||
Not affected at 6500 m (LT–HHC)
| Abraini (1998) [42] | |||
| Planning and decision making | Balloon Analogue Risk Taking task | Risk-taking not affected at 3269 m—session 4 (FT–A) with faster reaction times and higher earnings
| Falla (2021) [49] | |
Risk-taking not affected at 3800 m (FT–P) with faster reaction times
| Frost (2021) [43] | |||
Impaired, higher risk taking at 3000 m (LT–NHC)
| Pighin (2019) [69] | |||
| Computer-based Risk-Taking Task | Increased risk-taking for choices involving losses at 3000 m (LT–NHC)
| Pighin (2012) [70] | ||
| Game of Dice Task | Increased risk behavior at 4500 m (LT–NHC) with reduced risk behavior in preacclimatized subjects compared to unacclimatized subjects
| Niedermeier (2017) [67] | ||
| Further and mixed domains | ||||
| Affective flexibility | Lateralized Tachistoscopic Lexical Decision Task | Not affected at 3000/4500 m (LT–HHC)
| Pavlicek (2005) [78] | |
| Total Mood Disturbance | Impaired at 4300 m (LT–NHC)
| Seo (2017) [73] | ||
| Unspecified individual test outcome | Mini-Mental State Examination: concentration or working memory, language and praxis, orientation, memory, attention span, and other cognitive factors | Not affected at 4500 m (LT–HHC)
| Nakano (2015) [77] | |
| Research Condition | Neuropsychological Test Application N | |||
| Not affected | Impairment | Improvement | Total | |
| FT | 74 | 41 | 7 | 122 |
| A | 41 | 22 | 5 | 68 |
| NA | 9 | 6 | 1 | 16 |
| P | 24 | 13 | 1 | 38 |
| Maltitude FT | 4870 m (15,978 ft) | 4364 m (4364 ft) | 4811 m (15,784 ft) | 4696 m (15,407 ft) |
| Research condition | Not affected | Impairment | Improvement | Total |
| LT | 16 | 33 | 2 | 51 |
| BHG | 3 | 3 | 2 | 8 |
| HHC | 8 | 7 | 15 | |
| NHC | 5 | 23 | 28 | |
| Maltitude LT | 4539 m (14,892 ft) | 4748 m (15,577 ft) | 3725 m (12,221 ft) | 4642 m (15,230 ft) |
3.3.2. Classification According to the Numerical Frequency of Results
3.4. Overview of the Results
4. Discussion
4.1. Major Findings
4.1.1. Discussion of Threshold Altitude
4.1.2. Effects of the Ascent Mode
4.1.3. Differences of Field and Chamber Test Applications and Consequent Assumptions
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Article | Reasons | |
|---|---|---|
| 1. | Abeln V, MacDonald-Nethercott E, Piacentini MF, Meeusen R, Kleinert J, Strueder HK, et al. Exercise in isolation-A countermeasure for electrocortical, mental and cognitive impairments. PloS One. 2015; 10(5):e0126356 | Confounding variable: Physical exercise; +6-week interval between assessments |
| 2. | Algaze I, Phillips L, Inglis P, Lathrop G, Gadbois J, Rizzolo K, et al. Incidence of Mild Cognitive Impairment with Ascending Altitude. High Alt Med Biol. 2020 | No suitable control condition: First tests at 3500 m |
| 3. | Barkaszi I, Takacs E, Czigler I, Balazs L. Extreme Environment Effects on Cognitive Functions: A Longitudinal Study in High Altitude in Antarctica. Front Hum Neurosci. 2016; 10:331 | Without outcomes of interest: Delayed altitude testing |
| 4. | Bartholomew CJ, Jensen W, Petros TV, Ferraro FR, Fire KM, Biberdorf D, et al. The effect of moderate levels of simulated altitude on sustained cognitive performance. Int J Aviat Psychol. 1999; 9(4):351-9 | Without outcomes of interest: Only one administration of neuropsychological tests; + pilots |
| 5. | Baumgartner RW, Keller S, Regard M, Bartsch P. Flunarizine in prevention of headache, ataxia, and memory deficits during decompression to 4559 m. High Alt Med Biol. 2003; 4(3):333-9 | Drug-related confounding variable: Intake of Flunarizine vs. Placebo |
| 6. | Berry DT, McConnell JW, Phillips BA, Carswell CM, Lamb DG, Prine BC. Isocapnic hypoxemia and neuropsychological functioning. J Clin Exp Neuropsychol. 1989; 11(2):241-51 | Other reasons: No information on duration, exposure time, and corresponding altitude |
| 7. | Bhanushali D, Tyagi R, Limaye Rishi Nityapragya N, Anand A. Effect of mindfulness meditation protocol in subjects with various psychometric characteristics at high altitude. Brain Behav. 2020:e01604 | No control condition: Both assessments conducted at 3500 m; + Confounding variable mindfulness meditation |
| 8. | Blanchet A, Noel-Jorand MC, Bonaldi V. Discursive strategies of subjects with high altitude hypoxia: extreme environment. Stress medicine. 1997; 13(3):151-8 | Without outcomes of interest: no use of neuropsychological tests |
| 9. | Bolmont B, Bouquet C, Thullier F. Relationships of Personality Traits with Performance in Reaction Time, Psychomotor Ability, and Mental Efficiency during a 31-Day Simulated Climb of Mount Everest in a Hypobaric Chamber. Percept Mot Ski. 2001; 92(3_suppl):1022-30 | Not original research |
| 10. | Bolmont Bt, Thullier F, Abraini JH. Relationships between mood states and performances in reaction time, psychomotor ability, and mental efficiency during a 31-day gradual decompression in a hypobaric chamber from sea level to 8848 m equivalent altitude. Physiol Behav. 2000; 71(5):469-76 | Not original research |
| 11. | Bouquet CA, Gardette B, Gortan C, Abraini JH. Psychomotor skills learning under chronic hypoxia. NeuroReport. 1999; 10(14):3093-9 | Not original research |
| 12. | Bouzat P, Sechaud G, Banco P, Davranche K, Casini L, Baillieul S, et al. The effect of zolpidem on cognitive function and postural control at high altitude. Sleep. 2018; 41(10) | Drug-related confounding variable: Intake of Zolpidem and sleep |
| 13. | Cahoon RL. Simple Decision Making at High Altitude. Ergonomics. 1972; 15(2):157-63 | Other reasons: Professional military personnel |
| 14. | Carver RP, Winsmann FR. Effect of high elevation upon physical proficiency, cognitive functioning and subjective symptomatology. Percept Mot Skills. 1968; 26(1):223-30 | Drug-related confounding variable: intake of Acetazolamide vs. Placebo |
| 15. | Davis JE, Wagner DR, Garvin N, Moilanen D, Thorington J, Schall C. Cognitive and psychomotor responses to high-altitude exposure in sea level and high-altitude residents of Ecuador. J Physiol Anthropol. 2015; 34:2 | No control condition: Only one assessment of lowlanders at high altitude |
| 16. | Di Paola M, Bozzali M, Fadda L, Musicco M, Sabatini U, Caltagirone C. Reduced oxygen due to high-altitude exposure relates to atrophy in motor-function brain areas. Eur J Neurol. 2008; 15(10):1050-7 | Without outcomes of interest: No cognitive assessment under hypoxia, but 8 weeks before and 8 weeks after ascent |
| 17. | Dobashi S, Horiuchi M, Endo J, Kiuchi M, Koyama K. Cognitive Function and Cerebral Oxygenation During Prolonged Exercise Under Hypoxia in Healthy Young Males. High Alt Med Biol. 2016; 17(3):214-21 | Confounding variable: Physical exercise |
| 18. | Evans WO, Witt NF. The interaction of high altitude and psychotropic drug action. Psychopharmacologia. 1966; 10(2):184-8 | Drug-related confounding variable: Intake of psychotropic drugs |
| 19. | Feeback MR, Seo Y, Dancy M, Glickman EL. The Effect of Psychomotor Performance, Cerebral and Arterial Blood Saturation between African-American and Caucasian Males Before, During and After Normobaric Hypoxic Exercise. Int J Exerc Sci. 2017; 10(5):655-65 | Confounding variable: Physical exercise + no data of baseline assessments reported |
| 20. | Fowler B, Elcombe DD, Kelso B, Porlier G. The Threshold for Hypoxia Effects on Perceptual- Motor Performance. Hum Factors. 1987; 29(1):61-6 | No control condition |
| 21. | Gerard AB, McElroy MK, Taylor MJ, Grant I, Powell FL, Holverda S, et al. Six percent oxygen enrichment of room air at simulated 5000 m altitude improves neuropsychological function. High Alt Med Biol. 2000; 1(1):51-61 | No control condition |
| 22. | Guo W, Chen G, Qin J, Zhang J, Guo X, Yu J, et al. Short-term high-altitude pre-exposure improves neurobehavioral ability. Neuroreport. 2016; 27(6):367-73 | Confounding variable: High-altitude pre-exposure |
| 23. | Guo WY, Bian SZ, Zhang JH, Li QN, Yu J, Chen JF, et al. Physiological and psychological factors associated with onset of high-altitude headache in Chinese men upon acute high-altitude exposure at 3700 m. Cephalalgia. 2017; 37(4):336-47 | Confounding variable: High-altitude headache |
| 24. | Gustafsson C, Gennser M, Ornhagen H, Derefeldt G. Effects of normobaric hypoxic confinement on visual and motor performance. Aviat Space Environ Med. 1997; 68(11):985–92 | Confounding variable: Shift working |
| 25. | Hayashi R, Matsuzawa Y, Kubo K, Kobayashi T. Effects of simulated high altitude on event-related potential (P300) and auditory brain-stem responses. Clin Neurophysiol. 2005; 116(6):1471–6 | ERP measurement |
| 26. | Heinrich EC, Djokic MA, Gilbertson D, DeYoung PN, Bosompra NO, Wu L, et al. Cognitive function and mood at high altitude following acclimatization and use of supplemental oxygen and adaptive servoventilation sleep treatments. PloS One. 2019; 14(6):e0217089 | Confounding variable: Sleep treatments |
| 27. | Hoffman CE, Clark RT, Jr., Brown EB, Jr. Blood oxygen saturations and duration of consciousness in anoxia at high altitudes. Am J Physiol. 1946; 145:685-92 | No control condition |
| 28. | Hornbein TF, Townes BD, Schoene RB, Sutton JR, Houston CS. The cost to the central nervous system of climbing to extremely high altitude. New Engl J Med. 1989; 321(25):1714-9 | Not original research |
| 29. | Hu S, Shi J, Xiong W, Li W, Fang L, Feng H. Oxiracetam or fastigial nucleus stimulation reduces cognitive injury at high altitude. Brain Behav. 2017; 7(10):e00762 | Drug-related confounding variable: Intake of Oxiracetam or fastigial nucleus stimulation (FNS) |
| 30. | Jobe JB, Shukitt-Hale B, Banderet LE, Rock PB. Effects of dexamethasone and high terrestrial altitude on cognitive performance and affect. Aviat Space Environ Med. 1991; 62(8):727-32 | Drug-related confounding variable: Intake of Dexamethason vs. Placebo |
| 31. | Kammerer T, Faihs V, Hulde N, Bayer A, Hubner M, Brettner F, et al. Changes of hemodynamic and cerebral oxygenation after exercise in normobaric and hypobaric hypoxia: associations with acute mountain sickness. Ann Occup Environ Med. 2018; 30:66 | Confounding variable: Physical exercise |
| 32. | Kida M, Imai A. Cognitive performance and event-related brain potentials under simulated high altitudes. J Appl Physiol. 1993; 74(4):1735-41 | Confounding variable: Division of subjects into groups based on their reaction times |
| 33. | Kim C-H, Ryan EJ, Seo Y, Peacock C, Gunstad J, Muller MD, et al. Low intensity exercise does not impact cognitive function during exposure to normobaric hypoxia. Physiol Behav. 2015; 151:24-8 | Confounding variable: Physical exercise |
| 34. | Koller E, Bischoff M, Bührer A, Felder L, Schopen M. Respiratory, circulatory and neuropsychological responses to acute hypoxia in acclimatized and non-acclimatized subjects. Eur J Appl Physiol. 1991; 62(2):67-72 | Confounding variable: Preacclimatization |
| 35. | Lafleur J, Giron M, Demarco M, Kennedy R, BeLue R, Shields C. Cognitive effects of dexamethasone at high altitude. Wilderness Environ Med. 2003; 14(1):20-3 | Drug-related confounding variable: Intake of Dexamethasone |
| 36. | Leach J, Almond S. Ambient air, oxygen and nitrox effects on cognitive performance at altitude. Appl Human Sci. 1999; 18(5):175-9 | Drug-related confounding variable: Breathing of gas mixtures at surface ambient pressures |
| 37. | Lei OK, Kong Z, Loprinzi PD, Shi Q, Sun S, Zou L, et al. Severe Hypoxia Does Not Offset the Benefits of Exercise on Cognitive Function in Sedentary Young Women. Int J Environ Res Public Health. 2019; 16(6) | Confounding variable: Physical exercise |
| 38. | Li P, Zhang G, You HY, Zheng R, Gao YQ. Training-dependent cognitive advantage is suppressed at high altitude. Physiol Behav. 2012; 106(4):439-45 | Confounding variable: Physical training condition |
| 39. | Li Z, Xue X, Li X, Bao X, Yu S, Wang Z, et al. Neuropsychological effect of working memory capacity on mental rotation under hypoxia environment. Int J Psychophysiol. 2021; 165:18-28 | ERP measurement |
| 40. | Liao YH, Mundel T, Yang YT, Wei CC, Tsai SC. Effects of periodic carbohydrate ingestion on endurance and cognitive performances during a 40-km cycling time-trial under normobaric hypoxia in well-trained triathletes. J Sports Sci. 2019; 37(16):1805-15 | Confounding variable: Carbohydrate ingestion and physical exercise |
| 41. | Lieberman P, Protopapas A, Kanki BG. Speech production and cognitive deficits on Mt. Everest. Aviat Space Environ Med. 1995 | No suitable control condition |
| 42. | Luks AM, van Melick H, Batarse RR, Powell FL, Grant I, West JB. Room oxygen enrichment improves sleep and subsequent day-time perfor-mance at high altitude. Respir Physiol. 1998; 113(3):247-58 | Confounding variable: Room oxygen enrichment during sleep |
| 43. | Mairesse O, MacDonald-Nethercott E, Neu D, Tellez HF, Dessy E, Neyt X, et al. Preparing for Mars: human sleep and performance during a 13 month stay in Antarctica. Sleep. 2019; 42(1) | Without outcomes of interest: Delayed altitude testing |
| 44. | Malle C, Bourrilhon C, Quinette P, Laisney M, Eustache F, Pierard C. Physiological and Cognitive Effects of Acute Normobaric Hypoxia and Modulations from Oxygen Breathing. Aerosp Med Hum Perform. 2016; 87(1):3-12 | Confounding variable: Modification of oxygen breathing |
| 45. | Milne D, Gray D. Evidence bearing on the generalizability of laboratory findings relating to high-altitude mountaineering. Percept Mot Skills. 1983; 57(1):172-4 | Other reasons: Applied tests not further named |
| 46. | Morrison JD, Quinn K, MacDonald LA, Billaut F, Minahan C. Repeated Treadmill Sprints Impair Cognitive Performance in Amateur Team-Sport Athletes When Performed in Normobaric Hypoxia. J Sports Sci Med. 2019; 18(2):369-75 | Confounding variable: Physical exercise |
| 47. | Nakata H, Miyamoto T, Ogoh S, Kakigi R, Shibasaki M. Effects of acute hypoxia on human cognitive processing: a study using ERPs and SEPs. J Appl Physiol. 2017; 123(5):1246-55 | ERP measurement |
| 48. | Nickol AH, Leverment J, Richards P, Seal P, Harris GA, Cleland J, et al. Temazepam at high altitude reduces periodic breathing without impairing next-day performance: a randomized cross-over double-blind study. J Sleep Res. 2006; 15(4):445-54 | Drug-related confounding variable: Intake of Temazepam vs. Placebo |
| 49. | Nisha, S. N., Fathinul Fikri, A. S., Aida, A. R., Salasiah, M., Hamed, S., Rohit, T., Amei Farina, A. R., Loh, J. L., Mazlyfarina, M., & Subapriya, S. (2020). The objective assessment of the effects on cognition functioning among military personnel exposed to hypobaric-hypoxia: A pilot fMRI study. Med J Malaysia, 75(1), 62–67 | Confounding variable: Chronic intermittent exposure to high altitude |
| 50. | O’Keeffe K, Raccuglia G, Hodder S, Lloyd A. Mental fatigue independent of boredom and sleepiness does not impact self-paced physical or cognitive performance in normoxia or hypoxia. J Sports Sci. 2021; 39(15):1687-99 | Confounding variable: Physical exercise |
| 51. | Ochi G, Yamada Y, Hyodo K, Suwabe K, Fukuie T, Byun K, et al. Neural basis for reduced executive performance with hypoxic exercise. Neuroimage. 2018; 171:75-83 | Confounding variable: Physical exercise |
| 52. | Pagani M, Ravagnan G, Salmaso D. Effect of Acclimatisation to Altitude on Learning. Cortex. 1998; 34(2):243-51 | No control condition |
| 53. | Patrician A, Tymko MM, Caldwell HG, Howe CA, Coombs GB, Stone R, et al. The Effect of an Expiratory Resistance Mask with Dead Space on Sleep, Acute Mountain Sickness, Cognition, and Ventilatory Acclimatization in Normobaric Hypoxia. High Alt Med Biol. 2019; 20(1):61-70 | Confounding variable: Expiratory Resistance Masks during sleep |
| 54. | Phillips L, Basnyat B, Chang Y, Swenson ER, Harris NS. Findings of Cognitive Impairment at High Altitude: Relationships to Acetazolamide Use and Acute Mountain Sickness. High Alt Med Biol. 2017; 18(2):121-7 | No control condition |
| 55. | Remenyi A, Grosz A, Szabo SA, Totka Z, Molnar D, Helfferich F. Comparative study of the effect of bilastine and cetirizine on cognitive functions at ground level and at an altitude of 4000 m simulated in hypobaric chamber: a randomized, double-blind, placebo-controlled, cross-over study. Expert Opin Drug Saf. 2018; 17(9):859-68 | Drug-related confounding variable: Intake of Antihistamines vs. Placebo |
| 56. | Richalet JP. Operation Everest III: COMEX ‘97. High Alt Med Biol. 2010; 11(2):121-32 | Not original research |
| 57. | Schega L, Peter B, Brigadski T, Lessmann V, Isermann B, Hamacher D, et al. Effect of intermittent normobaric hypoxia on aerobic capacity and cognitive function in older people. J Sci Med Sport. 2016; 19(11):941-5 | Confounding variable: Intermittend normobaric hypoxia combined with subsequent aerobic training |
| 58. | Shannon OM, Duckworth L, Barlow MJ, Deighton K, Matu J, Williams EL, et al. Effects of Dietary Nitrate Supplementation on Physiological Responses, Cognitive Function, and Exercise Performance at Moderate and Very-High Simulated Altitude. Front Physiol. 2017; 8:401 | Drug-related confounding variable: Intake of beetroot juice |
| 59. | Shephard R. Physiological changes and psychomotor performance during acute hypoxia. J Appl Physiol. 1956; 9(3):343-51 | Without outcomes of interest: Baseline assessment under oxygen breathing |
| 60. | Shukitt-Hale B, Banderet LE, Lieberman HR. Elevation-dependent symptom, mood, and performance changes produced by exposure to hypobaric hypoxia. Int J Aviat Psychol. 1998; 8(4):319-34 | Drug-related confounding variable: Examination of group with placebo intake |
| 61. | Silva-Urra JA, Nunez-Espinosa CA, Nino-Mendez OA, Gaitan-Penas H, Altavilla C, Toro-Salinas A, et al. Circadian and Sex Differences After Acute High-Altitude Exposure: Are Early Acclimation Responses Improved by Blue Light? Wilderness Environ Med. 2015; 26(4):459-71 | Confounding variable: Circadian rhythm and lighting |
| 62. | Singh SB, Thakur L, Anand JP, Yadav D, Amitab, Banerjee PK. Effect of chronic hypobaric hypoxia on components of the human event related potential. Indian J Med Res. 2004; 120(2):94-9 | ERP measurement |
| 63. | Stamper DA, Kinsman RA, Evans WO. Subjective symptomatology and cognitive performance at high altitude. Percept Mot Skills. 1970; 31(1):247-61 | Drug-related confounding variable: Three drug groups (i.e., codeine, phenformin or placebo) |
| 64. | Stavres J, Gerhart HD, Kim JH, Glickman EL, Seo Y. Cerebral Hemodynamics and Executive Function During Exercise and Recovery in Normobaric Hypoxia. Aerosp Med Hum Perform. 2017; 88(10):911-7 | Confounding variable: Physical exercise |
| 65. | Stivalet P, Leifflen D, Poquin D, Savourey G, Launay JC, Barraud PA, et al. Positive expiratory pressure as a method for preventing the impairment of attentional processes by hypoxia. Ergonomics. 2000; 43(4):474-85 | Confounding variable: Positive expiratory pressure |
| 66. | Thakur L, Ray K, Anand JP, Panjwani U. Event related potential (ERP) P300 after 6 months residence at 4115 m. Indian J Med Res. 2011; 134:113-7 | ERP measurement |
| 67. | Tsarouchas N, Benedek K, Bezerianos A, Benedek G, Keri S. Effects of moderate hypobaric hypoxia on evoked categorical visuocognitive responses. Clin Neurophysiol. 2008; 119(7):1475-85 | ERP measurement |
| 68. | Van Dorp E, Los M, Dirven P, Sarton E, Valk P, Teppema L, et al. Inspired carbon dioxide during hypoxia: effects on task performance and cerebral oxygen saturation. Aviat Space Environ Med. 2007; 78(7):666-72 | Confounding variable: Change in inspired Carbon Dioxide |
| 69. | Walsh JJ, Drouin PJ, King TJ, D’Urzo KA, Tschakovsky ME, Cheung SS, et al. Acute aerobic exercise impairs aspects of cognitive function at high altitude. Physiol Behav. 2020; 223:112979 | Confounding variable: Physical exercise |
| 70. | Wang J, Ke T, Zhang X, Chen Y, Liu M, Chen J, et al. Effects of acetazolamide on cognitive performance during high-altitude exposure. Neurotoxicol Teratol. 2013; 35:28-33 | Drug-related confounding variable: Intake of Acetazolamide vs. Placebo |
| 71. | Zhang H, Lin J, Sun Y, Huang Y, Ye H, Wang X, et al. Compromised white matter microstructural integrity after mountain climbing: evidence from diffusion tensor imaging. High Alt Med Biol. 2012; 13(2):118-25 | Without outcomes of interest: No assessments at altitude, but prior and after climbing up to 6202 m |
References
- Schaffert, W. Akute Höhenkrankheit. In Alpin- und Höhenmedizin, 2nd ed.; Berghold, F., Brugger, H., Burtscher, M., Domej, W., Durrer, B., Fischer, R., Paal, P., Schaffert, W., Schobersberger, W., Sumann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 469–480. [Google Scholar]
- Fischer, R. Höhenlungenödem. In Alpin- und Höhenmedizin, 2nd ed.; Berghold, F., Brugger, H., Burtscher, M., Domej, W., Durrer, B., Fischer, R., Paal, P., Schaffert, W., Schobersberger, W., Sumann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 481–488. [Google Scholar]
- Berghold, F. Höhenhirnödem. In Alpin- und Höhenmedizin, 2nd ed.; Berghold, F., Brugger, H., Burtscher, M., Domej, W., Durrer, B., Fischer, R., Paal, P., Schaffert, W., Schobersberger, W., Sumann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 489–500. [Google Scholar]
- Hultgren, H.N. High Altitude Medicine; Hultgren Publications: Stanford, CA, USA, 1997. [Google Scholar]
- Domej, W.; Schwaberger, G. Physik der mittleren, großen und extremen Höhen: Die Erdatmosphäre. In Alpin- und Höhenmedizin, 2nd ed.; Berghold, F., Brugger, H., Burtscher, M., Domej, W., Durrer, B., Fischer, R., Paal, P., Schaffert, W., Schobersberger, W., Sumann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 327–335. [Google Scholar]
- Berghold, F. Praxis der alpinistischen Höhentaktik: Höhenakklimatisation. In Alpin- und Höhenmedizin, 2nd ed.; Berghold, F., Brugger, H., Burtscher, M., Domej, W., Durrer, B., Fischer, R., Paal, P., Schaffert, W., Schobersberger, W., Sumann, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 459–468. [Google Scholar]
- Falla, M.; Giardini, G.; Angelini, C. Recommendations for traveling to altitude with neurological disorders. J. Cent. Nerv. Syst. Dis. 2021, 13, 11795735211053448. [Google Scholar] [CrossRef] [PubMed]
- Hüfner, K.; Brugger, H.; Kuster, E.; Dünsser, F.; Stawinoga, A.E.; Turner, R.; Tomazin, I.; Sperner-Unterweger, B. Isolated psychosis during exposure to very high and extreme altitude—Characterisation of a new medical entity. Psychol. Med. 2018, 48, 1872–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falla, M.; Micarelli, A.; Hüfner, K.; Strapazzon, G. The Effect of Cold Exposure on Cognitive Performance in Healthy Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9725. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; McLeod, E.; Periard, J.; Rattray, B.; Keegan, R.; Pyne, D.B. The Impact of Environmental Stress on Cognitive Performance: A Systematic Review. Hum. Factors 2019, 61, 1205–1246. [Google Scholar] [CrossRef]
- Ando, S.; Komiyama, T.; Sudo, M.; Higaki, Y.; Ishida, K.; Costello, J.T.; Katayama, K. The interactive effects of acute exercise and hypoxia on cognitive performance: A narrative review. Scand. J. Med. Sci. Sports 2020, 30, 384–398. [Google Scholar] [CrossRef]
- Killgore, W.; Weber, M. Sleep Deprivation and Cognitive Performance. In Sleep Deprivation and Disease: Effects on the Body, Brain and Behavior; Bianchi, M., Ed.; Springer Science + Business Media: New York, NY, USA, 2014; pp. 209–229. [Google Scholar]
- Bloch, K.E.; Buenzli, J.C.; Latshang, T.D.; Ulrich, S. Sleep at high altitude: Guesses and facts. J. Appl. Physiol. 2015, 119, 1466–1480. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Vaitl, D. Veränderte Bewusstseinszustände: Grundlagen-Techniken-Phänomenologie; Schattauer Verlag: Stuttgart, Germany, 2012. [Google Scholar]
- Di Paola, M.; Caltagirone, C.; Fadda, L.; Sabatini, U.; Serra, L.; Carlesimo, G.A. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus 2008, 18, 719–728. [Google Scholar] [CrossRef]
- Caine, D.; Watson, J.D. Neuropsychological and neuropathological sequelae of cerebral anoxia: A critical review. J. Int. Neuropsychol. Soc. 2000, 6, 86–99. [Google Scholar] [CrossRef]
- Chauhan, P.; Jethwa, K.; Rathawa, A.; Chauhan, G.; Mehra, S. The Anatomy of the Hippocampus. In Cerebral Ischemia; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Virués-Ortega, J.; Buela-Casal, G.; Garrido, E.; Alcázar, B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol. Rev. 2004, 14, 197–224. [Google Scholar] [CrossRef]
- Petrassi, F.A.; Hodkinson, P.D.; Walters, P.L.; Gaydos, S.J. Hypoxic hypoxia at moderate altitudes: Review of the state of the science. Aviat. Space Environ. Med. 2012, 83, 975–984. [Google Scholar] [CrossRef]
- Taylor, L.; Watkins, S.L.; Marshall, H.; Dascombe, B.J.; Foster, J. The Impact of Different Environmental Conditions on Cognitive Function: A Focused Review. Front. Physiol. 2016, 6, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.N.; Herman, N.M.; Wentz, R.J.; Taylor, B.J.; Summerfield, D.C.; Johnson, B.D. Association of Cognitive Performance with Time at Altitude, Sleep Quality, and Acute Mountain Sickness Symptoms. Wilderness Environ. Med. 2016, 27, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davranche, K.; Casini, L.; Arnal, P.J.; Rupp, T.; Perrey, S.; Verges, S. Cognitive functions and cerebral oxygenation changes during acute and prolonged hypoxic exposure. Physiol. Behav. 2016, 164 Pt A, 189–197. [Google Scholar] [CrossRef]
- Harris, G.A.; Cleland, J.; Collie, A.; McCrory, P. Cognitive Assessment of a Trekking Expedition to 5100 m: A Comparison of Computerized and Written Testing Methods. Wilderness Environ. Med. 2009, 20, 261–268. [Google Scholar] [CrossRef]
- Hornbein, T.F.; Townes, B.D.; Schoene, R.B.; Sutton, J.R.; Houston, C.S. The cost to the central nervous system of climbing to extremely high altitude. N. Engl. J. Med. 1989, 321, 1714–1719. [Google Scholar] [CrossRef] [Green Version]
- Asmaro, D.; Ferguson, S.; Mayall, J. Cognition at altitude: Impairment in executive and memory processes under hypoxic conditions. Aviat. Space Environ. Med. 2013, 84, 1159–1165. [Google Scholar] [CrossRef]
- Kramer, A.F.; Coyne, J.T.; Strayer, D.L. Cognitive function at high altitude. Hum. Factors 1993, 35, 329–344. [Google Scholar] [CrossRef]
- Pagani, M.; Ravagnan, G.; Salmaso, D. Effect of Acclimatisation to Altitude on Learning. Cortex 1998, 34, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Pun, M.; Guadagni, V.; Bettauer, K.M.; Drogos, L.L.; Aitken, J.; Hartmann, S.E.; Furian, M.; Muralt, L.; Lichtblau, M.; Bader, P.R.; et al. Effects on Cognitive Functioning of Acute, Subacute and Repeated Exposures to High Altitude. Front. Physiol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pun, M.; Hartmann, S.E.; Furian, M.; Dyck, A.M.; Muralt, L.; Lichtblau, M.; Bader, P.R.; Rawling, J.M.; Ulrich, S.; Bloch, K.E.; et al. Effect of Acute, Subacute, and Repeated Exposure to High Altitude (5050 m) on Psychomotor Vigilance. Front. Physiol. 2018, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Roach, E.B.; Bleiberg, J.; Lathan, C.E.; Wolpert, L.; Tsao, J.W.; Roach, R.C. AltitudeOmics: Decreased reaction time after high altitude cognitive testing is a sensitive metric of hypoxic impairment. Neuroreport 2014, 25, 814–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subudhi, A.W.; Bourdillon, N.; Bucher, J.; Davis, C.; Elliott, J.E.; Eutermoster, M.; Evero, O.; Fan, J.L.; Houten, S.J.; Julian, C.G.; et al. AltitudeOmics: The integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PLoS ONE 2014, 9, e92191. [Google Scholar] [CrossRef] [PubMed]
- Brodmann Maeder, M.; Brugger, H.; Pun, M.; Strapazzon, G.; Dal Cappello, T.; Maggiorini, M.; Hackett, P.; Bärtsch, P.; Swenson, E.R.; Zafren, K. The STAR Data Reporting Guidelines for Clinical High Altitude Research. High Alt. Med. Biol. 2018, 19, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limmer, M.; Platen, P. The influence of hypoxia and prolonged exercise on attentional performance at high and extreme altitudes: A pilot study. PLoS ONE 2018, 13, e0205285. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, T.E.; Bartsch, P.; Fisch, H.U. Paradoxical effects of mild hypoxia and moderate altitude on human visual perception. Clin. Sci. 1992, 83, 633–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latshang, T.D.; Lo Cascio, C.M.; Stowhas, A.C.; Grimm, M.; Stadelmann, K.; Tesler, N.; Achermann, P.; Huber, R.; Kohler, M.; Bloch, K.E. Are nocturnal breathing, sleep, and cognitive performance impaired at moderate altitude (1630–2590 m)? Sleep 2013, 36, 1969–1976. [Google Scholar] [CrossRef]
- Malle, C.; Ginon, B.; Bourrilhon, C. Brief Working Memory and Physiological Monitoring During a High-Altitude Expedition. High Alt. Med. Biol. 2016, 17, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Karinen, H.M.; Tuomisto, M.T. Performance, Mood, and Anxiety During a Climb of Mount Everest. High Alt. Med. Biol. 2017, 18, 400–410. [Google Scholar] [CrossRef]
- Kourtidou-Papadeli, C.; Papadelis, C.; Koutsonikolas, D.; Boutzioukas, S.; Styliadis, C.; Guiba-Tziampiri, O. High altitude cognitive performance and COPD interaction. Hippokratia 2008, 12 (Suppl. 1), 84–90. [Google Scholar] [PubMed]
- Abraini, J.H.; Bouquet, C.; Joulia, F.; Nicolas, M.; Kriem, B. Cognitive performance during a simulated climb of Mount Everest: Implications for brain function and central adaptive processes under chronic hypoxic stress. Pflügers Arch. 1998, 436, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Orr, J.E.; Oeung, B.; Puvvula, N.; Pham, K.; Brena, R.; DeYoung, P.; Jain, S.; Sun, S.; Malhotra, A.; et al. Improvements in sleep-disordered breathing during acclimatization to 3800 m and the impact on cognitive function. Physiol. Rep. 2021, 9, e14827. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, T.D.; Tymko, M.M.; Thomas, K.N.; Wilson, L.C.; Stembridge, M.; Caldwell, H.G.; Howe, C.A.; Hoiland, R.L.; Akerman, A.P.; Dawkins, T.G.; et al. Global REACH 2018: The influence of acute and chronic hypoxia on cerebral haemodynamics and related functional outcomes during cold and heat stress. J. Physiol. 2020, 598, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.H.; Ge, D.; Zhao, W.; Ma, X.; Hu, K.Y.; Lu, Y.; Liu, Z.X.; Ran, J.H.; Li, X.L.; Zhou, Y.; et al. A Computerized Evaluation of Sensory Memory and Short-term Memory Impairment After Rapid Ascent to 4280 m. Biomed. Environ. Sci. 2016, 29, 457–460. [Google Scholar] [PubMed]
- Bjursten, H.; Ederoth, P.; Sigurdsson, E.; Gottfredsson, M.; Syk, I.; Einarsson, O.; Gudbjartsson, T. S100B profiles and cognitive function at high altitude. High Alt. Med. Biol. 2010, 11, 31–38. [Google Scholar] [CrossRef]
- Bonnon, M.; Noel-Jorand, M.C.; Therme, P. Criteria for psychological adaptation to high-altitude hypoxia. Percept. Mot. Ski. 1999, 89, 3–18. [Google Scholar] [CrossRef]
- Dykiert, D.; Hall, D.; van Gemeren, N.; Benson, R.; Der, G.; Starr, J.M.; Deary, I.J. The effects of high altitude on choice reaction time mean and intra-individual variability: Results of the Edinburgh Altitude Research Expedition of 2008. Neuropsychology 2010, 24, 391–401. [Google Scholar] [CrossRef]
- Falla, M.; Papagno, C.; Dal Cappello, T.; Vögele, A.; Hüfner, K.; Kim, J.; Weiss, E.M.; Weber, B.; Palma, M.; Mrakic-Sposta, S.; et al. A Prospective Evaluation of the Acute Effects of High Altitude on Cognitive and Physiological Functions in Lowlanders. Front. Physiol. 2021, 12, 670278. [Google Scholar] [CrossRef]
- Griva, K.; Stygall, J.; Wilson, M.H.; Martin, D.; Levett, D.; Mitchell, K.; Mythen, M.; Montgomery, H.E.; Grocott, M.P.; Aref-Adib, G.; et al. Caudwell Xtreme Everest: A prospective study of the effects of environmental hypoxia on cognitive functioning. PLoS ONE 2017, 12, e0174277. [Google Scholar] [CrossRef]
- Lefferts, W.K.; DeBlois, J.P.; White, C.N.; Day, T.A.; Heffernan, K.S.; Brutsaert, T.D. Changes in cognitive function and latent processes of decision-making during incremental ascent to high altitude. Physiol. Behav. 2019, 201, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lefferts, W.K.; DeBlois, J.P.; Soriano, J.E.; Mann, L.; Rampuri, Z.; Herrington, B.; Thrall, S.; Bird, J.; Harman, T.S.; Day, T.A.; et al. Preservation of Neurovascular Coupling to Cognitive Activity in Anterior Cerebrovasculature During Incremental Ascent to High Altitude. High Alt. Med. Biol. 2020, 21, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Merz, T.M.; Bosch, M.M.; Barthelmes, D.; Pichler, J.; Hefti, U.; Schmitt, K.-U.; Bloch, K.E.; Schoch, O.D.; Hess, T.; Turk, A.J.; et al. Cognitive performance in high-altitude climbers: A comparative study of saccadic eye movements and neuropsychological tests. Eur. J. Appl. Physiol. 2013, 113, 2025–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, T.O.; Dunlosky, J.; White, D.M.; Steinberg, J.; Townes, B.D.; Anderson, D. Cognition and metacognition at extreme altitudes on Mount Everest. J. Exp. Psychol. Gen. 1990, 119, 367–374. [Google Scholar] [CrossRef]
- Pelamatti, G.; Pascotto, M.; Semenza, C. Verbal Free Recall in High Altitude: Proper Names vs. Common Names. Cortex 2003, 39, 97–103. [Google Scholar] [CrossRef]
- Petiet, C.A.; Townes, B.D.; Brooks, R.J.; Kramer, J.H. Neurobehavioral and psychosocial functioning of women exposed to high altitude in mountaineering. Percept. Mot. Ski. 1988, 67, 443–452. [Google Scholar] [CrossRef]
- Phillips, L.W.; Griswold, R.L.; Pace, N. Cognitive changes at high altitude. Psychol. Rep. 1963, 13, 423E–430E. [Google Scholar] [CrossRef]
- Phillips, L.W.; Pace, N. Performance changes at moderately high altitude: Short-term memory measured by free recall. Psychol. Rep. 1966, 19, 655–665. [Google Scholar] [CrossRef]
- Weigle, D.S.; Buben, A.; Burke, C.C.; Carroll, N.D.; Cook, B.M.; Davis, B.S.; Dubowitz, G.; Fisher, R.E.; Freeman, T.C.; Gibbons, S.M.; et al. Adaptation to altitude as a vehicle for experiential learning of physiology by university undergraduates. Adv. Physiol. Educ. 2007, 31, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhou, S.M.; Yuan, C.; Tian, H.J.; Li, P.; Gao, Y.Q. The effects of short-term and long-term exposure to a high altitude hypoxic environment on neurobehavioral function. High Alt. Med. Biol. 2013, 14, 338–341. [Google Scholar] [CrossRef]
- Altbacker, A.; Takacs, E.; Barkaszi, I.; Kormos, T.; Czigler, I.; Balazs, L. Differential impact of acute hypoxia on event related potentials: Impaired task-irrelevant, but preserved task-relevant processing and response inhibition. Physiol. Behav. 2019, 206, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chroboczek, M.; Kostrzewa, M.; Micielska, K.; Grzywacz, T.; Laskowski, R. Effect of Acute Normobaric Hypoxia Exposure on Executive Functions among Young Physically Active Males. J. Clin. Med. 2021, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Matalgah, A.; Crawford, L.; Yu, J.J.; Kong, Z.; Wang, B.; Liu, S.; Zou, L. Effects of Acute Normobaric Hypoxia on Memory Interference. Brain Sci. 2019, 9, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochi, G.; Kanazawa, Y.; Hyodo, K.; Suwabe, K.; Shimizu, T.; Fukuie, T.; Byun, K.; Soya, H. Hypoxia-induced lowered executive function depends on arterial oxygen desaturation. J. Physiol. Sci. 2018, 68, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, J.; Cocco, D.; Pradhan, G.N.; Smith, B.E.; Bartlett, J.; Studer, M.; Kuhn, F.; Cevette, M.J. Early detection of hypoxia-induced cognitive impairment using the King-Devick test. Aviat. Space Environ. Med. 2013, 84, 1017–1022. [Google Scholar] [CrossRef] [Green Version]
- de Aquino Lemos, V.; Antunes, H.K.; dos Santos, R.V.; Lira, F.S.; Tufik, S.; de Mello, M.T. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 2012, 49, 1298–1306. [Google Scholar] [CrossRef]
- Niedermeier, M.; Weisleitner, A.; Lamm, C.; Ledochowski, L.; Frühauf, A.; Wille, M.; Burtscher, M.; Kopp, M. Is decision making in hypoxia affected by pre-acclimatisation? A randomized controlled trial. Physiol. Behav. 2017, 173, 236–242. [Google Scholar] [CrossRef]
- Parker, P.J.; Manley, A.J.; Shand, R.; O’Hara, J.P.; Mellor, A. Working Memory Capacity and Surgical Performance While Exposed to Mild Hypoxic Hypoxemia. Aerosp. Med. Hum. Perform. 2017, 88, 918–923. [Google Scholar] [CrossRef] [Green Version]
- Pighin, S.; Bonini, N.; Hadjichristidis, C.; Schena, F.; Savadori, L. Decision making under stress: Mild hypoxia leads to increased risk-taking. Stress 2019, 23, 290–297. [Google Scholar] [CrossRef]
- Pighin, S.; Bonini, N.; Savadori, L.; Hadjichristidis, C.; Antonetti, T.; Schena, F. Decision making under hypoxia: Oxygen depletion increases risk seeking for losses but not for gains. Judgm. Decis. Mak. 2012, 7, 472. [Google Scholar]
- Pramsohler, S.; Wimmer, S.; Kopp, M.; Gatterer, H.; Faulhaber, M.; Burtscher, M.; Netzer, N.C. Normobaric hypoxia overnight impairs cognitive reaction time. BMC Neurosci. 2017, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Burns, K.; Fennell, C.; Kim, J.H.; Gunstad, J.; Glickman, E.; McDaniel, J. The Influence of Exercise on Cognitive Performance in Normobaric Hypoxia. High Alt. Med. Biol. 2015, 16, 298–305. [Google Scholar] [CrossRef]
- Seo, Y.; Gerhart, H.D.; Stavres, J.; Fennell, C.; Draper, S.; Glickman, E.L. Normobaric Hypoxia and Submaximal Exercise Effects on Running Memory and Mood State in Women. Aerosp. Med. Hum. Perform. 2017, 88, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Barker-Collo, S.L.; Connell, C.J.; Gant, N. Acute hypoxic gas breathing severely impairs cognition and task learning in humans. Physiol. Behav. 2015, 142, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.B.; Corbett, J.; McMorris, T.; Young, J.S.; Dicks, M.; Ando, S.; Thelwell, R.C.; Tipton, M.J.; Costello, J.T. Cognitive performance is associated with cerebral oxygenation and peripheral oxygen saturation, but not plasma catecholamines, during graded normobaric hypoxia. Exp. Physiol. 2019, 104, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- De Bels, D.; Pierrakos, C.; Bruneteau, A.; Reul, F.; Crevecoeur, Q.; Marrone, N.; Vissenaeken, D.; Borgers, G.; Balestra, C.; Honoré, P.M.; et al. Variation of Cognitive Function During a Short Stay at Hypobaric Hypoxia Chamber (Altitude: 3842 M). Front. Physiol. 2019, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Iwazaki, M.; Sasao, G.; Nagai, A.; Ebihara, A.; Iwamoto, T.; Kuwahira, I. Hypobaric hypoxia is not a direct dyspnogenic factor in healthy individuals at rest. Respir. Physiol. Neurobiol. 2015, 218, 28–31. [Google Scholar] [CrossRef]
- Pavlicek, V.; Schirlo, C.; Nebel, A.; Regard, M.; Koller, E.A.; Brugger, P. Cognitive and emotional processing at high altitude. Aviat. Space Environ. Med. 2005, 76, 28–33. [Google Scholar]
- Conway, A.R.; Kane, M.J.; Engle, R.W. Working memory capacity and its relation to general intelligence. Trends Cogn. Sci. 2003, 7, 547–552. [Google Scholar] [CrossRef]
- Lim, J.; Dinges, D.F. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 2008, 1129, 305–322. [Google Scholar] [CrossRef]
- Schear, J.M.; Sato, S.D. Effects of visual acuity and visual motor speed and dexterity on cognitive test performance. Arch. Clin. Neuropsychol. 1989, 4, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Mediation of adult age differences in cognition by reductions in working memory and speed of processing. Psychol. Sci. 1991, 2, 179–183. [Google Scholar] [CrossRef]
- Jones, R.N.; Gallo, J.J. Dimensions of the Mini-Mental State Examination among community dwelling older adults. Psychol. Med. 2000, 30, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Bernasch, D.; Weymann, J.; Holle, R.; Bartsch, P. Acute mountain sickness: Influence of susceptibility, preexposure, and ascent rate. Med. Sci. Sports Exerc. 2002, 34, 1886–1891. [Google Scholar] [CrossRef]
- Roach, R.C.; Hackett, P.H.; Oelz, O.; Bärtsch, P.; Luks, A.M.; MacInnis, M.J.; Baillie, J.K.; Achatz, E.; Albert, E.; Andrews, J.; et al. The 2018 Lake Louise Acute Mountain Sickness Score. High Alt. Med. Biol. 2018, 19, 4–6. [Google Scholar] [CrossRef]
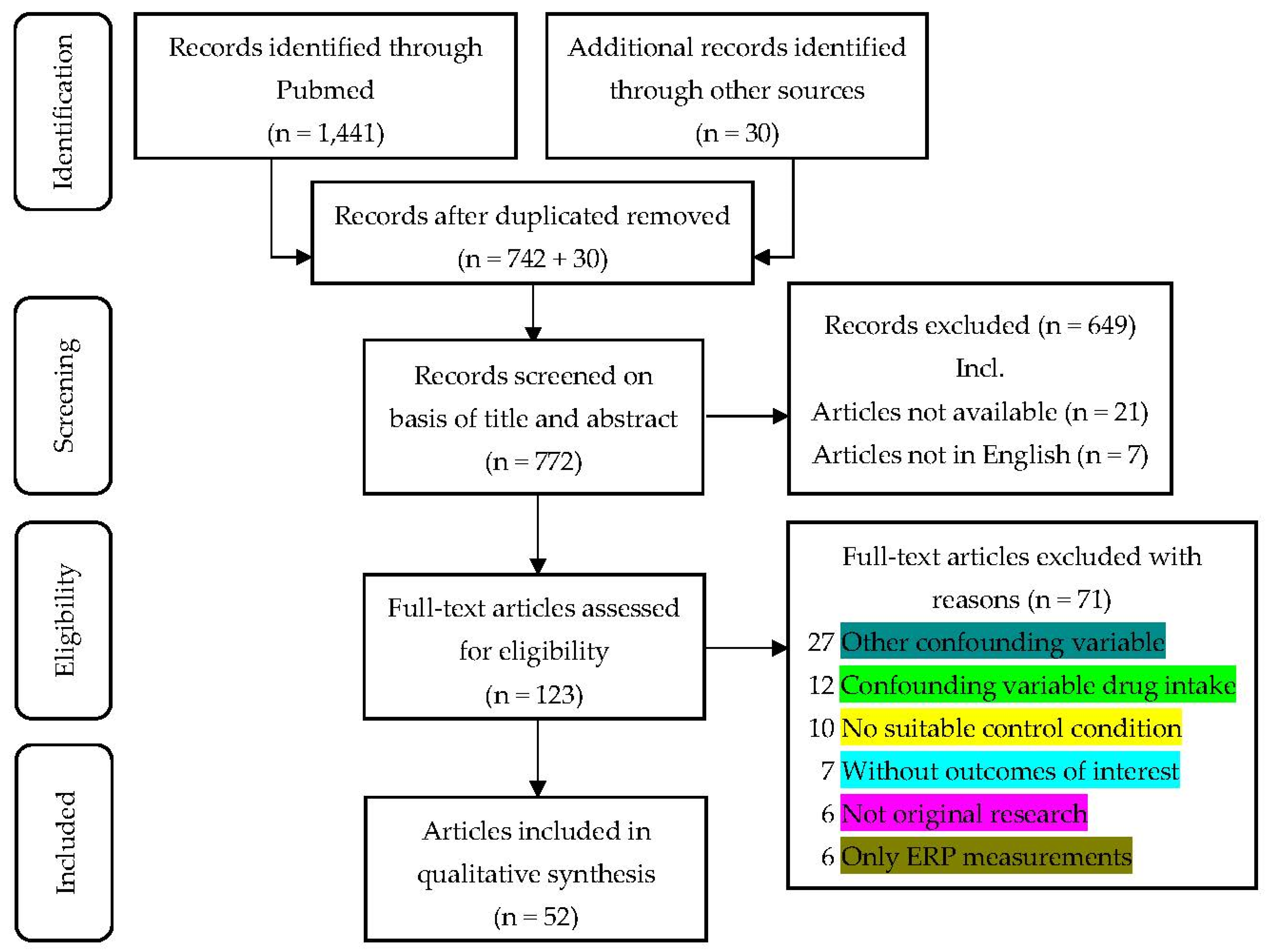


| Field Studies (n = 29) | ||
|---|---|---|
| Passive ascent only (n = 8) | Davranche et al. (2016) [24] Frost et al. (2021) [43] Gibbons et al. (2020) [44] Latshang et al. (2013) [38] Pun, Guadagni et al. (2018) *1 [31] and Pun, Hartmann et al. (2018) *1 [32] Roach et al. (2014) *2 [33] and Subudhi et al. (2014) *2 [34] Schlaepfer, Bärtsch, and Fisch (1992) ** [37] Shi et al. (2016) [45] | |
| Passive and active ascent (n = 21) | Bjursten et al. (2010) [46] Bonnon, Noël-Jorand, and Therme (1999) [47] Dykiert et al. (2010) [48] Falla et al. (2021) [49] Griva et al. (2017) [50] Harris, Cleland, Collie, and McCrory (2009) [25] Issa et al. (2016) [23] Karinen and Tuomisto (2017) [40] Kramer, Coyne, and Strayer (1993) [28] Lefferts et al. (2019) [51] Lefferts et al. (2020) [52] Limmer and Platen (2018) ** [36] Malle, Ginon, and Bourrilhon (2016) [39] Merz et al. (2013) [53] Nelson et al. (1990) [54] Pelamatti, Pascotto, and Semenza (2003) [55] Petiet, Townes, Brooks, and Kramer (1988) [56] Phillips, Griswold, and Pace (1963) [57] Phillips and Pace (1966) [58] Weigle et al. (2007) [59] Zhang et al. (2013) [60] | |
| Laboratory studies (n = 23) | ||
| Breathing of hypoxic gas mixture (n = 7) | Altbäcker et al. (2019) [61] Chroboczek, Kostrzewa, Micielska, Grzywacz, and Laskowski (2021) [62] Kourtidou-Papadeli et al. (2008) [41] Loprinzi et al. (2019) [63] Ochi et al. (2018) [64] Schlaepfer, Bärtsch, and Fisch (1992) ** [37] Stepanek et al. (2013) [65] | |
| Chamber studies (n = 16) | ||
| Normobaric hypoxia (n = 11) | De Aquino Lemos et al. (2012) [66] Limmer and Platen (2018) ** [36] Niedermeier et al. (2017) [67] Parker, Manley, Shand, O’Hara, and Mellor (2017) [68] Pighin, Bonini, Hadjichristidis, Schena, and Savadori (2019) [69] Pighin et al. (2012) [70] Pramsohler et al. (2017) [71] Seo et al. (2015) [72] Seo et al. (2017) [73] Turner, Barker-Collo, Connell, and Gant (2015) [74] Williams et al. (2019) [75] | |
| Hypobaric hypoxia (n = 5) | Abraini, Bouquet, Joulia, Nicolas, and Kriem (1998) [42] Asmaro, Mayall, and Ferguson (2013) [27] De Bels et al. (2019) [76] Nakano et al. (2015) [77] Pavlicek et al. (2005) [78] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bliemsrieder, K.; Weiss, E.M.; Fischer, R.; Brugger, H.; Sperner-Unterweger, B.; Hüfner, K. Cognition and Neuropsychological Changes at Altitude—A Systematic Review of Literature. Brain Sci. 2022, 12, 1736. https://doi.org/10.3390/brainsci12121736
Bliemsrieder K, Weiss EM, Fischer R, Brugger H, Sperner-Unterweger B, Hüfner K. Cognition and Neuropsychological Changes at Altitude—A Systematic Review of Literature. Brain Sciences. 2022; 12(12):1736. https://doi.org/10.3390/brainsci12121736
Chicago/Turabian StyleBliemsrieder, Kathrin, Elisabeth Margarete Weiss, Rainald Fischer, Hermann Brugger, Barbara Sperner-Unterweger, and Katharina Hüfner. 2022. "Cognition and Neuropsychological Changes at Altitude—A Systematic Review of Literature" Brain Sciences 12, no. 12: 1736. https://doi.org/10.3390/brainsci12121736
APA StyleBliemsrieder, K., Weiss, E. M., Fischer, R., Brugger, H., Sperner-Unterweger, B., & Hüfner, K. (2022). Cognition and Neuropsychological Changes at Altitude—A Systematic Review of Literature. Brain Sciences, 12(12), 1736. https://doi.org/10.3390/brainsci12121736







