Immunohistochemical Expression of p27Kip1, p57Kip2, Cyclin D1, Nestin, and Ki-67 in Ependymoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry
2.2. Statistical Methods
2.3. Ethics
3. Results
3.1. Clinical Characteristics
3.2. Tumoral Characteristics
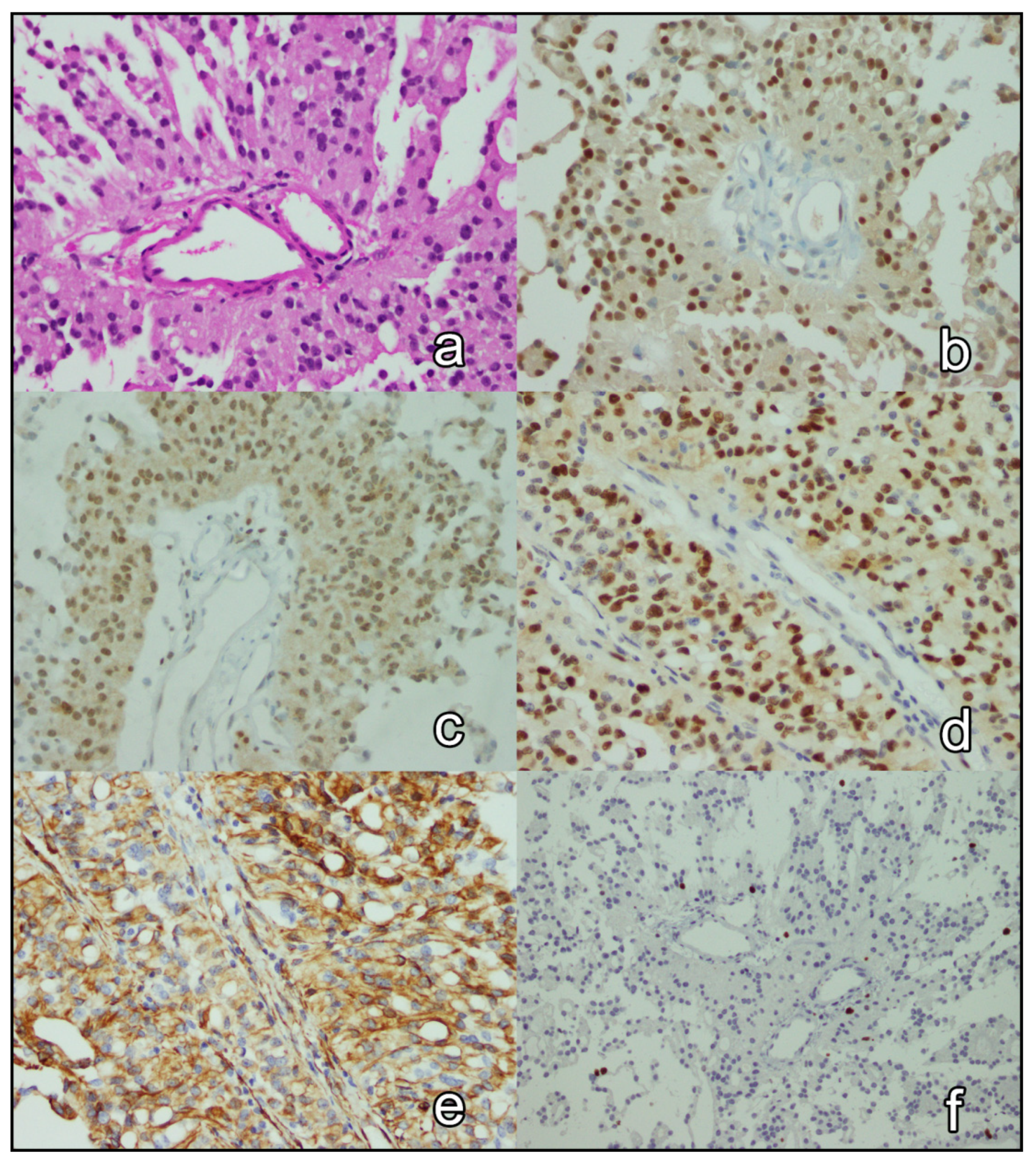
3.3. Immunohistochemical Analysis
3.4. Survival Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chamberlain, M.C. Ependymomas. Curr. Neurol. Neurosci. Rep. 2003, 3, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncol. 2018, 20 (Suppl. S4), iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellison, D.W.; Kocak, M.; Figarella-Branger, D.; Felice, G.; Catherine, G.; Pietsch, T.; Frappaz, D.; Massimino, M.; Grill, J.; Boyett, J.M.; et al. Histopathological grading of pediatric ependymoma: Reproducibility and clinical relevance in European trial cohorts. J. Negat. Results Biomed. 2011, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Mack, S.C.; Ramaswamy, V.; Smith, C.A.; Witt, H.; Smith, A.; Hansford, J.R.; von Hoff, K.; Wright, K.D.; Hwang, E.; et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol. 2017, 133, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in Cancer. Annu. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Pezuk, J.A.; Salomão, K.B.; Baroni, M.; Pereira, C.A.; Geron, L.; Brassesco, M.S. Aberrantly expressed microRNAs and their implications in childhood central nervous system tumors. Cancer Metastasis Rev. 2019, 38, 813–828. [Google Scholar] [CrossRef]
- Ahram, M.; Amarin, J.Z.; Suradi, H.H.; Abdelhamid, S.S.; Makhamreh, M.M.; Bawadi, R.M.; Al-Hussaini, M. Association of MicroRNAs with the Clinicopathologic Characteristics of Ependymoma. J. Mol. Neurosci. 2018, 66, 307–313. [Google Scholar] [CrossRef]
- Medina, R.; Zaidi, S.K.; Liu, C.G.; Stein, J.L.; van Wijnen, A.J.; Croce, C.M.; Stein, G.S. MicroRNAs 221 and 222 bypass quiescence and compromise cell survival. Cancer Res. 2008, 68, 2773–2780. [Google Scholar] [CrossRef] [Green Version]
- Bencivenga, D.; Caldarelli, I.; Stampone, E.; Mancini, F.P.; Balestrieri, M.L.; Della Ragione, F.; Borriello, A. p27Kip1 and human cancers: A reappraisal of a still enigmatic protein. Cancer Lett. 2017, 403, 354–365. [Google Scholar] [CrossRef]
- Besson, A.; Dowdy, S.F.; Roberts, J.M. CDK inhibitors: Cell cycle regulators and beyond. Dev. Cell 2008, 14, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Fu, Z.; Wu, P.; Zheng, D.; Zhang, X. The clinicopathological and prognostic significance of P27kip in hepatocellular carcinoma patients: A systemic review and meta-analysis. Gene 2020, 734, 144351. [Google Scholar] [CrossRef] [PubMed]
- Fornari, F.; Gramantieri, L.; Ferracin, M.; Veronese, A.; Sabbioni, S.; Calin, G.A.; Grazi, G.L.; Giovannini, C.; Croce, C.M.; Bolondi, L.; et al. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 2008, 27, 5651–5661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Willingham, T.; Shuford, M.; Nisen, P.D. Tumor suppression and inhibition of aneuploid cell accumulation in human brain tumor cells by ectopic overexpression of the cyclin-dependent kinase inhibitor p27KIP1. J. Clin. Investig. 1996, 97, 1983–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumatsu, S.; Tamiya, T.; Ono, Y.; Abe, T.; Matsumoto, K.; Furuta, T.; Ohmoto, T. Expression of cell cycle regulator p27Kip1 is correlated with survival of patients with astrocytoma. Clin. Cancer Res. 1999, 5, 551–557. [Google Scholar]
- Kirla, R.M.; Haapasalo, H.K.; Kalimo, H.; Salminen, E.K. Low expression of p27 indicates a poor prognosis in patients with high-grade astrocytomas. Cancer 2003, 97, 644–648. [Google Scholar] [CrossRef]
- Pesutić-Pisac, V.; Punda, A.; Gluncić, I.; Bedeković, V.; Pranić-Kragić, A.; Kunac, N. Cyclin D1 and p27 expression as prognostic factor in papillary carcinoma of thyroid: Association with clinicopathological parameters. Croat. Med. J. 2008, 49, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Khoo, M.L.; Beasley, N.J.; Ezzat, S.; Freeman, J.L.; Asa, S.L. Overexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2002, 87, 1814–1818. [Google Scholar] [CrossRef]
- Rosenberg, E.; Demopoulos, R.I.; Zeleniuch-Jacquotte, A.; Yee, H.; Sorich, J.; Speyer, J.L.; Newcomb, E.W. Expression of cell cycle regulators p57(KIP2), cyclin D1, and cyclin E in epithelial ovarian tumors and survival. Hum. Pathol. 2001, 32, 808–813. [Google Scholar] [CrossRef]
- Guan, G.; Bakr, M.M.; Firth, N.; Love, R.M. Expression of cyclin D1 correlates with p27KIP1 and regulates the degree of oral dysplasia and squamous cell carcinoma differentiation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Zamecnik, J.; Snuderl, M.; Eckschlager, T.; Chanova, M.; Hladikova, M.; Tichy, M.; Kodet, R. Pediatric intracranial ependymomas: Prognostic relevance of histological, immunohistochemical, and flow cytometric factors. Mod. Pathol. 2003, 16, 980–991. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, F.G.; Marie, S.K.; Uno, M.; Matushita, H.; Taricco, M.A.; Teixeira, M.J.; Rosemberg, S.; Oba-Shinjo, S.M. Immunohistochemical expression of cyclin D1 is higher in supratentorial ependymomas and predicts relapses in gross total resection cases. Neuropathology 2015, 35, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Milde, T.; Hielscher, T.; Witt, H.; Kool, M.; Mack, S.C.; Deubzer, H.E.; Oehme, I.; Lodrini, M.; Benner, A.; Taylor, M.D.; et al. Nestin expression identifies ependymoma patients with poor outcome. Brain Pathol. 2012, 22, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Fischer, I.; Höftberger, R.; Birner, P.; Slavc, I.; Dieckmann, K.; Czech, T.; Budka, H.; Hainfellner, J. Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am. J. Surg. Pathol. 2004, 28, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Sunabori, T.; Tokunaga, A.; Nagai, T.; Sawamoto, K.; Okabe, M.; Miyawaki, A.; Matsuzaki, Y.; Miyata, T.; Okano, H. Cell-cycle-specific nestin expression coordinates with morphological changes in embryonic cortical neural progenitors. J. Cell Sci. 2008, 121, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Attiga, F.; Mansour, A. Cancer care in Jordan. Hematol. Oncol Stem Cell Ther. 2015, 8, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Ellison, D.W.; Aldape, K.D.; Capper, D.; Fouladi, M.; Gilbert, M.R.; Gilbertson, R.J.; Hawkins, C.; Merchant, T.E.; Pajtler, K.; Venneti, S.; et al. cIMPACT-NOW update 7: Advancing the molecular classification of ependymal tumors. Brain Pathol. 2020, 30, 863–866. [Google Scholar] [CrossRef]
- Korshunov, A.; Golanov, A.; Timirgaz, V. Immunohistochemical markers for intracranial ependymoma recurrence. An analysis of 88 cases. J. Neurol. Sci. 2000, 177, 72–82. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Stefanaki, K.; Moschovi, M.; Patereli, A.; Prodromou, N.; Karentzou, O. Immunohistochemical expression of cell cycle/apoptosis regulators and epidermal growth factor receptor in pediatric intracranial ependymomas. J. Child. Neurol. 2011, 26, 195–198. [Google Scholar] [CrossRef]
- Schiffer, D.; Bortolotto, S.; Bosone, I.; Cancelli, I.; Cavalla, P.; Schiffer, P.; Piva, R. Cell-cycle inhibitor p27/Kip-1 expression in non-astrocytic and non-oligodendrocytic human nervous system tumors. Neurosci. Lett. 1999, 264, 29–32. [Google Scholar] [CrossRef]
- Wu, J.; Armstrong, T.S.; Gilbert, M.R. Biology and management of ependymomas. Neuro-Oncol. 2016, 18, 902–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pateras, I.S.; Apostolopoulou, K.; Niforou, K.; Kotsinas, A.; Gorgoulis, V.G. p57KIP2: “Kip”ing the cell under control. Mol. Cancer Res. 2009, 7, 1902–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaishi, K.; Nakayama, J.; Sakai, K.; Kobayashi, T.; Rutka, J.T. Antigen p57/Kip2 as a potential negative regulator of human astrocytoma growth. J. Clin. Neurosci. 2009, 16, 1615–1618. [Google Scholar] [CrossRef] [Green Version]
- Musgrove, E.A.; Caldon, C.E.; Barraclough, J.; Stone, A.; Sutherland, R.L. Cyclin D as a therapeutic target in cancer. Nat. Rev. Cancer 2011, 11, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.; Lange, S. Adjusting for multiple testing--when and how? J. Clin. Epidemiol. 2001, 54, 343–349. [Google Scholar] [CrossRef]


| Antibody | Clone | Retrieval | Concentration | Company |
|---|---|---|---|---|
| p27Kip1 | SX53G8 | CC1 standard (64 min) | Ready-to-use | Roche |
| p57Kip2 | Kp10 | CC1 standard (64 min) | Ready-to-use | Roche |
| Cyclin D1 | SP4 | CC1 standard (64 min) | Ready-to-use | Roche |
| Nestin | NE029 | CC1 mild (52 min) | Ready-to-use | Quartett |
| Ki-67 | MIB-1 | CC1 mild (48 min) | 1:200 | Dako |
| p27 | p57 | Cyclin D1 | Nestin | Ki-67 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| −, n (%) | +, n (%) | −, n (%) | +, n (%) | −, n (%) | +, n (%) | −, n (%) | +, n (%) | −, n (%) | +, n (%) | |
| Age group | ||||||||||
| <18 years | 7 (43.8) | 30 (61.2) | 21 (50.0) | 16 (69.6) | 28 (53.8) | 4 (69.2) | 9 (42.9) | 28 (63.6) | 25 (51.0) | 12 (75.0) |
| ≥18 years | 9 (56.2) | 19 (38.8) | 21 (50.0) | 7 (30.4) | 24 (46.2) | 9 (30.8) | 12 (57.1) | 16 (36.4) | 24 (49.0) | 4 (25.0) |
| p value | 0.22 | 0.13 | 0.32 | 0.11 | 0.093 | |||||
| Anatomic site | ||||||||||
| Supratentorium | 3 (18.8) | 12 (24.5) | 11 (26.2) | 4 (17.4) | 10 (19.2) | 5 (38.5) | 1 (4.8) | 14 (31.8) | 8 (16.3) | 7 (43.8) |
| Infratentorium | 10 (62.5) | 20 (40.8) | 18 (42.9) | 12 (52.2) | 23 (44.2) | 7 (53.8) | 10 (47.6) | 20 (45.5) | 23 (46.9) | 7 (43.8) |
| Spine | 3 (18.8) | 17 (34.7) | 13 (31.0) | 7 (30.4) | 19 (36.5) | 1 (7.7) | 10 (47.6) | 10 (22.7) | 18 (36.7) | 2 (12.5) |
| p value | 0.30 | 0.68 | 0.096 | 0.025 | 0.044 | |||||
| Anatomic site, collapsed | ||||||||||
| Intracranium | 13 (81.2) | 32 (65.3) | 29 (69.0) | 16 (69.6) | 33 (63.5) | 12 (92.3) | 11 (52.4) | 34 (77.3) | 31 (63.3) | 14 (87.5) |
| Spine | 3 (18.8) | 17 (34.7) | 13 (31.0) | 7 (30.4) | 19 (36.5) | 1 (7.7) | 10 (47.6) | 10 (22.7) | 18 (36.7) | 2 (12.5) |
| p value | 0.23 | 0.97 | 0.044 | 0.042 | 0.068 | |||||
| Metastasis at presentation | ||||||||||
| No | 15 (100.0) | 33 (78.6) | 31 (86.1) | 17 (81.0) | 40 (87.0) | 8 (72.7) | 15 (83.3) | 33 (83.3) | 36 (87.8) | 12 (75.0) |
| Yes | 0 (0.0) | 9 (21.4) | 5 (13.9) | 4 (19.0) | 6 (13.0) | 3 (27.3) | 3 (16.7) | 6 (16.7) | 5 (12.2) | 4 (25.0) |
| p value | 0.051 | 0.61 | 0.24 | 0.90 | 0.23 | |||||
| Recurrence | ||||||||||
| No | 10 (66.7) | 23 (54.8) | 21 (58.3) | 12 (47.1) | 30 (65.2) | 3 (27.3) | 11 (61.1) | 22 (56.4) | 24 (58.5) | 9 (56.2) |
| Yes | 5 (33.3) | 19 (45.2) | 15 (41.7) | 9 (42.9) | 16 (34.8) | 8 (72.7) | 7 (38.9) | 17 (43.6) | 17 (41.5) | 7 (43.8) |
| p value | 0.42 | 0.93 | 0.022 | 0.74 | 0.88 | |||||
| Mitotic count | ||||||||||
| <3 | 11 (68.8) | 37 (75.5) | 31 (73.8) | 17 (26.2) | 41 (78.8) | 7 (53.8) | 21 (100.0) | 27 (61.4) | 42 (85.7) | 6 (37.5) |
| ≥3 | 5 (31.2) | 12 (24.5) | 11 (73.9) | 6 (26.1) | 11 (21.2) | 6 (46.2) | 0 (0.0) | 17 (38.6) | 7 (14.3) | 10 (62.5) |
| p value | 0.59 | 0.99 | 0.067 | <0.001 | <0.001 | |||||
| Microvascular proliferation | ||||||||||
| Absent | 8 (50.0) | 29 (59.2) | 22 (52.4) | 15 (47.6) | 32 (61.5) | 5 (38.5) | 15 (71.4) | 22 (50.0) | 33 (67.3) | 4 (25.0) |
| Present | 8 (50.0) | 20 (40.8) | 20 (65.2) | 8 (34.8) | 20 (38.5) | 8 (61.5) | 6 (28.6) | 22 (50.0) | 16 (32.7) | 12 (75.0) |
| p value | 0.52 | 0.32 | 0.13 | 0.10 | 0.003 | |||||
| Necrosis | ||||||||||
| Absent | 7 (43.8) | 17 (34.7) | 19 (45.2) | 5 (21.7) | 20 (38.5) | 4 (30.8) | 11 (52.4) | 13 (29.5) | 23 (46.9) | 1 (6.2) |
| Present | 9 (56.2) | 32 (65.3) | 23 (54.8) | 18 (78.3) | 32 (61.5) | 9 (69.2) | 10 (47.6) | 31 (70.5) | 26 (53.1) | 15 (93.8) |
| p value | 0.51 | 0.06 | 0.61 | 0.074 | 0.003 | |||||
| Type of necrosis | ||||||||||
| Nonpalisading | 2 (22.2) | 24 (75.0) | 12 (52.2) | 14 (77.8) | 22 (68.8) | 4 (44.4) | 7 (70.0) | 19 (61.3) | 19 (73.1) | 7 (46.7) |
| Pseudopalisading | 7 (77.8) | 8 (25.0) | 11 (47.8) | 4 (22.2) | 10 (31.2) | 5 (55.6) | 3 (30.0) | 12 (38.7) | 7 (26.9) | 8 (53.3) |
| p value | 0.004 | 0.09 | 0.18 | 0.62 | 0.091 | |||||
| Nodules | ||||||||||
| Absent | 7 (63.6) | 27 (81.8) | 20 (74.1) | 14 (82.4) | 29 (76.3) | 5 (83.3) | 12 (70.6) | 22 (81.5) | 30 (78.9) | 4 (66.7) |
| Present | 4 (36.4) | 6 (18.2) | 7 (25.9) | 3 (17.6) | 9 (23.7) | 1 (16.7) | 5 (29.4) | 5 (18.5) | 8 (21.1) | 2 (33.3) |
| p value | 0.21 | 0.52 | 0.70 | 0.40 | 0.50 | |||||
| Grade | ||||||||||
| II | 12 (75.0) | 39 (79.6) | 32 (76.2) | 19 (82.6) | 44 (84.6) | 7 (53.8) | 21 (100.0) | 30 (68.2) | 45 (91.8) | 6 (37.5) |
| III | 4 (25.0) | 10 (20.4) | 10 (23.8) | 4 (17.4) | 8 (15.4) | 6 (46.2) | 0 (0.0) | 14 (31.8) | 4 (8.2) | 10 (62.5) |
| p value | 0.70 | 0.55 | 0.016 | 0.004 | <0.001 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqneibi, S.; Nazzal, J.; Owda, B.; Sultan, H.; Amoudi, R.; Amarin, J.Z.; Al-Ghnimat, S.; Ahram, M.; Al-Hussaini, M. Immunohistochemical Expression of p27Kip1, p57Kip2, Cyclin D1, Nestin, and Ki-67 in Ependymoma. Brain Sci. 2022, 12, 282. https://doi.org/10.3390/brainsci12020282
Iqneibi S, Nazzal J, Owda B, Sultan H, Amoudi R, Amarin JZ, Al-Ghnimat S, Ahram M, Al-Hussaini M. Immunohistochemical Expression of p27Kip1, p57Kip2, Cyclin D1, Nestin, and Ki-67 in Ependymoma. Brain Sciences. 2022; 12(2):282. https://doi.org/10.3390/brainsci12020282
Chicago/Turabian StyleIqneibi, Shahad, Jamil Nazzal, Basma Owda, Hala Sultan, Runa Amoudi, Justin Z. Amarin, Sura Al-Ghnimat, Mamoun Ahram, and Maysa Al-Hussaini. 2022. "Immunohistochemical Expression of p27Kip1, p57Kip2, Cyclin D1, Nestin, and Ki-67 in Ependymoma" Brain Sciences 12, no. 2: 282. https://doi.org/10.3390/brainsci12020282







