Autocrine Neuromodulation and Network Activity Patterns in the Locus Coeruleus of Newborn Rat Slices
Abstract
1. Introduction
1.1. Connectivity of the Adult LC
1.2. Modular Organization of Adult LC
1.3. Current Knowledge of Neonatal LC Network Properties
1.4. Aim of Present Study
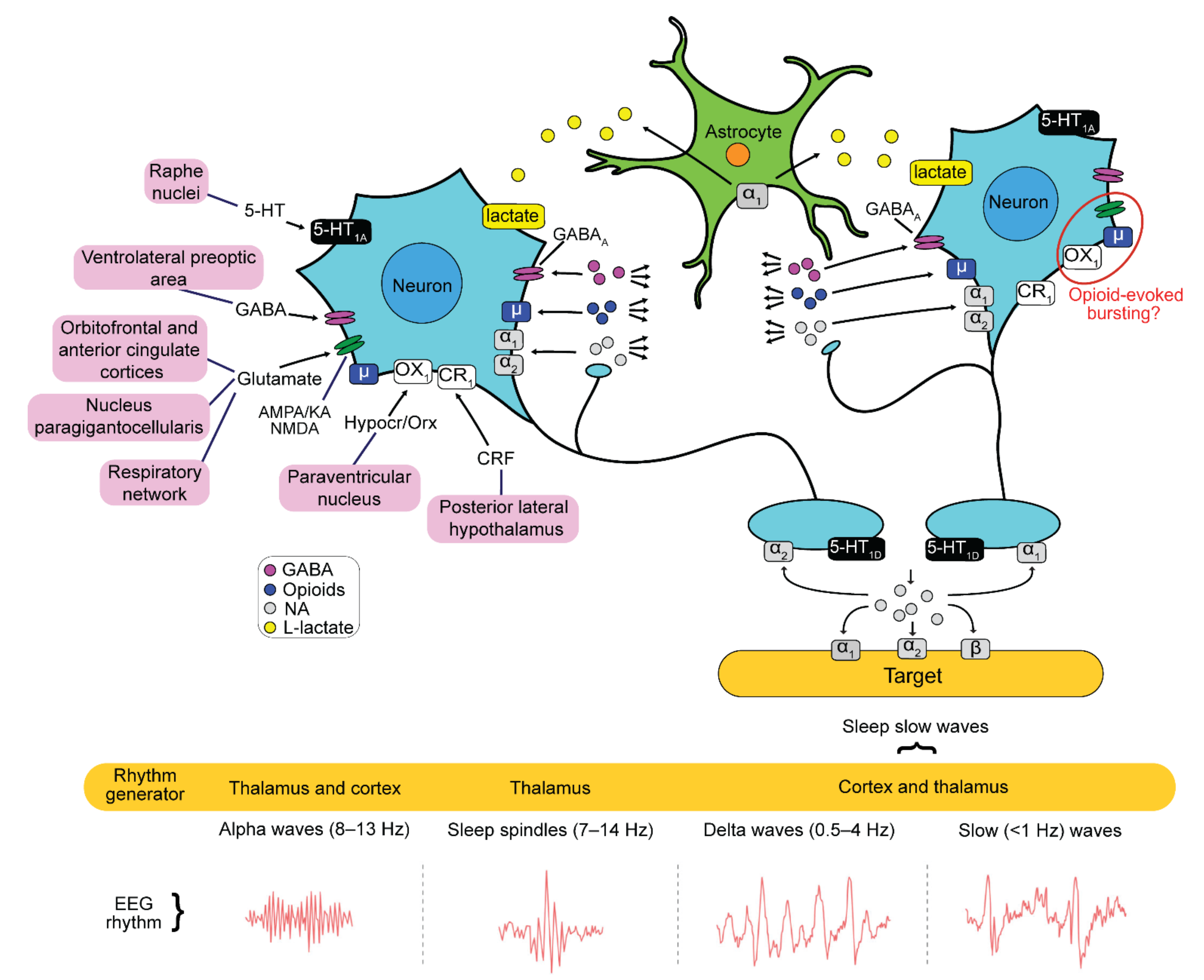
2. Results
2.1. Anatomical and Intrinsic LC Properties
2.2. Role of iGluR in Neonatal LC Rhythm
2.3. Role of Inhibition on Neonatal LC Rhythm
2.4. µR- and ɑ2(Auto)R-Mediated LFP Pattern Transformations
2.5. Cai Changes in LC Neurons and Astrocytes
3. Discussion
3.1. Intrinsic Neonatal LC Properties
3.2. Independence of LC Network Rhythm on Anion Channel-Mediated Inhibition and iGluR
3.3. iGluR-Mediated LFP Pattern Transformation
3.4. LFP Pattern Transformations by μ-Opioid and α2 Receptors
3.5. Cai Responses in the Neonatal LC
3.6. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foote, S.L.; Bloom, F.E.; Aston-Jones, G. Nucleus Locus Ceruleus: New Evidence of Anatomical and Physiological Specificity. Physiol. Rev. 1983, 63, 844–914. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The Locus Coeruleus–Noradrenergic System: Modulation of Behavioral State and State-Dependent Cognitive Processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the Locus Coeruleus-Norepinephrine System. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, K.S.; Spiller, P.F.; da Silva, M.P.; Kuntze, L.B.; Paton, J.F.R.; Machado, B.H.; Moraes, D.J.A. Locus Coeruleus as a Vigilance Centre for Active Inspiration and Expiration in Rats. Sci. Rep. 2018, 8, 15654. [Google Scholar] [CrossRef]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus Coeruleus: A New Look at the Blue Spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Nakamura, S.; Sakaguchi, T. Development and Plasticity of the Locus Coeruleus: A Review of Recent Physiological and Pharmacological Experimentation. Prog. Neurobiol. 1990, 34, 505–526. [Google Scholar] [CrossRef]
- Benarroch, E.E. The Locus Ceruleus Norepinephrine System: Functional Organization and Potential Clinical Significance. Neurology 2009, 73, 1699–1704. [Google Scholar] [CrossRef]
- Delaville, C.; De Deurwaerdère, P.; Benazzouz, A. Noradrenaline and Parkinson’s Disease. Front. Syst. Neurosci. 2011, 5, 31. [Google Scholar] [CrossRef]
- Halassa, M.M.; Haydon, P.G. Integrated Brain Circuits: Astrocytic Networks Modulate Neuronal Activity and Behavior. Ann. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef]
- Teschemacher, A.G.; Kasparov, S. Dialogue Between Astrocytes and Noradrenergic Neurons Via l-Lactate. In Noradrenergic Signaling and Astroglia; Vardjan, N., Zorec, R., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 8; pp. 167–182. ISBN 978-0-12-805088-0. [Google Scholar]
- Crunelli, V.; Lőrincz, M.L.; Connelly, W.M.; David, F.; Hughes, S.W.; Lambert, R.C.; Leresche, N.; Errington, A.C. Dual Function of Thalamic Low-Vigilance State Oscillations: Rhythm-Regulation and Plasticity. Nat. Rev. Neurosci. 2018, 19, 107–118. [Google Scholar] [CrossRef]
- Loughlin, S.E.; Foote, S.L.; Bloom, F.E. Efferent Projections of Nucleus Locus Coeruleus: Topographic Organization of Cells of Origin Demonstrated by Three-Dimensional Reconstruction. Neuroscience 1986, 18, 291–306. [Google Scholar] [CrossRef]
- Christie, M.J. Generators of Synchronous Activity of the Locus Coeruleus during Development. Sem. Cell Dev. Biol. 1997, 8, 29–34. [Google Scholar] [CrossRef]
- Totah, N.K.B.; Logothetis, N.K.; Eschenko, O. Noradrenergic Ensemble-Based Modulation of Cognition over Multiple Timescales. Brain Res. 2019, 1709, 50–66. [Google Scholar] [CrossRef]
- Chandler, D.J.; Gao, W.-J.; Waterhouse, B.D. Heterogeneous Organization of the Locus Coeruleus Projections to Prefrontal and Motor Cortices. Proc. Natl. Acad. Sci. USA 2014, 111, 6816–6821. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Bondy, B.; Zhong, W.; Oginsky, M.F.; Wu, Y.; Johnson, C.M.; Zhang, S.; Cui, N.; Jiang, C. Identification of a Group of GABAergic Neurons in the Dorsomedial Area of the Locus Coeruleus. PLoS ONE 2016, 11, e0146470. [Google Scholar] [CrossRef]
- Li, Y.; Hickey, L.; Perrins, R.; Werlen, E.; Patel, A.A.; Hirschberg, S.; Jones, M.W.; Salinas, S.; Kremer, E.J.; Pickering, A.E. Retrograde Optogenetic Characterization of the Pontospinal Module of the Locus Coeruleus with a Canine Adenoviral Vector. Brain Res. 2016, 1641, 274–290. [Google Scholar] [CrossRef]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.J.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular Organization of the Brainstem Noradrenaline System Coordinates Opposing Learning States. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef]
- Totah, N.K.; Neves, R.M.; Panzeri, S.; Logothetis, N.K.; Eschenko, O. The Locus Coeruleus Is a Complex and Differentiated Neuromodulatory System. Neuron 2018, 99, 1055–1068.e6. [Google Scholar] [CrossRef]
- Waselenchuk, Q.; Rawal, B.; Ballanyi, K. Gi/o Signaling-Related Activity Pattern Transformations in the Locus Coeruleus of Newborn Rath Slices. University of Alberta: Edmonton, AB, Canada, 2022; manuscript in preparation. [Google Scholar]
- Oyamada, Y.; Ballantyne, D.; Mückenhoff, K.; Scheid, P. Respiration-Modulated Membrane Potential and Chemosensitivity of Locus Coeruleus Neurones in the in Vitro Brainstem-Spinal Cord of the Neonatal Rat. J. Physiol. 1998, 513, 381–398. [Google Scholar] [CrossRef]
- Kantor, C.; Panaitescu, B.; Kuribayashi, J.; Ruangkittisakul, A.; Jovanovic, I.; Leung, V.; Lee, T.-F.; MacTavish, D.; Jhamandas, J.H.; Cheung, P.-Y.; et al. Spontaneous Neural Network Oscillations in Hippocampus, Cortex, and Locus Coeruleus of Newborn Rat and Piglet Brain Slices. In Isolated Central Nervous System Circuits; Neuromethods; Ballanyi, K., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 73, pp. 315–356. ISBN 978-1-62703-019-9. [Google Scholar]
- Olson, L.; Seiger, Å. Early Prenatal Ontogeny of Central Monoamine Neurons in the Rat: Fluorescence Histochemical Observations. Z. Anat. Entwickl. Gesch. 1972, 137, 301–316. [Google Scholar] [CrossRef]
- Lauder, J.M.; Bloom, F.E. Ontogeny of Monoamine Neurons in the Locus Coeruleus, Raphe Nuclei and Substantia Nigra of the Rat. I. Cell Differentiation. J. Comp. Neurol. 1974, 155, 469–481. [Google Scholar] [CrossRef]
- Kimura, F.; Nakamura, S. Locus Coeruleus Neurons in the Neonatal Rat: Electrical Activity and Responses to Sensory Stimulation. Dev. Brain Res. 1985, 23, 301–305. [Google Scholar] [CrossRef]
- Marshall, K.C.; Christie, M.J.; Finlayson, P.G.; Williams, J.T. Developmental Aspects of the Locus Coeruleus-Noradrenaline System. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 1991; Volume 88, pp. 173–185. ISBN 978-0-444-81394-7. [Google Scholar]
- Patel, M.; Joshi, B. Modeling the Evolving Oscillatory Dynamics of the Rat Locus Coeruleus through Early Infancy. Brain Res. 2015, 1618, 181–193. [Google Scholar] [CrossRef]
- Nakamura, S.; Kimura, F.; Sakaguchi, T. Postnatal Development of Electrical Activity in the Locus Ceruleus. J. Neurophysiol. 1987, 58, 510–524. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nakamura, S. Some in Vivo Electrophysiological Properties of Locus Coeruleus Neurones in Fetal Rats. Exp. Brain Res. 1987, 68, 122–130. [Google Scholar] [CrossRef]
- Williams, J.T.; North, R.A.; Shefner, S.A.; Nishi, S.; Egan, T.M. Membrane Properties of Rat Locus Coeruleus Neurones. Neuroscience 1984, 13, 137–156. [Google Scholar] [CrossRef]
- Williams, J.T.; Marshall, K.C. Membrane Properties and Adrenergic Responses in Locus Coeruleus Neurons of Young Rats. J. Neurosci. 1987, 7, 3687–3694. [Google Scholar] [CrossRef]
- Christie, M.J.; Williams, J.T.; North, R.A. Electrical Coupling Synchronizes Subthreshold Activity in Locus Coeruleus Neurons in Vitro from Neonatal Rats. J. Neurosci. 1989, 9, 3584–3589. [Google Scholar] [CrossRef]
- Christi, M.J.; Jelinek, H.F. Dye-Coupling among Neurons of the Rat Locus Coeruleus during Postnatal Development. Neuroscience 1993, 56, 129–137. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Chow, C.C.; Van Bockstaele, E.J.; Williams, J.T. Frequency-Dependent Synchrony in Locus Ceruleus: Role of Electrotonic Coupling. Proc. Natl. Acad. Sci. USA 2002, 99, 4032–4036. [Google Scholar] [CrossRef]
- Rawal, B.; Rancic, V.; Ballanyi, K. TARP Mediation of Accelerated and More Regular Locus Coeruleus Network Bursting in Neonatal Rat Brain Slices. Neuropharmacology 2019, 148, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rancic, V.; Rawal, B.; Panaitescu, B.; Ruangkittisakul, A.; Ballanyi, K. Suction Electrode Recording in Locus Coeruleus of Newborn Rat Brain Slices Reveals Network Bursting Comprising Summated Non-Synchronous Spiking. Neurosci. Lett. 2018, 671, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Panaitescu, B.; Ballanyi, K. Methylxanthine Acceleration and Countering of Opioid depression of Synchronous Locus Coeruleus Network Oscillations in Newborn Rat Brain Slices. University of Alberta: Edmonton, AB, Canada, 2022; to be submitted. [Google Scholar]
- Williams, J.T.; Bobker, D.H.; Harris, G.C. Synaptic Potentials in Locus Coeruleus Neurons in Brain Slices. In Progress in Brain Research; Neurobiology of the Locus Coeruleus; Barnes, C.D., Pompeiano, O., Eds.; Elsevier: Cambridge, MA, USA, 1991; Chapter 11; Volume 88, pp. 167–172. [Google Scholar]
- Melnychuk, M.C.; Robertson, I.H.; Plini, E.R.G.; Dockree, P.M. A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State. Brain Sci. 2021, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Poil, S.-S.; Jansen, R.; van Aerde, K.; Timmerman, J.; Brussaard, A.B.; Mansvelder, H.D.; Linkenkaer-Hansen, K. Fast Network Oscillations In Vitro Exhibit a Slow Decay of Temporal Auto-Correlations. Eur. J. Neurosci. 2011, 34, 394–403. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The Origin of Extracellular Fields and Currents—EEG, ECoG, LFP and Spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Einevoll, G.T.; Kayser, C.; Logothetis, N.K.; Panzeri, S. Modelling and Analysis of Local Field Potentials for Studying the Function of Cortical Circuits. Nat. Rev. Neurosci. 2013, 14, 770–785. [Google Scholar] [CrossRef]
- Garaschuk, O.; Linn, J.; Eilers, J.; Konnerth, A. Large-Scale Oscillatory Calcium Waves in the Immature Cortex. Nat. Neurosci. 2000, 3, 452–459. [Google Scholar] [CrossRef]
- Ishimatsu, M.; Williams, J.T. Synchronous Activity in Locus Coeruleus Results from Dendritic Interactions in Pericoerulear Regions. J. Neurosci. 1996, 16, 5196–5204. [Google Scholar] [CrossRef]
- Ballantyne, D.; Andrzejewski, M.; Mückenhoff, K.; Scheid, P. Rhythms, Synchrony and Electrical Coupling in the Locus Coeruleus. Respir. Physiol. Neurobiol. 2004, 143, 199–214. [Google Scholar] [CrossRef]
- Sanchez-Padilla, J.; Guzman, J.N.; Ilijic, E.; Kondapalli, J.; Galtieri, D.J.; Yang, B.; Schieber, S.; Oertel, W.; Wokosin, D.; Schumacker, P.T.; et al. Mitochondrial Oxidant Stress in Locus Coeruleus Is Regulated by Activity and Nitric Oxide Synthase. Nat. Neurosci. 2014, 17, 832–840. [Google Scholar] [CrossRef]
- Matschke, L.A.; Bertoune, M.; Roeper, J.; Snutch, T.P.; Oertel, W.H.; Rinné, S.; Decher, N. A Concerted Action of L- and T-Type Ca2+ Channels Regulates Locus Coeruleus Pacemaking. Mol. Cell. Neurosci. 2015, 68, 293–302. [Google Scholar] [CrossRef]
- Matschke, L.A.; Rinné, S.; Snutch, T.P.; Oertel, W.H.; Dolga, A.M.; Decher, N. Calcium-Activated SK Potassium Channels Are Key Modulators of the Pacemaker Frequency in Locus Coeruleus Neurons. Mol. Cell. Neurosci. 2018, 88, 330–341. [Google Scholar] [CrossRef]
- Rawal, B.; Ballanyi, K. AMPA Receptor-Evoked Transformation of Locus Coeruleus Network Bursting in Newborn Rat Slices into Faster and More Regular Spindle-Shaped Oscillations. University of Alberta, Edmonton, AB, Canada. 2022; to be submitted. [Google Scholar]
- Rawal, B.; Rancic, V.; Ballanyi, K. NMDA Enhances and Glutamate Attenuates Synchronization of Spontaneous Phase-Locked Locus Coeruleus Network Bursting in Newborn Rat Brain Slices. University of Alberta: Edmonton, AB, Canada, 2022; to be submitted. [Google Scholar]
- Alvarez-Maubecin, V.; García-Hernández, F.; Williams, J.T.; Van Bockstaele, E.J. Functional Coupling between Neurons and Glia. J. Neurosci. 2000, 20, 4091–4098. [Google Scholar] [CrossRef]
- Rash, J.E.; Olson, C.O.; Davidson, K.G.V.; Yasumura, T.; Kamasawa, N.; Nagy, J.I. Identification of Connexin36 in Gap Junctions between Neurons in Rodent Locus Coeruleus. Neuroscience 2007, 147, 938–956. [Google Scholar] [CrossRef][Green Version]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Sipilä, S.T.; Kaila, K. GABAergic Control of CA3-Driven Network Events in the Developing Hippocampus. In Inhibitory Regulation of Excitatory Neurotransmission; Darlison, M.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 99–121. ISBN 978-3-540-72602-9. [Google Scholar]
- Jackson, A.C.; Nicoll, R.A. Stargazin (TARP-2) Is Required for Compartment-Specific AMPA Receptor Trafficking and Synaptic Plasticity in Cerebellar Stellate Cells. J. Neurosci. 2011, 31, 3939–3952. [Google Scholar] [CrossRef]
- Greger, I.H.; Watson, J.F.; Cull-Candy, S.G. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 2017, 94, 713–730. [Google Scholar] [CrossRef]
- Maher, M.P.; Matta, J.A.; Gu, S.; Seierstad, M.; Bredt, D.S. Getting a Handle on Neuropharmacology by Targeting Receptor-Associated Proteins. Neuron 2017, 96, 989–1001. [Google Scholar] [CrossRef]
- Ruangkittisakul, A.; Ballanyi, K. Methylxanthine Reversal of Opioid-Evoked Inspiratory Depression via Phosphodiesterase-4 Blockade. Respir. Physiol. Neurobiol. 2010, 172, 94–105. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, W. Excitatory Amino Acid Receptors Are Involved in Morphine-Induced Synchronous Oscillatory Discharges in the Locus Coeruleus of Rats. Eur. J. Pharmacol. 2005, 528, 73–78. [Google Scholar] [CrossRef]
- Ballanyi, K.; Eschenko, O. Anesthesia-Related LC Neuron Bursting in Adult Rats In Vivo. University of Alberta, Edmonton, AB, Canada. 2022; manuscript in preparation. [Google Scholar]
- Ruangkittisakul, A.; Okada, Y.; Oku, Y.; Koshiya, N.; Ballanyi, K. Fluorescence Imaging of Active Respiratory Networks. Respir. Physiol. Neurobiol. 2009, 168, 26–38. [Google Scholar] [CrossRef]
- Carrillo-Reid, L.; Yang, W.; Kang Miller, J.; Peterka, D.S.; Yuste, R. Imaging and Optically Manipulating Neuronal Ensembles. Ann. Rev. Biophys. 2017, 46, 271–293. [Google Scholar] [CrossRef]
- Pires, J.; Nelissen, R.; Mansvelder, H.D.; Meredith, R.M. Spontaneous Synchronous Network Activity in the Neonatal Development of MPFC in Mice. Dev. Neurobiol. 2021, 81, 207–225. [Google Scholar] [CrossRef]
- Tang, F.; Lane, S.; Korsak, A.; Paton, J.F.R.; Gourine, A.V.; Kasparov, S.; Teschemacher, A.G. Lactate-Mediated Glia-Neuronal Signalling in the Mammalian Brain. Nat. Commun. 2014, 5, 3284. [Google Scholar] [CrossRef]
- Jefferys, J.G. Experimental Neurobiology of Epilepsies. Curr. Opin. Neurol. 1994, 7, 113–122. [Google Scholar] [CrossRef]
- Bartos, M.; Vida, I.; Jonas, P. Synaptic Mechanisms of Synchronized Gamma Oscillations in Inhibitory Interneuron Networks. Nat. Rev. Neurosci. 2007, 8, 45–56. [Google Scholar] [CrossRef]
- Gonzalez, O.J.A.; van Aerde, K.I.; van Elburg, R.A.J.; Poil, S.-S.; Mansvelder, H.D.; Linkenkaer-Hansen, K.; van Pelt, J.; van Ooyen, A. External Drive to Inhibitory Cells Induces Alternating Episodes of High- and Low-Amplitude Oscillations. PLoS Comp. Biol. 2012, 8, e1002666. [Google Scholar] [CrossRef]
- Avramiea, A.-E.; Masood, A.; Mansvelder, H.D.; Linkenkaer-Hansen, K. Long-Range Amplitude Coupling Is Optimized for Brain Networks That Function at Criticality. J. Neurosci. 2022, 42, 2221–2233. [Google Scholar] [CrossRef]
- Funk, G.D.; Smith, J.C.; Feldman, J.L. Generation and Transmission of Respiratory Oscillations in Medullary Slices: Role of Excitatory Amino Acids. J. Neurophysiol. 1993, 70, 1497–1515. [Google Scholar] [CrossRef]
- Bracci, E.; Ballerini, L.; Nistri, A. Localization of Rhythmogenic Networks Responsible for Spontaneous Bursts Induced by Strychnine and Bicuculline in the Rat Isolated Spinal Cord. J. Neurosci. 1996, 16, 7063–7076. [Google Scholar] [CrossRef]
- Ballanyi, K.; Ruangkittisakul, A. Structure–Function Analysis of Rhythmogenic Inspiratory Pre-Bötzinger Complex Networks in “Calibrated” Newborn Rat Brainstem Slices. Respir. Physiol. Neurobiol. 2009, 168, 158–178. [Google Scholar] [CrossRef] [PubMed]
- Lieske, S.P.; Thoby-Brisson, M.; Telgkamp, P.; Ramirez, J.M. Reconfiguration of the Neural Network Controlling Multiple Breathing Patterns: Eupnea, Sighs and Gasps. Nat. Neurosci. 2000, 3, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Devor, A.; Yarom, Y. Electrotonic Coupling in the Inferior Olivary Nucleus Revealed by Simultaneous Double Patch Recordings. J. Neurophysiol. 2002, 87, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Ruangkittisakul, A.; Sharopov, S.; Kantor, C.; Kuribayashi, J.; Mildenberger, E.; Luhmann, H.J.; Kilb, W.; Ballanyi, K. Methylxanthine-Evoked Perturbation of Spontaneous and Evoked Activities in Isolated Newborn Rat Hippocampal Networks. Neuroscience 2015, 301, 106–120. [Google Scholar] [CrossRef]
- Ahmadi-Soleimani, S.M.; Ghaemi-Jandabi, M.; Azizi, H.; Semnanian, S. Orexin Type 1 Receptor Antagonism in Lateral Paragigantocellularis Nucleus Attenuates Naloxone Precipitated Morphine Withdrawal Symptoms in Rats. Neurosci. Lett. 2014, 558, 62–66. [Google Scholar] [CrossRef]
- Ahmadi-Soleimani, S.M.; Azizi, H.; Gompf, H.S.; Semnanian, S. Role of Orexin Type-1 Receptors in Paragiganto-Coerulear Modulation of Opioid Withdrawal and Tolerance: A Site Specific Focus. Neuropharmacology 2017, 126, 25–37. [Google Scholar] [CrossRef]
- Kaeidi, A.; Azizi, H.; Javan, M.; Soleimani, S.M.A.; Fathollahi, Y.; Semnanian, S. Direct Facilitatory Role of Paragigantocellularis Neurons in Opiate Withdrawal-Induced Hyperactivity of Rat Locus Coeruleus Neurons: An In Vitro Study. PLoS ONE 2015, 10, e0134873. [Google Scholar] [CrossRef]
- Mohammad Ahmadi Soleimani, S.; Azizi, H.; Mirnajafi-Zadeh, J.; Semnanian, S. Orexin Type 1 Receptor Antagonism in Rat Locus Coeruleus Prevents the Analgesic Effect of Intra-LC Met-Enkephalin Microinjection. Pharmacol. Biochem. Behav. 2015, 136, 102–106. [Google Scholar] [CrossRef]
- Van Drongelen, W.; Koch, H.; Elsen, F.P.; Lee, H.C.; Mrejeru, A.; Doren, E.; Marcuccilli, C.J.; Hereld, M.; Stevens, R.L.; Ramirez, J.-M. Role of Persistent Sodium Current in Bursting Activity of Mouse Neocortical Networks In Vitro. J. Neurophysiol. 2006, 96, 2564–2577. [Google Scholar] [CrossRef]
- Alreja, M.; Aghajanian, G.K. Pacemaker Activity of Locus Coeruleus Neurons: Whole-Cell Recordings in Brain Slices Show Dependence on CAMP and Protein Kinase A. Brain Res. 1991, 556, 339–343. [Google Scholar] [CrossRef]
- Andrade, R.; Aghajanian, G.K. Locus Coeruleus Activity in Vitro: Intrinsic Regulation by a Calcium-Dependent Potassium Conductance but Not Alpha 2-Adrenoceptors. J. Neurosci. 1984, 4, 161–170. [Google Scholar] [CrossRef]
- Taccola, G.; Olivieri, D.; D’Angelo, G.; Blackburn, P.; Secchia, L.; Ballanyi, K. A1 Adenosine Receptor Modulation of Chemically and Electrically Evoked Lumbar Locomotor Network Activity in Isolated Newborn Rat Spinal Cords. Neuroscience 2012, 222, 191–204. [Google Scholar] [CrossRef]
- Feldman, J.L.; Smith, J.C. Cellular Mechanisms Underlying Modulation of Breathing Pattern in Mammalsa. Ann. N. Y. Acad. Sci. 1989, 563, 114–130. [Google Scholar] [CrossRef]
- Shao, X.M.; Feldman, J.L. Respiratory Rhythm Generation and Synaptic Inhibition of Expiratory Neurons in Pre-Bötzinger Complex: Differential Roles of Glycinergic and GABAergic Neural Transmission. J. Neurophysiol. 1997, 77, 1853–1860. [Google Scholar] [CrossRef]
- Brockhaus, J.; Ballanyi, K. Synaptic Inhibition in the Isolated Respiratory Network of Neonatal Rats. Eur. J. Neurosci. 1998, 10, 3823–3839. [Google Scholar] [CrossRef]
- Janczewski, W.A.; Tashima, A.; Hsu, P.; Cui, Y.; Feldman, J.L. Role of Inhibition in Respiratory Pattern Generation. J. Neurosci. 2013, 33, 5454–5465. [Google Scholar] [CrossRef]
- Rancic, V.; Gosgnach, S. Recent Insights into the Rhythmogenic Core of the Locomotor CPG. Int. J. Mol. Sci. 2021, 22, 1394. [Google Scholar] [CrossRef]
- Olpe, H.-R.; Steinmann, M.W.; Hall, R.G.; Brugger, F.; Pozza, M.F. GABAA and GABAB Receptors in Locus Coeruleus: Effects of Blockers. Eur. J. Pharmacol. 1988, 149, 183–185. [Google Scholar] [CrossRef]
- Olpe, H.-R.; Steinmann, M.W.; Brugger, F.; Pozza, M.F. Excitatory Amino Acid Receptors in Rat Locus Coeruleus: An Extracellular in Vitro Study. Naunyn Schmiedeberg’s Arch. Pharmacol. 1989, 339, 312–314. [Google Scholar] [CrossRef]
- Zamalloa, T.; Bailey, C.P.; Pineda, J. Glutamate-Induced Post-Activation Inhibition of Locus Coeruleus Neurons Is Mediated by AMPA/Kainate Receptors and Sodium-Dependent Potassium Currents. Brit. J. Pharmacol. 2009, 156, 649–661. [Google Scholar] [CrossRef]
- Singewald, N.; Philippu, A. Release of Neurotransmitters in the Locus Coeruleus. Prog. Neurobiol. 1998, 56, 237–267. [Google Scholar] [CrossRef]
- Tsintsadze, V.; Minlebaev, M.; Suchkov, D.; Cunningham, M.; Khazipov, R. Ontogeny of Kainate-Induced Gamma Oscillations in the Rat CA3 Hippocampus in Vitro. Front. Cell. Neurosci. 2015, 9, 195. [Google Scholar] [CrossRef]
- Lüthi, A. Sleep Spindles: Where They Come From, What They Do. Neuroscientist 2014, 20, 243–256. [Google Scholar] [CrossRef]
- Sullivan, D.; Mizuseki, K.; Sorgi, A.; Buzsaki, G. Comparison of Sleep Spindles and Theta Oscillations in the Hippocampus. J. Neurosci. 2014, 34, 662–674. [Google Scholar] [CrossRef]
- Posłuszny, A. The Contribution of Electrical Synapses to Field Potential Oscillations in the Hippocampal Formation. Front. Neural Circ. 2014, 8, 32. [Google Scholar]
- Turecek, J.; Yuen, G.S.; Han, V.Z.; Zeng, X.-H.; Bayer, K.U.; Welsh, J.P. NMDA Receptor Activation Strengthens Weak Electrical Coupling in Mammalian Brain. Neuron 2014, 81, 1375–1388. [Google Scholar] [CrossRef][Green Version]
- Neuman, R.S.; Cherubini, E.; Ben-Ari, Y. Endogenous and Network Bursts Induced by N-Methyl-d-Aspartate and Magnesium Free Medium in the CA3 Region of the Hippocampal Slice. Neuroscience 1989, 28, 393–399. [Google Scholar] [CrossRef]
- Zhu, Z.-T.; Munhall, A.; Shen, K.-Z.; Johnson, S.W. Calcium-Dependent Subthreshold Oscillations Determine Bursting Activity Induced by N-Methyl-d-Aspartate in Rat Subthalamic Neurons in Vitro. Eur. J. Neurosci. 2004, 19, 1296–1304. [Google Scholar] [CrossRef]
- Sharifullina, E.; Ostroumov, K.; Grandolfo, M.; Nistri, A. N-Methyl-d-Aspartate Triggers Neonatal Rat Hypoglossal Motoneurons in Vitro to Express Rhythmic Bursting with Unusual Mg2+ Sensitivity. Neuroscience 2008, 154, 804–820. [Google Scholar] [CrossRef]
- Mrejeru, A.; Wei, A.; Ramirez, J.M. Calcium-Activated Non-Selective Cation Currents Are Involved in Generation of Tonic and Bursting Activity in Dopamine Neurons of the Substantia Nigra Pars Compacta. J. Physiol. 2011, 589, 2497–2514. [Google Scholar] [CrossRef]
- North, R.A.; Williams, J.T. On the Potassium Conductance Increased by Opioids in Rat Locus Coeruleus Neurones. J. Physiol. 1985, 364, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Wang, Y.-Y. Common A2- and Opiate Effector Mechanisms in the Locus Coeruleus: Intracellular Studies in Brain Slices. Neuropharmacology 1987, 26, 793–799. [Google Scholar] [CrossRef]
- Metzger, F.; Kulik, A.; Sendtner, M.; Ballanyi, K. Contribution of Ca 2+ -Permeable AMPA/KA Receptors to Glutamate-Induced Ca2+ Rise in Embryonic Lumbar Motoneurons In Situ. J. Neurophysiol. 2000, 83, 50–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; de Lecea, L. Tuning Arousal with Optogenetic Modulation of Locus Coeruleus Neurons. Nat. Neurosci 2010, 13, 1526–1533. [Google Scholar] [CrossRef]
- McCall, J.G.; Al-Hasani, R.; Siuda, E.R.; Hong, D.Y.; Norris, A.J.; Ford, C.P.; Bruchas, M.R. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 2015, 87, 605–620. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hopf, F.W.; Li, S.-B.; de Lecea, L. In Vivo Cell Type-Specific CRISPR Knockdown of Dopamine Beta Hydroxylase Reduces Locus Coeruleus Evoked Wakefulness. Nat. Commun. 2018, 9, 5211. [Google Scholar] [CrossRef]
- Bitzenhofer, S.H.; Ahlbeck, J.; Hanganu-Opatz, I.L. Methodological Approach for Optogenetic Manipulation of Neonatal Neuronal Networks. Front. Cell. Neurosci. 2017, 11, 239. [Google Scholar] [CrossRef]
- Eschenko, O.; Magri, C.; Panzeri, S.; Sara, S.J. Noradrenergic Neurons of the Locus Coeruleus Are Phase Locked to Cortical Up-Down States during Sleep. Cereb. Cortex 2012, 22, 426–435. [Google Scholar] [CrossRef]
- Totah, N.K.; Logothetis, N.K.; Eschenko, O. Synchronous Spiking Associated with Prefrontal High γ Oscillations Evokes a 5-Hz Rhythmic Modulation of Spiking in Locus Coeruleus. J. Neurophysiol. 2021, 125, 1191–1201. [Google Scholar] [CrossRef]
- Ivanov, A.; Aston-Jones, G. Extranuclear Dendrites of Locus Coeruleus Neurons: Activation by Glutamate and Modulation of Activity by Alpha Adrenoceptors. J. Neurophysiol. 1995, 74, 2427–2436. [Google Scholar] [CrossRef]
- Brofiga, M.; Pisano, M.; Raiteri, R.; Massobrio, P. On the Road to the Brain-on-a-Chip: A Review on Strategies, Methods, and Applications. J. Neural Eng. 2021, 18, 041005. [Google Scholar] [CrossRef]
- Sucher, N.J.; Deitcher, D.L. PCR and Patch-Clamp Analysis of Single Neurons. Neuron 1995, 14, 1095–1100. [Google Scholar] [CrossRef]
- Latorre, R.; Aguirre, C.; Rabinovich, M.; Varona, P. Transient Dynamics and Rhythm Coordination of Inferior Olive Spatio-Temporal Patterns. Front. Neural Circ. 2013, 7, 138. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waselenchuk, Q.; Ballanyi, K. Autocrine Neuromodulation and Network Activity Patterns in the Locus Coeruleus of Newborn Rat Slices. Brain Sci. 2022, 12, 437. https://doi.org/10.3390/brainsci12040437
Waselenchuk Q, Ballanyi K. Autocrine Neuromodulation and Network Activity Patterns in the Locus Coeruleus of Newborn Rat Slices. Brain Sciences. 2022; 12(4):437. https://doi.org/10.3390/brainsci12040437
Chicago/Turabian StyleWaselenchuk, Quinn, and Klaus Ballanyi. 2022. "Autocrine Neuromodulation and Network Activity Patterns in the Locus Coeruleus of Newborn Rat Slices" Brain Sciences 12, no. 4: 437. https://doi.org/10.3390/brainsci12040437
APA StyleWaselenchuk, Q., & Ballanyi, K. (2022). Autocrine Neuromodulation and Network Activity Patterns in the Locus Coeruleus of Newborn Rat Slices. Brain Sciences, 12(4), 437. https://doi.org/10.3390/brainsci12040437




