Exploring the Effects of Brain Stimulation on Musical Taste: tDCS on the Left Dorso-Lateral Prefrontal Cortex—A Null Result
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.1.1. Participants
2.1.2. Stimuli
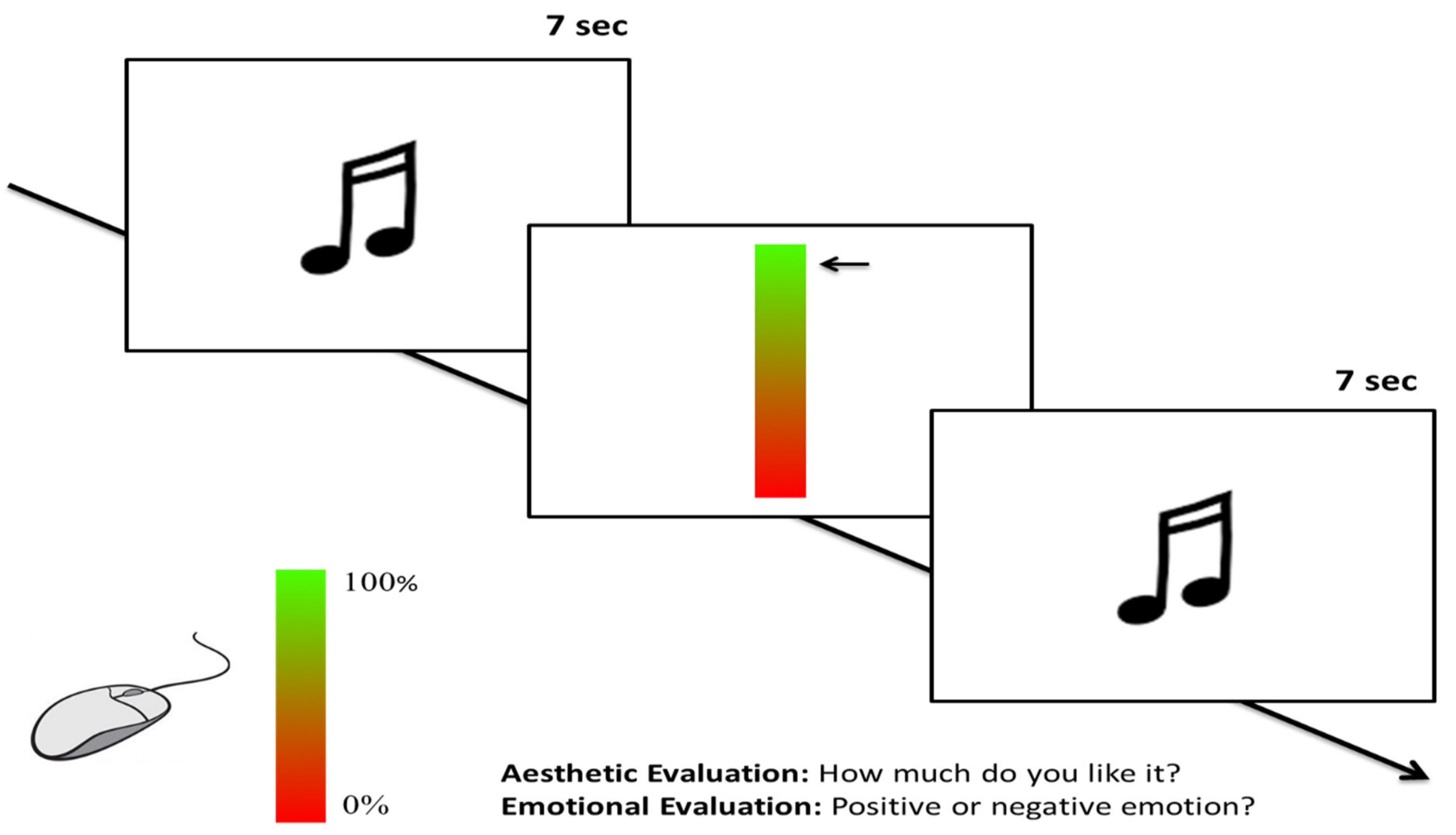
2.1.3. Procedure
2.1.4. Data Analysis
2.2. Experiment 2
2.2.1. Participants
2.2.2. Stimuli
2.2.3. Procedure
3. Results
3.1. Experiment #1
3.1.1. Esthetic Evaluation
Rating Scores
Response Latencies
3.1.2. Emotional Evaluation
Rating Scores
Response Latencies
3.1.3. Brief Discussion
3.2. Experiment 2
3.2.1. Comparison between Esthetic Rating Scores of Experiments #1 and #2
3.2.2. tDCS Side Effects
3.2.3. Rating Scores
3.2.4. Response Latencies
3.2.5. Brief Discussion
4. General Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattaneo, Z.; Lega, C.; Flexas, A.; Nadal, M.; Munar, E.; Cela-Conde, C.J. The world can look better: Enhancing beauty experience with brain stimulation. Soc. Cogn. Affect. Neurosci. 2013, 9, 1713–1721. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Vartanian, O. Neuroaesthetics. Trends Cogn. Sci. 2014, 18, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Skov, M.; Nadal, M. A Farewell to Art: Aesthetics as a Topic in Psychology and Neuroscience. Perspect. Psychol. Sci. 2020, 15, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, Z. Neural correlates of visual aesthetic appreciation: Insights from non-invasive brain stimulation. Exp. Brain Res. 2020, 238, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brattico, E.; Pearce, M. The neuroaesthetics of music. Psychol. Aesthet. Creat. Arts 2013, 7, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Mas-Herrero, E.; Dagher, A.; Zatorre, R.J. Modulating musical reward sensitivity up and down with transcranial magnetic stimulation. Nat. Hum. Behav. 2018, 2, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bever, T.G.; Chiarello, R.J. Cerebral Dominance in Musicians and Nonmusicians. Science 1974, 185, 537–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaab, N.; Tallal, P.; Kim, H.; Lakshminarayanan, K.; Archie, J.J.; Glover, G.H.; Gabrieli, J.D.E. Neural Correlates of Rapid Spectrotemporal Processing in Musicians and Nonmusicians. Ann. N. Y. Acad. Sci. 2005, 1060, 82–88. [Google Scholar] [CrossRef]
- Ohnishi, T.; Matsuda, H.; Asada, T.; Aruga, M.; Hirakata, M.; Nishikawa, M.; Katoh, A.; Imabayashi, E. Functional anatomy of musical perception in musicians. Cereb. Cortex 2001, 11, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Krzyżak, A. Different pattern of auditory processing lateralization in musicians and non-musicians. Acta Neuropsychol. 2021, 19, 105–119. [Google Scholar] [CrossRef]
- Criscuolo, A.; Pando-Naude, V.; Bonetti, L.; Vuust, P.; Brattico, E. Rediscovering the musician’s brain: A systematic review and meta-analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Fedorenko, E.; Patel, A.; Casasanto, D.; Winawer, J.; Gibson, E. Structural integration in language and music: Evidence for a shared system. Mem. Cogn. 2009, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zhong, S.; Cui, Z.; Li, J.; Gong, G.; Dong, Q.; Nan, Y. The lateralized arcuate fasciculus in developmental pitch disorders among mandarin amusics: Left for speech and right for music. Brain Struct. Funct. 2018, 223, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Asano, R.; Boeckx, C.; Seifert, U. Hierarchical control as a shared neurocognitive mechanism for language and music. Cognition 2021, 216, 104847. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, E.G.; Weiss, M.W. Music and Cognitive Abilities. In The Psychology of Music; Deutsch, D., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013; pp. 499–550. [Google Scholar]
- Forgeard, M.; Schlaug, G.; Norton, A.; Rosam, C.; Iyengar, U.; Winner, E. The relation between music and phonological processing in normal-reading children and children with dyslexia. Music Percept. 2008, 25, 383–390. [Google Scholar] [CrossRef]
- Anvari, S.H.; Trainor, L.J.; Woodside, J.; Levy, B.A. Relations among musical skills, phonological processing, and early reading ability in preschool children. J. Exp. Child Psychol. 2002, 83, 111–130. [Google Scholar] [CrossRef]
- Patston, L.M.; Tippett, L.J. The effect of background music on cognitive performance in musicians and nonmusicians. Music Percept. 2011, 29, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Sluming, V.; Brooks, J.; Howard, M.; Downes, J.J.; Roberts, N. Broca’s area supports enhanced visuospatial cognition in orchestral musicians. J. Neurosci. 2007, 27, 3799–3806. [Google Scholar] [CrossRef]
- Jakobson, L.; Lewycky, S.; Kilgour, A.; Stoesz, B. Memory for verbal and visual material in highly trained musicians. Music Percept. 2008, 26, 41–55. [Google Scholar] [CrossRef]
- Moreno, S.; Marques, C.; Santos, A.; Santos, M.; Castro, S.L.; Besson, M. Musical training influences linguistic abilities in 8-year-old children: More evidence for brain plasticity. Cereb. Cortex 2009, 19, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, T.; Trofimovich, P. Phonological memory, attention control, and musical ability: Effects of individual differences on rater judgments of second language speech. Appl. Psycholinguist. 2011, 32, 113–140. [Google Scholar] [CrossRef] [Green Version]
- Gaser, C.; Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 2003, 23, 9240–9245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konecni, V.J. The aesthetic trinity: Awe, being moved, thrills. Bull. Psychol. Arts 2005, 5, 27–44. [Google Scholar]
- Bigand, E.; Vieillard, S.; Madurell, F.; Marozeau, J.; Dacquet, A. Multidimensional scaling of emotional responses to music: The effect of musical expertise and of the duration of the excerpts. Cogn. Emot. 2005, 19, 1113–1139. [Google Scholar] [CrossRef]
- Anglada-Tort, M.; Müllensiefen, D. The repeated recording illusion: The effects of extrinsic and individual difference factors on musical judgments. Music Percept. 2017, 35, 94–117. [Google Scholar] [CrossRef] [Green Version]
- Silvia, P.J.; Nusbaum, E.C. On personality and piloerection: Individual differences in aesthetic chills and other unusual aesthetic experiences. Psychol. Aesthet. Creat. Arts 2011, 5, 208. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Gutyrchik, E.; Bao, Y.; Zaytseva, Y.; Carl, P.; Welker, L.; Pöppel, E.; Reiser, M.; Blautzik, J.; Meindl, T. Differences between musicians and non-musicians in neuro-affective processing of sadness and fear expressed in music. Neurosci. Lett. 2014, 566, 120–124. [Google Scholar] [CrossRef]
- Fredrickson, W.E. Perception of tension in music: Musicians versus nonmusicians. J. Music. Ther. 2000, 37, 40–50. [Google Scholar] [CrossRef]
- Müller, M.; Höfel, L.; Brattico, E.; Jacobsen, T. Aesthetic judgments of music in experts and laypersons—An ERP study. Int. J. Psychophysiol. 2010, 76, 40–51. [Google Scholar] [CrossRef]
- Pearce, M.T. Effects of Expertise on The Cognitive and Neural Processes Involved In Musical Appreciation. In Art, Aesthetics and the Brain; Huston, J.P., Nadal, M., Mora, F., Agnati, L.F., Cela-Conde, C.J., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 319–338. [Google Scholar]
- Juslin, P.N.; Isaksson, S. Subjective criteria for choice and aesthetic judgment of music: A comparison of psychology and music students. Res. Stud. Music. Educ. 2014, 36, 179–198. [Google Scholar] [CrossRef]
- Brattico, E.; Bogert, B.; Alluri, V.; Tervaniemi, M.; Eerola, T.; Jacobsen, T. It’s sad but I like it: The neural dissociation between musical emotions and liking in experts and laypersons. Front. Hum. Neurosci. 2016, 9, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lychner, J.A. A comparison of non-musicians’ and musicians’ aesthetic response to music experienced with and without video. Int. J. Music. Educ. 2008, 26, 21–32. [Google Scholar] [CrossRef]
- Rentfrow, P.J.; Gosling, S.D. The do re mi’s of everyday life: The structure and personality correlates of music preferences. J. Personal. Soc. Psychol. 2003, 84, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosselin, N.; Peretz, I.; Noulhiane, M.; Hasboun, D.; Beckett, C.; Baulac, M.; Samson, S. Impaired recognition of scary music following unilateral temporal lobe excision. Brain 2005, 128, 628–640. [Google Scholar] [CrossRef] [Green Version]
- Mas-Herrero, E.; Marco-Pallares, J.; Lorenzo-Seva, U.; Zatorre, R.J.; Rodriguez-Fornells, A. Individual differences in music reward experiences. Music Percept. 2013, 31, 118–138. [Google Scholar] [CrossRef] [Green Version]
- Nadal, M.; Munar, E.; Capó, M.À.; Rosselló, J.; Cela-Conde, C.J. Towards a framework for the study of the neural correlates of aesthetic preference. Spat. Vis. 2008, 21, 379–396. [Google Scholar]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef] [Green Version]
- Mas-Herrero, E.; Dagher, A.; Farrés-Franch, M.; Zatorre, R.J. Unraveling the temporal dynamics of reward signals in music-induced pleasure with TMS. J. Neurosci. 2021, 41, 3889–3899. [Google Scholar] [CrossRef]
- Zentner, M.; Grandjean, D.; Scherer, K.R. Emotions evoked by the sound of music: Characterization, classification, and measurement. Emotion 2008, 8, 494. [Google Scholar] [CrossRef] [Green Version]
- Sachs, M.E.; Damasio, A.; Habibi, A. The pleasures of sad music: A systematic review. Front. Hum. Neurosci. 2015, 9, 404. [Google Scholar] [CrossRef] [Green Version]
- Salimpoor, V.N.; van den Bosch, I.; Kovacevic, N.; McIntosh, A.R.; Dagher, A.; Zatorre, R.J. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 2013, 340, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Molina, N.; Mas-Herrero, E.; Rodríguez-Fornells, A.; Zatorre, R.J.; Marco-Pallarés, J. Neural correlates of specific musical anhedonia. Proc. Natl. Acad. Sci. USA 2016, 113, E7337–E7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.S.; Strafella, A.P. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE 2009, 4, e6725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Ko, J.H.; Strafella, A.P.; Dagher, A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc. Natl. Acad. Sci. USA 2013, 110, 4422–4427. [Google Scholar] [CrossRef] [Green Version]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2015, 127, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, L.; Koslowsky, M.; Lavidor, M. tDCS polarity effects in motor and cognitive domains: A meta-analytical review. Exp. Brain Res. 2012, 216, 1–10. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Van den Bergh, D.; Van Doorn, J.; Marsman, M.; Draws, T.; Van Kesteren, E.J.; Derks, K.; Dablander, F.; Gronau, Q.F.; Kucharský, Š.; Gupta, A.R.K.N.; et al. A tutorial on conducting and interpreting a Bayesian ANOVA in JASP. LAnnee Psychol. 2020, 120, 73–96. [Google Scholar] [CrossRef]
- Jeffreys, H. The Theory of Probability; Oxford University Press: Oxford, UK, 1961. [Google Scholar]
- Rusjan, P.M.; Barr, M.S.; Farzan, F.; Arenovich, T.; Maller, J.J.; Fitzgerald, P.B.; Daskalakis, Z.J. Optimal Transcranial Magnetic Stimulation Coil Placement for Targeting the Dorsolateral Prefrontal Cortex Using Novel Magnetic Resonance Image-Guided Neuronavigation; Wiley Company: Hoboken, NJ, USA, 2010; Volume 31, pp. 1643–1652. [Google Scholar]
- Kincses, T.Z.; Antal, A.; Nitsche, M.A.; Bártfai, O.; Paulus, W. Facilitation of probabilistic classification learning by transcranial direct current stimulation of the prefrontal cortex in the human. Neuropsychologia 2004, 42, 113–117. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.P.; Silva, M.T.; Paulus, W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef]
- Fecteau, S.; Pascual-Leone, A.; Zald, D.H.; Liguori, P.; Théoret, H.; Boggio, P.S.; Fregni, F. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J. Neurosci. 2007, 27, 6212–6218. [Google Scholar] [CrossRef] [PubMed]
- Moos, K.; Vossel, S.; Weidner, R.; Sparing, R.; Fink, G.R. Modulation of top-down control of visual attention by cathodal tDCS over right IPS. J. Neurosci. 2012, 32, 16360–16368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, R.; Wallace, D.; Fitzgerald, P.B.; Cooper, N.R. Perception of comfort during active and sham transcranial direct current stimulation: A double-blind study. Brain Stimul. 2013, 6, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Jovančević, A.; Milićević, N.; Zdravić-Mihailović, D. Differences in music preferences between musicians and non-musicians. Facta Univ. Ser. Vis. Arts Music 2019, 5, 31–39. [Google Scholar] [CrossRef]
- Fung, C.V. Musicians’ and nonmusicians’ preferences for world musics: Relation to musical characteristics and familiarity. J. Res. Music. Educ. 1996, 44, 60–83. [Google Scholar] [CrossRef]
- McDermott, J.H.; Schultz, A.F.; Undurraga, E.A.; Godoy, R.A. Indifference to dissonance in native Amazonians reveals cultural variation in music perception. Nature 2016, 535, 547–550. [Google Scholar] [CrossRef]
- Trainor, L.J.; Heinmiller, B.M. The development of evaluative responses to music: Infants prefer to listen to consonance over dissonance. Infant Behav. Dev. 1998, 21, 77–88. [Google Scholar] [CrossRef]
- Brattico, E.; Jacobsen, T. Subjective appraisal of music. Ann. N. Y. Acad. Sci. 2009, 1169, 308–317. [Google Scholar] [CrossRef]
- Porflitt, F.; Rosas, R. Core music elements: Rhythmic, melodic and harmonic musicians show differences in cognitive performance. Stud. Psychol. 2020, 41, 532–562. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J.; Kleinsorge, T. Short-term mobilization of processing resources is revealed in the event-related potential. Psychophysiology 2003, 40, 914–923. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Salimpoor, V.N. From perception to pleasure: Music and its neural substrates. Proc. Natl. Acad. Sci. USA 2013, 110 (Suppl. 2), 10430–10437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cela-Conde, C.J.; Lombardo, R.G.; Avise, J.C.; Ayala, F.J. In the Light of Evolution: Volume VII: The Human Mental Machinery; National Academies Press (US): Washington, DC, USA, 2014. [Google Scholar]
- Blood, A.J.; Zatorre, R.J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. USA 2001, 98, 11818–11823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juslin, P.N.; Sloboda, J. (Eds.) Handbook of Music and Emotion: Theory, Research, Applications; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Brattico, E.; Bogert, B.; Jacobsen, T. Toward a neural chronometry for the aesthetic experience of music. Front. Psychol. 2013, 4, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantono, K.; Hamid, N.; Shepherd, D.; Yoo, M.J.; Carr, B.T.; Grazioli, G. The effect of background music on food pleasantness ratings. Psychol. Music 2016, 44, 1111–1125. [Google Scholar] [CrossRef]






| Effects (Liking—Rating Scores) | Statistics Results | p Values |
|---|---|---|
| Judgment | F (2, 76) = 87.16 | p < 0.01, ηp2 = 0.69 |
| Group | F (1, 38) = 0.24 | p = 0.63, ηp2 = 0.01 |
| Group × Judgment | F (2, 76) = 2.85 | p = 0.06, ηp2 = 0.07 |
| Effects (Liking—Response Latencies) | ||
| Judgment | F (2, 76) = 3.35 | p = 0.04, ηp2 = 0.08 |
| Group | F (1, 38) = 4.11 | p = 0.05, ηp2 = 0.1 |
| Group × Judgment | F (2, 76) = 0.16 | p = 0.85, ηp2 = 0.004 |
| Effects (Emotion—Rating Scores) | ||
| Judgment | F (2, 76) = 184.1 | p < 0.01, ηp2 = 0.83 |
| Group | F (1, 38) = 0.57 | p = 0.46, ηp2 = 0.02 |
| Group × Judgment | F (2, 76) = 0.45 | p = 0.65, ηp2 = 0.011 |
| Effects (Emotion—Response Latencies) | ||
| Judgment | F (2, 76) = 10.5 | p < 0.01, ηp2 = 0.22 |
| Group | F (1, 38) = 7.78 | p = 0.01, ηp2 = 0.17 |
| Group × Judgment | F (2, 76) = 0.62 | p = 0.54, ηp2 = 0.02 |
| Effects (Rating Scores) | Statistics Results | p Values |
|---|---|---|
| Condition | F (1, 21) = 1.02 | p = 0.32, ηp2 = 0.05 |
| Session | F (1, 21) = 8.47 | p < 0.01, ηp2 = 0.29 |
| Judgment | F (1, 21) = 92.88 | p < 0.001 ηp2 = 0.82 |
| Session × Judgment | F (1, 21) = 4.51 | p = 0.017 ηp2 = 0.31 |
| Condition × Session | F (1, 21) = 0.08 | p = 0.79, ηp2 < 0.01) |
| Condition × Judgment | F (1, 21) = 0.72 | p = 0.5 ηp2 = 0.07 |
| Session × Condition × Judgment | F (1, 21) = 0.57 | p = 0.57 ηp2 = 0.05 |
| Effects (Response Latencies) | ||
| Condition | F (1, 21) = 0.55 | p = 0.47, ηp2 = 0.03 |
| Session | F (1, 21) = 4.49 | p = 0.046, ηp2 = 0.18 |
| Judgment | F (1, 21) = 6.35 | p < 0.01, ηp2 = 0.23 |
| Session × Judgment | F (1, 21) = 0.07 | p = 0.93, ηp2 = 0.003 |
| Condition × Session | F (1, 21) = 0.89 | p = 0.36, ηp2 = 0.04 |
| Condition × Judgment | F (1, 21) = 0.18 | p = 0.83, ηp2 = 0.01 |
| Session × Condition × Judgment | F (1, 21) = 0.91 | p = 0.41, ηp2 = 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massetti, G.; Lega, C.; Cattaneo, Z.; Gallace, A.; Vallar, G. Exploring the Effects of Brain Stimulation on Musical Taste: tDCS on the Left Dorso-Lateral Prefrontal Cortex—A Null Result. Brain Sci. 2022, 12, 467. https://doi.org/10.3390/brainsci12040467
Massetti G, Lega C, Cattaneo Z, Gallace A, Vallar G. Exploring the Effects of Brain Stimulation on Musical Taste: tDCS on the Left Dorso-Lateral Prefrontal Cortex—A Null Result. Brain Sciences. 2022; 12(4):467. https://doi.org/10.3390/brainsci12040467
Chicago/Turabian StyleMassetti, Gemma, Carlotta Lega, Zaira Cattaneo, Alberto Gallace, and Giuseppe Vallar. 2022. "Exploring the Effects of Brain Stimulation on Musical Taste: tDCS on the Left Dorso-Lateral Prefrontal Cortex—A Null Result" Brain Sciences 12, no. 4: 467. https://doi.org/10.3390/brainsci12040467
APA StyleMassetti, G., Lega, C., Cattaneo, Z., Gallace, A., & Vallar, G. (2022). Exploring the Effects of Brain Stimulation on Musical Taste: tDCS on the Left Dorso-Lateral Prefrontal Cortex—A Null Result. Brain Sciences, 12(4), 467. https://doi.org/10.3390/brainsci12040467







