Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Analytical Procedures
2.3. Statistical Analysis
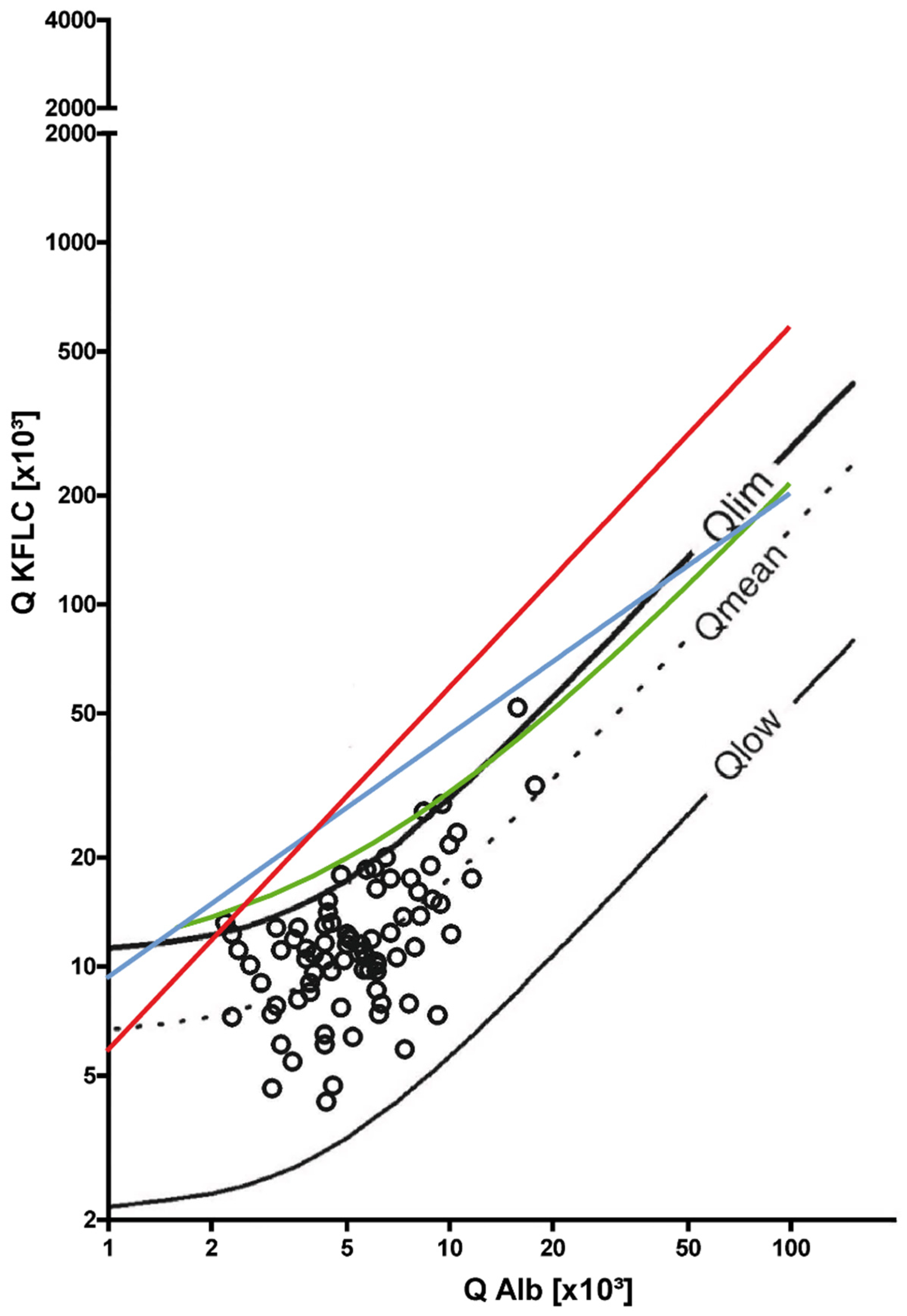
3. Results
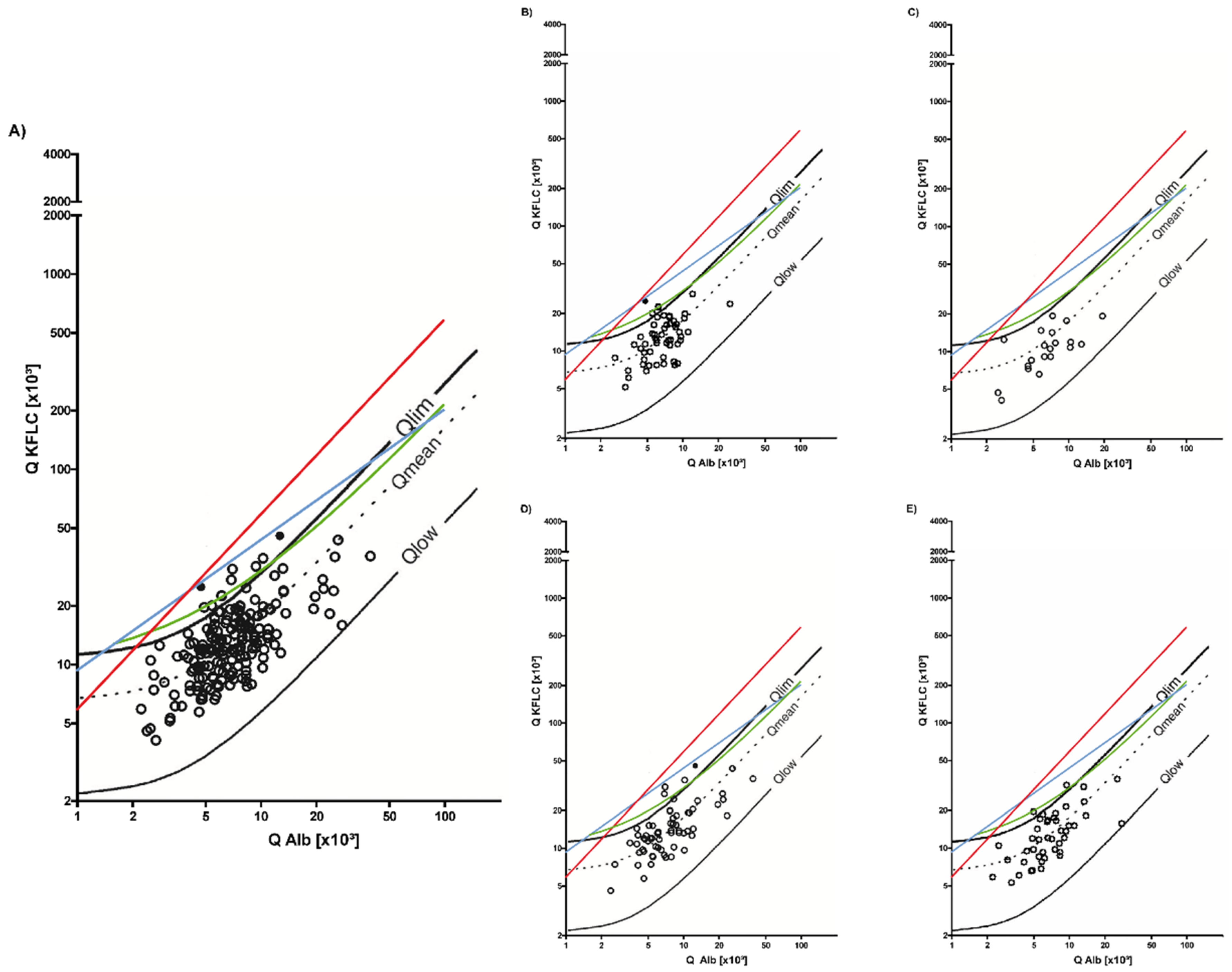
3.1. Symptomatic Controls
3.2. Non-Inflammatory Neurological Diseases
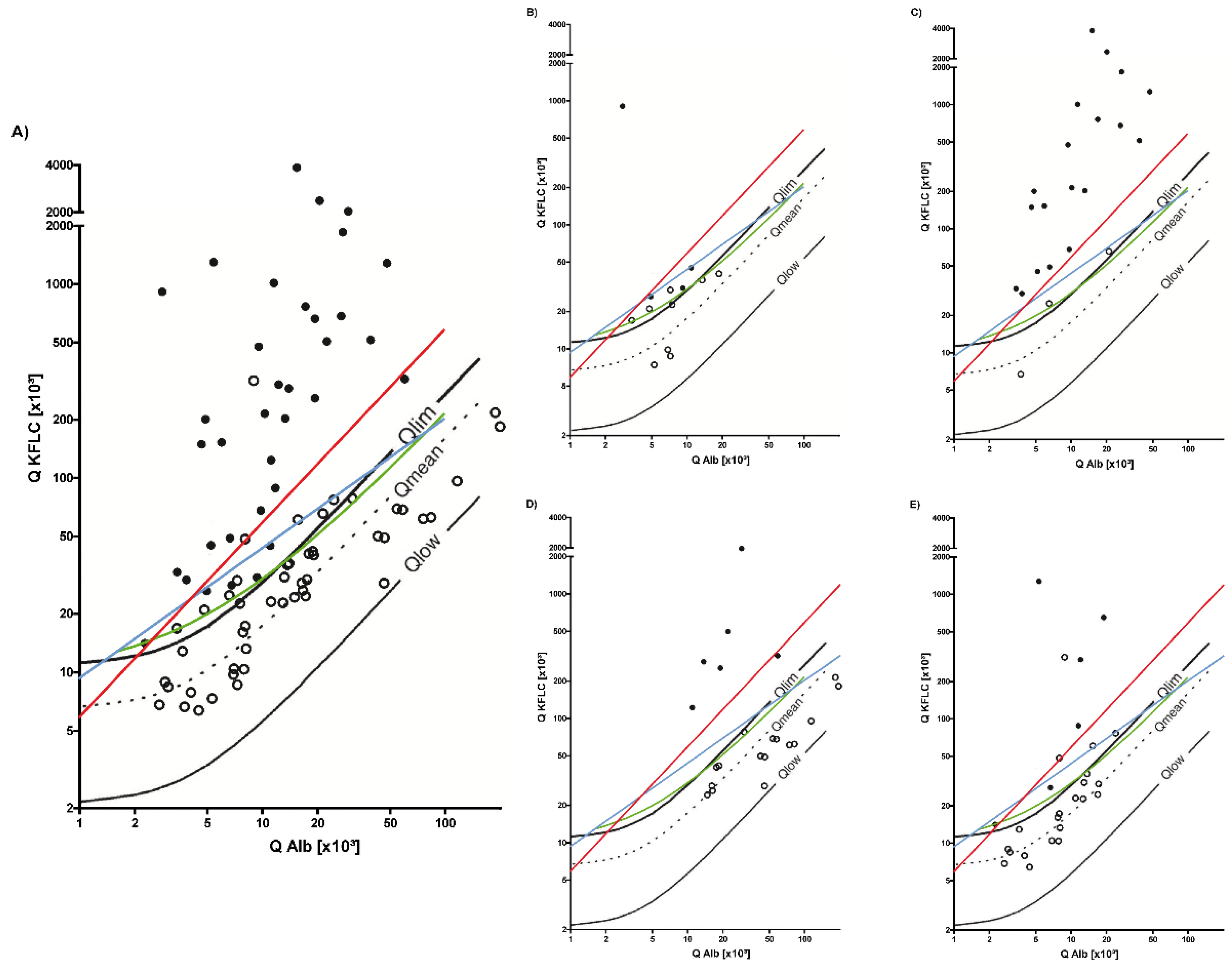
3.3. Inflammatory Neurological Diseases
3.4. Sensitivity and Specificity of KFLC IF According to Reiber and Oligoclonal Band Determination to Distinguish Inflammatory and Non-inflammatory Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Stangel, M.; Fredrikson, S.; Meinl, E.; Petzold, A.; Stüve, O.; Tumani, H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat. Rev. Neurol. 2013, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Luxton, R.W.; McLean, B.N.; Thompson, E.J. Isoelectric focusing versus quantitative measurements in the detection of intrathecal local synthesis of IgG. Clin. Chim. Acta 1990, 187, 297–308. [Google Scholar] [CrossRef]
- Konen, F.F.; Schwenkenbecher, P.; Jendretzky, K.F.; Gingele, S.; Sühs, K.-W.; Tumani, H.; Süße, M.; Skripuletz, T. The Increasing Role of Kappa Free Light Chains in the Diagnosis of Multiple Sclerosis. Cells 2021, 10, 3056. [Google Scholar] [CrossRef] [PubMed]
- Schwenkenbecher, P.; Konen, F.F.; Wurster, U.; Witte, T.; Gingele, S.; Sühs, K.-W.; Stangel, M.; Skripuletz, T. Reiber’s Diagram for Kappa Free Light Chains: The New Standard for Assessing Intrathecal Synthesis? Diagnostics 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Süße, M.; Reiber, H.; Grothe, M.; Petersmann, A.; Nauck, M.; Dressel, A.; Hannich, M.J. Free light chain kappa and the polyspecific immune response in MS and CIS—Application of the hyperbolic reference range for most reliable data interpretation. J. Neuroimmunol. 2020, 346, 577287. [Google Scholar] [CrossRef]
- Presslauer, S.; Milosavljevic, D.; Huebl, W.; Aboulenein-Djamshidian, F.; Krugluger, W.; Deisenhammer, F.; Senel, M.; Tumani, H.; Hegen, H. Validation of kappa free light chains as a diagnostic biomarker in multiple sclerosis and clinically isolated syndrome: A multicenter study. Mult. Scler. J. 2015, 22, 502–510. [Google Scholar] [CrossRef]
- Senel, M.; Mojib-Yezdani, F.; Braisch, U.; Bachhuber, F.; Lewerenz, J.; Ludolph, A.C.; Otto, M.; Tumani, H. CSF Free Light Chains as a Marker of Intrathecal Immunoglobulin Synthesis in Multiple Sclerosis: A Blood-CSF Barrier Related Evaluation in a Large Cohort. Front. Immunol. 2019, 10, 641. [Google Scholar] [CrossRef]
- Zeman, D.; Kušnierová, P.; Švagera, Z.; Všianský, F.; Byrtusová, M.; Hradílek, P.; Kurková, B.; Zapletalová, O.; Bartoš, V. Assessment of Intrathecal Free Light Chain Synthesis: Comparison of Different Quantitative Methods with the Detection of Oligoclonal Free Light Chains by Isoelectric Focusing and Affinity-Mediated Immunoblotting. PLoS ONE 2016, 11, e0166556. [Google Scholar] [CrossRef]
- Emersic, A.; Anadolli, V.; Krsnik, M.; Rot, U. Intrathecal immunoglobulin synthesis: The potential value of an adjunct test. Clin. Chim. Acta 2019, 489, 109–116. [Google Scholar] [CrossRef]
- Combined use of Kappa Free Light Chain Index and Isoelectrofocusing of Cerebro-Spinal Fluid in Diagnosing Multiple Sclerosis: Performances and Costs. Clin. Lab. 2017, 63, 551–559. [CrossRef]
- Valencia-Vera, E.; Garcia-Ripoll, A.M.-E.; Enguix, A.; Abalos-Garcia, C.; Segovia-Cuevas, M.J. Application of κ free light chains in cerebrospinal fluid as a biomarker in multiple sclerosis diagnosis: Development of a diagnosis algorithm. Clin. Chem. Lab. Med. (CCLM) 2017, 56, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, K.M.; Shosha, E.; Bryant, S.C.; Andreguetto, B.D.; Murray, D.L.; Pittock, S.J.; Willrich, M.A.V. CSF free light chain identification of demyelinating disease: Comparison with oligoclonal banding and other CSF indexes. Clin. Chem. Lab. Med. (CCLM) 2018, 56, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, S.; Milosavljevic, D.; Brücke, T.; Bayer, P.; Hübl, W. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J. Neurol. 2008, 255, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Zeman, D.; Kušnierová, P.; Mundwiler, E.; Bernasconi, L. Diagnostic relevance of free light chains in cerebrospinal fluid—The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta 2019, 497, 153–162. [Google Scholar] [CrossRef]
- Tjernberg, I.; Johansson, M.; Henningsson, A.J. Diagnostic performance of cerebrospinal fluid free light chains in Lyme neuroborreliosis—A pilot study. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 2008–2018. [Google Scholar] [CrossRef]
- Hegen, H.; Milosavljevic, D.; Schnabl, C.; Manowiecka, A.; Walde, J.; Deisenhammer, F.; Presslauer, S. Cerebrospinal fluid free light chains as diagnostic biomarker in neuroborreliosis. Clin. Chem. Lab. Med. (CCLM) 2018, 56, 1383–1391. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Czupryna, P.; Moniuszko-Malinowska, A.; Pancewicz, S.; Mroczko, B. Free Immunoglobulin Light Chains in Patients with Tick-Borne Encephalitis: Before and after Treatment. J. Clin. Med. 2021, 10, 2922. [Google Scholar] [CrossRef]
- Agnello, L.; Sasso, B.L.; Salemi, G.; Altavilla, P.; Pappalardo, E.M.; Caldarella, R.; Meli, F.; Scazzone, C.; Bivona, G.; Ciaccio, M. Clinical Use of κ Free Light Chains Index as a Screening Test for Multiple Sclerosis. Lab. Med. 2020, 51, 402–407. [Google Scholar] [CrossRef]
- Cavalla, P.; Caropreso, P.; Limoncelli, S.; Bosa, C.; Pasanisi, M.; Schillaci, V.; Alteno, A.; Costantini, G.; Giordana, M.; Mengozzi, G.; et al. Kappa free light chains index in the differential diagnosis of Multiple Sclerosis from Neuromyelitis optica spectrum disorders and other immune-mediated central nervous system disorders. J. Neuroimmunol. 2020, 339, 577122. [Google Scholar] [CrossRef]
- Süße, M.; Feistner, F.; Grothe, M.; Nauck, M.; Dressel, A.; Hannich, M.J. Free light chains kappa can differentiate between myelitis and noninflammatory myelopathy. Neurol.-Neuroimmunol. Neuroinflamm. 2020, 7, e892. [Google Scholar] [CrossRef]
- Vecchio, D.; Bellomo, G.; Serino, R.; Virgilio, E.; Lamonaca, M.; Dianzani, U.; Cantello, R.; Comi, C.; Crespi, I. Intrathecal kappa free light chains as markers for multiple sclerosis. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Süße, M.; Feistner, F.; Holbe, C.; Grothe, M.; Nauck, M.; Dressel, A.; Hannich, M.J. Diagnostic value of kappa free light chains in patients with one isolated band in isoelectric focusing. Clin. Chim. Acta 2020, 507, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Süße, M.; Hannich, M.; Petersmann, A.; Zylla, S.; Pietzner, M.; Nauck, M.; Dressel, A. Kappa free light chains in cerebrospinal fluid to identify patients with oligoclonal bands. Eur. J. Neurol. 2018, 25, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Konen, F.F.; Schwenkenbecher, P.; Wurster, U.; Jendretzky, K.F.; Möhn, N.; Gingele, S.; Sühs, K.-W.; Hannich, M.J.; Grothe, M.; Witte, T.; et al. The Influence of Renal Function Impairment on Kappa Free Light Chains in Cerebrospinal Fluid. J. Central Nerv. Syst. Dis. 2021, 13, 11795735211042166. [Google Scholar] [CrossRef]
- Teunissen, C.; Menge, T.; Altintas, A.; Álvarez-Cermeño, J.C.; Bertolotto, A.; Berven, F.S.; Brundin, L.; Comabella, M.; Degn, M.; Deisenhammer, F.; et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult. Scler. J. 2013, 19, 1802–1809. [Google Scholar] [CrossRef]
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor. Neurol. Neurosci. 2003, 21, 79–96. [Google Scholar]
- Andersson, M.; Alvarez-Cermeno, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Gronning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Saadeh, R.S.; Bryant, S.C.; McKeon, A.; Weinshenker, B.; Murray, D.L.; Pittock, S.J.; Willrich, M.A.V. CSF Kappa Free Light Chains: Cutoff Validation for Diagnosing Multiple Sclerosis. Mayo Clin. Proc. 2021, 97, 738–751. [Google Scholar] [CrossRef]
- Hannich, M.; Dressel, A.; Budde, K.; Petersmann, A.; Nauck, M.; Süße, M. Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern. Cells 2021, 10, 616. [Google Scholar] [CrossRef]
- Chu, A.B.; Sever, J.L.; Madden, D.L.; Iivanainen, M.; Leon, M.; Wallen, W.; Brooks, B.R.; Lee, Y.J.; Houff, S. Oligoclonal IgG bands in cerebrospinal fluid in various neurological diseases. Ann. Neurol. 1983, 13, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Petzold, A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J. Neuroimmunol. 2013, 262, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pannewitz-Makaj, K.; Wurster, U.; Jendretzky, K.F.; Gingele, S.; Sühs, K.-W.; Stangel, M.; Skripuletz, T.; Schwenkenbecher, P. Evidence of Oligoclonal Bands Does Not Exclude Non-Inflammatory Neurological Diseases. Diagnostics 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Konen, F.F.; Wurster, U.; Witte, T.; Jendretzky, K.F.; Gingele, S.; Tumani, H.; Sühs, K.-W.; Stangel, M.; Schwenkenbecher, P.; Skripuletz, T. The Impact of Immunomodulatory Treatment on Kappa Free Light Chains as Biomarker in Neuroinflammation. Cells 2020, 9, 842. [Google Scholar] [CrossRef] [Green Version]
- Topping, J.; Dobson, R.; Lapin, S.; Maslyanskiy, A.; Kropshofer, H.; Leppert, D.; Giovannoni, G.; Evdoshenko, E. The effects of intrathecal rituximab on biomarkers in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 6, 49–53. [Google Scholar] [CrossRef]
- Anderson, T.J.; Donaldson, I.M.; Shear, J.M.; George, P.M. Methylprednisolone in multiple sclerosis exacerbation: Changes in CSF parameters. Aust. New Zealand J. Med. 1990, 20, 794–797. [Google Scholar] [CrossRef]
- Piccio, L.; Naismith, R.T.; Trinkaus, K.; Klein, R.S.; Parks, B.J.; Lyons, J.A.; Cross, A.H. Changes in B- and T-Lymphocyte and Chemokine Levels With Rituximab Treatment in Multiple Sclerosis. Arch. Neurol. 2010, 67, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Cross, A.H.; Stark, J.L.; Lauber, J.; Ramsbottom, M.J.; Lyons, J.-A. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 2006, 180, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Zrzavy, T.; Höftberger, R.; Wimmer, I.; Berger, T.; Rommer, P.; Macher, S. Longitudinal CSF Findings in Autoimmune Encephalitis—A Monocentric Cohort Study. Front. Immunol. 2021, 12, 646940. [Google Scholar] [CrossRef]
- Blinder, T.; Lewerenz, J. Cerebrospinal Fluid Findings in Patients With Autoimmune Encephalitis—A Systematic Analysis. Front. Neurol. 2019, 10, 804. [Google Scholar] [CrossRef] [Green Version]
- Bonadio, W.A.; Smith, D. Cerebrospinal fluid changes after 48 hours of effective therapy for Hemophilus influenzae type B meningitis. Am. J. Clin. Pathol. 1990, 94, 426–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigrovic, L.E.; Malley, R.; Macias, C.G.; Kanegaye, J.T.; Moro-Sutherland, D.M.; Schremmer, R.D.; Schwab, S.H.; Agrawal, D.; Mansour, K.M.; Bennett, J.E.; et al. Effect of Antibiotic Pretreatment on Cerebrospinal Fluid Profiles of Children With Bacterial Meningitis. Pediatr. 2008, 122, 726–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiber, H.; Peter, J.B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001, 184, 101–122. [Google Scholar] [CrossRef]
| Characteristics | Symptomatic Controls, n = 80 | Non-Inflammatory Neurological Diseases, n = 182 | Inflammatory Neurological Diseases, n = 84 | p Value |
|---|---|---|---|---|
| Females, n (%) | 30/80 (38%) | 95/182 (52%) | 39/84 (46%) | <0.0001 |
| Age [years], median (min–max) | 40 (18–87) | 61 (19–89) | 48.5 (18–87) | 0.0882 |
| Oligoclonal bands, n (%) | 0/80 | 2/182 (1%) | 35/84 (42%) | <0.0001 |
| Intrathecal IgG-synthesis, n (%) | 0/80 | 0/182 | 17/84 (20%) | <0.0001 |
| Intrathecal IgA-synthesis, n (%) | 0/80 | 0/182 | 11/84 (13%) | <0.0001 |
| Intrathecal IgM-synthesis, n (%) | 0/80 | 0/182 | 22/84 (26%) | <0.0001 |
| Reiber’s diagram for KFLC, n (%) | 7/80 (9%) | 10/182 (5%) | 44/84 (52%) | <0.0001 |
| KFLC index > 5.9, n (%) | 1/80 (1%) | 0/182 | 32/84 (38%) | <0.0001 |
| KFLC index, mean (min–max) | 2.3 (0.8–6) | 2.2 (0.4–19.8) | 21.5 (0.6–325.7) | <0.0001 |
| KFLC CSF [mg/L], mean (min–max) | 0.2 (0.04–0.7) | 0.3 (0.03–2) | 3.5 (0.03–35.2) | <0.0001 |
| KFLC serum [mg/L], mean (min–max) | 12.6 (5.6–53.2) | 17.7 (4–95.2) | 15.5 (3.7–73.8) | 0.0767 |
| Presslauer’s exponential curve, n (%) | 0/80 | 0/182 | 33/84 (39%) | <0.0001 |
| Senel’s linear curve, n (%) | 1/80 (1%) | 8/182 (4%) | 45/84 (54%) | <0.0001 |
| CSF cell count [cells/µL CSF], mean (min–max) | 1 (0–4) | 2 (0–15) | 825 (0–14666) | <0.0001 |
| CSF lactate concentration [mmol/L], mean (min–max) | 1.7 (1.1–2.1) | 2 (1.3–6.5) | 3.5 (1.2–29.8) | < 0.0001 |
| QAlbumin, mean (min–max) | 5.8 (2.2–17.8) | 7.6 (2.2–46.7) | 21.9 (2.2–196.6) | < 0.0001 |
| Diagnosis | Headache (unclassified); sensory disturbances; dizziness | Epilepsy (n = 54); IIH/NPH (n = 9); non-inflammatory peripheral nerve pathology (n = 52); non-inflammatory vascular diseases of the CNS (n = 35); ALS (n = 11); Parkinson’s disease (n = 9); dementia syndromes (n = 12) | Bacterial meningitis (n = 22); viral encephalitis (n = 27); neuroborreliosis (n = 17); neurosyphilis (n = 5); vasculitis with affection of the CNS (n = 5); autoimmune- mediated encephalitis (n = 8) |
| Characteristics | Autoimmune-mediated Encephalitis, n = 8 | Vasculitis with Affection of the CNS, n = 5 | Viral Encephalitis, n = 27 | Bacterial Meningitis, n = 22 | Neuroborreliosis, n = 17 | Neurosyphilis, n = 5 |
|---|---|---|---|---|---|---|
| Oligoclonal bands, n (%) | 1/8 (13%) | 1/5 (20%) | 6/27 (22%) | 6/22 (27%) | 15/17 (88%) | 4/5 (80%) |
| Intrathecal IgG-synthesis, n (%) | 1/8 (13%) | 0/5 | 2/27 (7%) | 4/22 (18%) | 8/17 (47%) | 2/5 (40%) |
| Intrathecal IgA-synthesis, n (%) | 0/8 | 0/5 | 3/27 (11%) | 2/22 (9%) | 6/17 (35%) | 0/5 |
| Intrathecal IgM-synthesis, n (%) | 2/8 (25%) | 0/5 | 4/27 (15%) | 2/22 (9%) | 12/17 (71%) | 2/5 (40%) |
| Reiber’s diagram for KFLC, n (%) | 5/8 (63%) | 2/5 (40%) | 10/27 (36%) | 6/22 (27%) | 16/17 (94%) | 5/5 |
| KFLC index > 5.9, n (%) | 1/8 (13%) | 0/5 | 7/27 (26%) | 5/22 (23%) | 15/17 (88%) | 4/5 (80%) |
| KFLC index, mean (min–max) | 43.9 (1.4–325.7) | 2.6 (1.2–4.3) | 15 (1.3–242.3) | 7.5 (0.3–69.2) | 33.4 (1.8–116.1) | 61.2 (3.8–254) |
| KFLC CSF [mg/L], mean (min–max) | 1.3 (0.1–8.7) | 0.6 (0.1–1.9) | 2 (0.05–19.3) | 3.3 (0.2–34.9) | 6.7 (0.03–21.6) | 7.9 (0.4–35.2) |
| KFLC serum [mg/L], mean (min–max) | 11.4 (4.3–18.2) | 22.3 (7.6–42.4) | 18.2 (6.6–73.8) | 13.6 (3.7–25) | 13.5 (5.2–38.7) | 15.8 (9.1–19.4) |
| Presslauer’s exponential curve, n (%) | 1/8 (13%) | 0/5 | 7/27 (26%) | 6/22 (27%) | 15/17 (88%) | 4/5 (80%) |
| Senel’s linear curve, n (%) | 5/8 (63%) | 2/5 (40%) | 10/27 (36%) | 7/22 (32%) | 16/17 (94%) | 5/5 |
| CSF cell count [cells/µL CSF], mean (min–max) | 12 (1–36) | 10 (1–40) | 56 (7–304) | 2951 (223–14666) | 155 (0–1056) | 13 (6–17) |
| CSF lactate concentration [mmol/L], mean (min–max) | 1.6 (1.3–2.4) | 1.8 (1.5–2.6) | 2 (1.2–3.8) | 8.2 (1.5–29.8) | 2.2 (1.3–3.9) | 1.7 (1.5–2) |
| QAlbumin, mean (min–max) | 6.6 (2.8–13.5) | 9.8 (5.2–18.8) | 9.5 (2.2–24.2) | 53 (11–196.6) | 16.1 (3.4–47.3) | 7.3 (3.8–15.2) |
| Inflammatory Diseases n = 84 | Non-Inflammatory Diseases n = 262 | n = 346 | |
|---|---|---|---|
| QKFLC > Qlim (KFLC) | n = 44 | n = 17 | PPV 72% |
| Oligoclonal bands positive | n = 35 | n = 2 | PPV 95% |
| QKFLC < Qlim (KFLC) | n = 40 | n = 245 | NPV 14% |
| Oligoclonal bands negative | n = 49 | n = 260 | NPV 16% |
| Sens KFLC 52% Sens OCB 41% | Spec KFLC 94% Spec OCB 99% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konen, F.F.; Schwenkenbecher, P.; Jendretzky, K.F.; Gingele, S.; Witte, T.; Sühs, K.-W.; Grothe, M.; Hannich, M.J.; Süße, M.; Skripuletz, T. Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain Sci. 2022, 12, 475. https://doi.org/10.3390/brainsci12040475
Konen FF, Schwenkenbecher P, Jendretzky KF, Gingele S, Witte T, Sühs K-W, Grothe M, Hannich MJ, Süße M, Skripuletz T. Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain Sciences. 2022; 12(4):475. https://doi.org/10.3390/brainsci12040475
Chicago/Turabian StyleKonen, Franz Felix, Philipp Schwenkenbecher, Konstantin Fritz Jendretzky, Stefan Gingele, Torsten Witte, Kurt-Wolfram Sühs, Matthias Grothe, Malte Johannes Hannich, Marie Süße, and Thomas Skripuletz. 2022. "Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases" Brain Sciences 12, no. 4: 475. https://doi.org/10.3390/brainsci12040475
APA StyleKonen, F. F., Schwenkenbecher, P., Jendretzky, K. F., Gingele, S., Witte, T., Sühs, K.-W., Grothe, M., Hannich, M. J., Süße, M., & Skripuletz, T. (2022). Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain Sciences, 12(4), 475. https://doi.org/10.3390/brainsci12040475







