Co-Occurrence of Multiple Sclerosis and Amyotrophic Lateral Sclerosis in an FUS-Mutated Patient: A Case Report
Abstract
1. Introduction
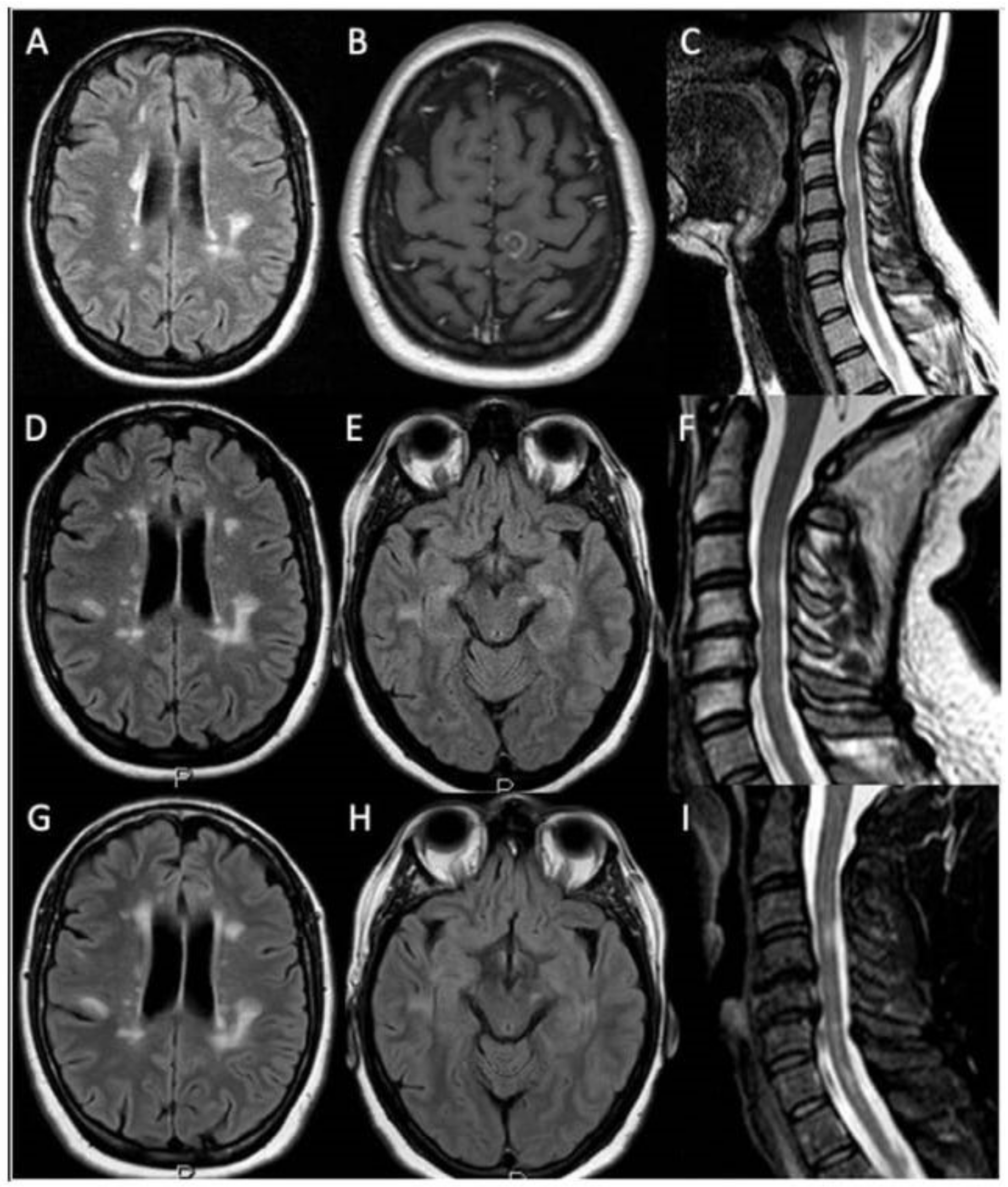
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Sawcer, S.; Franklin, R.J.M.; Ban, M. Multiple sclerosis genetics. Lancet Neurol. 2014, 13, 700–709. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L.; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Mathis, S.; Goizet, C.; Soulages, A.; Vallat, J.-M.; Masson, G.L. Genetics of amyotrophic lateral sclerosis: A review. J. Neurol. Sci. 2019, 399, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.R.; Goldacre, R.; Ramagopalan, S.; Talbot, K.; Goldacre, M.J. Autoimmune disease preceding amyotrophic lateral sclerosis. Neurology 2013, 81, 1222–1225. [Google Scholar] [CrossRef]
- Dynes, G.J.; Schwimer, C.J.; Staugaitis, S.M.; Doyle, J.J.; Hays, A.P.; Mitsumoto, H. Amyotrophic lateral sclerosis with multiple sclerosis: A clinical and pathological report. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 2000, 1, 349–353. [Google Scholar] [CrossRef]
- Trojsi, F.; Sagnelli, A.; Cirillo, G.; Piccirillo, G.; Femiano, C.; Izzo, F.; Monsurro, M.R.; Tedeschi, G. Amyotrophic lateral sclerosis and multiple sclerosis overlap: A case report. Case Rep. Med. 2012, 2012, 324685. [Google Scholar] [CrossRef]
- Borisow, N.; Meyer, T.; Paul, F. Concomitant amyotrophic lateral sclerosis and paraclinical laboratory features of multiple sclerosis: Coincidence or causal relationship? BMJ Case Rep. 2013, 2013, bcr2012007975. [Google Scholar] [CrossRef]
- Hader, W.J.; Rpzdilsky, B.; Nair, C.P. The concurrence of multiple sclerosis and amyotrophic lateral sclerosis. Can. J. Neurol. Sci. 1986, 13, 66–69. [Google Scholar] [CrossRef]
- Ismail, A.; Cooper-Knock, J.; Highley, J.R.; Milano, A.; Kirby, J.; Goodall, E.; Lowe, J.; Scott, I.; Constantinescu, C.S.; Walters, S.J.; et al. Concurrence of multiple sclerosis and amyotrophic lateral sclerosis in patients with hexanucleotide repeat expansions of C9ORF72. J. Neurol. Neurosurg. Psychiatry 2013, 84, 79–87. [Google Scholar] [CrossRef]
- Kwiatkowski, T.J., Jr.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef]
- Vance, C.; Rogelj, B.; Hortobágyi, T.; De Vos, K.J.; Nishimura, A.L.; Sreedharan, J.; Hu, X.; Smith, B.; Ruddy, D.; Wright, P.; et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 2009, 323, 1208–1211. [Google Scholar] [CrossRef]
- Pozzessere, G.; Valle, E.; Tomaselli, M.; D’Alessio, M.; Bianco, F.; Pierelli, F.; Morocutti, C. Crural amyotrophy associated with a parietal lesion: A case report. Acta Neurol. Belg. 1995, 95, 96–100. [Google Scholar] [PubMed]
- Bouche, P.; Moulonguet, A.; Younes-Chennoufi, A.B.; Adams, D.; Baumann, N.; Meininger, V.; Leger, J.M.; Said, G. Multifocal motor neuropathy with conduction block: A study of 24 patients. J. Neurol. Neurosurg. Psychiatry 1995, 59, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, K. Juvenile Muscular Atrophy of Distal Upper Extremity (Hirayama Disease). Intern. Med. 2000, 39, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Abramzon, Y.; Schymick, J.C.; Stephan, D.A.; Dunckley, T.; Dillman, A.; Cookson, M.; Calvo, A.; Battistini, S.; Giannini, F.; et al. FUS mutations in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging 2011, 32, 550.e1–550.e4. [Google Scholar] [CrossRef]
- Lattante, S.; Rouleau, G.A.; Kabashi, E. TARDBP and FUS mutations associated with amyotrophic lateral sclerosis: Summary and update. Hum. Mutat. 2013, 34, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.; Kirby, J.; Highley, J.R.; Hartley, J.A.; Hibberd, R.; Hollinger, H.C.; Williams, T.L.; Ince, P.G.; McDermott, C.J.; Shaw, P.J. Novel FUS/TLS Mutations and Pathology in Familial and Sporadic Amyotrophic Lateral Sclerosis. Arch Neurol. 2010, 67, 455–461. [Google Scholar] [CrossRef]
- Sproviero, W.; La Bella, V.; Mazzei, R.; Valentino, P.; Rodolico, C.; Simone, I.L.; Logroscino, G.; Ungaro, C.; Magariello, A.; Patitucci, A.; et al. FUS mutations in sporadic amyotrophic lateral sclerosis: Clinical and genetic analysis. Neurobiol. Aging 2012, 33, 837.e1–837.e5. [Google Scholar] [CrossRef][Green Version]
- Confavreux, C.; Moreau, T.; Jouvet, A.; Tommasi, M.; Aimard, G. Association sclérose latérale amyotrophique et sclérose en plaques [Association of amyotrophic lateral sclerosis and multiple sclerosis]. Rev. Neurol. 1993, 149, 351–353. [Google Scholar]
- Li, G.; Esiri, M.M.; Ansorge, O.; DeLuca, G.C. Concurrent multiple sclerosis and amyotrophic lateral sclerosis: Where inflammation and neurodegeneration meet? J. Neuroinflamm. 2012, 9, 20. [Google Scholar] [CrossRef]
- Machner, B.; Gottschalk, S.; Kimmig, H.; Helmchen, C. Kombiniertes Auftreten von amyotropher Lateralsklerose und Multipler Sklerose [Simultaneous presentation of amyotrophic lateral sclerosis and multiple sclerosis]. Der Nervenarzt 2007, 78, 1440–1443. (In Germany) [Google Scholar] [CrossRef] [PubMed]
- Dattola, V.; Famà, F.; Russo, M.; Calabrò, R.S.; Logiudice, A.L.; Grasso, M.G.; Patti, F.; Buccafusca, M. Multiple sclerosis and amyotrophic lateral sclerosis: A human leukocyte antigen challenge. Neurol. Sci. 2017, 38, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Guennoc, A.M.; Pallix-Guyot, M.; Le Page, E.; Le Port, D.; Daryabin, M.; Hergesheimer, R.; Beltran, S.; Tourbah, A.; Edan, G.; Corcia, P. Co-occurrence of MS and ALS: A clue in favor of common pathophysiological findings? Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Stein, R.; Baker, R.A.; Royden Jones, H. Muscle atrophy associated with multiple sclerosis: A benign condition or the onset of amyotrophic lateral sclerosis? J. Clin. Neurosci. 2008, 15, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Pocock, K.; Baysal, I.; Scanlan, J.; Elliott, M.; Mayadev, A. Amyotrophic Lateral Sclerosis and Multiple Sclerosis: More Evidence Suggesting a Link. RRNMF Neuromuscul. J. 2021, 2, 23–26. [Google Scholar] [CrossRef]
- Nonneman, A.; Robberecht, W.; Van Den Bosch, L. The role of oligodendroglial dysfunction in amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2014, 4, 223–239. [Google Scholar] [CrossRef]
- Pohl, H.B.; Porcheri, C.; Mueggler, T.; Bachmann, L.C.; Martino, G.; Riethmacher, D.; Franklin, R.J.; Rudin, M.; Suter, U. Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage. J. Neurosci. 2011, 31, 1069–1080. [Google Scholar] [CrossRef]
- Oluich, L.J.; Stratton, J.A.; Xing, Y.L.; Ng, S.W.; Cate, H.S.; Sah, P.; Windels, F.; Kilpatrick, T.J.; Merson, T.D. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. J. Neurosci. 2012, 32, 8317–8330. [Google Scholar] [CrossRef]
- Ghosh, A.; Manrique-Hoyos, N.; Voigt, A.; Schulz, J.B.; Kreutzfeldt, M.; Merkler, D.; Simons, M. Targeted ablation of oligodendrocytes triggers axonal damage. PLoS ONE 2011, 6, e22735. [Google Scholar]
- Mackenzie, I.R.; Ansorge, O.; Strong, M.; Bilbao, J.; Zinman, L.; Ang, L.C.; Baker, M.; Stewart, H.; Eisen, A.; Rademakers, R.; et al. Pathological heterogeneity in amyotrophic lateral sclerosis with FUS mutations: Two distinct patterns correlating with disease severity and mutation. Acta Neuropathol. 2011, 122, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Scekic-Zahirovic, J.; Oussini, H.E.; Mersmann, S.; Drenner, K.; Wagner, M.; Sun, Y.; Allmeroth, K.; Dieterlé, S.; Sinniger, J.; Dirrig-Grosch, S.; et al. Motor neuron intrinsic and extrinsic mechanisms contribute to the pathogenesis of FUS-associated amyotrophic lateral sclerosis. Acta Neuropathol. 2017, 133, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Guzman, K.M.; Brink, L.E.; Rodriguez-Bey, G.; Bodnar, R.J.; Kuang, L.; Xing, B.; Sullivan, M.; Park, H.J.; Koppes, E.; Zhu, H.; et al. Conditional depletion of Fus in oligodendrocytes leads to motor hyperactivity and increased myelin deposition associated with Akt and cholesterol activation. Glia 2020, 68, 2040–2056. [Google Scholar] [CrossRef] [PubMed]

| Reference | Multiple Sclerosis | ALS | ||||
|---|---|---|---|---|---|---|
| Gender | AO | SO | Form | AO | Site of Onset | |
| Confavreux [20] | F | 25 | Hemiparesis | RRMS | 35 | Limb |
| Li [21] | M | 37 | VI nerve palsy | SPMS | 39 | Bulbar |
| Hader [9] | M | 21 | UNK | RRMS | 56 | Bulbar |
| Borisow [8] | M | UNK | UNK | UNK | 55 | Limb |
| Machner [22] | F | 55 | Gait disorder | UNK | 56 | Limb |
| Dynes [6] | F | 61 | UNK | UNK | 61 | Limb |
| Trojsi [7] | F | 33 | UNK | PPMS | 34 | Limb |
| Ismail [10] | F | 22 | UNK | SPSM | 62 | Bulbar |
| Ismail | F | 49 | UNK | RRMS | 52 | Bulbar |
| Ismail | F | 43 | UNK | RRMS | 52 | Limb |
| Ismail | M | 46 | UNK | PPMS | 67 | Bulbar |
| Ismail | M | 39 | UNK | PPMS | 40 | Bulbar |
| Ismail | F | 40 | Pain-weakness upper limbs | SPMS | 41 | Limb |
| Ismail | F | 56 | Left upper limb and lower limbs | PPMS | 56 | Limb |
| Dattola [23] | F | UNK | UNK | UNK | 47 | UNK |
| Dattola | F | 35 | Diplopia | RRMS | 38 | Bulbar |
| Dattola | F | UNK | UNK | RRMS | 52 | Bulbar |
| Dattola | F | 25 | UNK | RRMS | 49 | UNK |
| Guennoc [24] | F | 41 | Optic neuritis | SPMS | 52 | Bulbar |
| Guennoc | F | 39 | Sensory | SPMS | 51 | Limb |
| Guennoc | M | 34 | Sensory | SPMS | 50 | Limb |
| Guennoc | F | 41 | Weakness lower limbs | PPMS | 66 | Limb |
| Guennoc | F | 27 | Optic neuritis | RRMS | 59 | Limb |
| Guennoc | F | 54 | Spastic gait | PPMS | 55 | Limb |
| Allen [25] | M | 27 | Sensory | RRMS | 51 | Limb |
| Hewitt [18] | F | UNK | UNK | RRMS | 62 | Bulbar |
| Sproviero [19] | F | UNK | Sensory | RRMS | 45 | Limb |
| Pocock [26] | F | UNK | Right leg weakness | RRMS | 70 | Limb |
| Pocock | F | 49 | UNK | RRMS | 72 | Bulbar |
| Pocock | F | 44 | UNK | SPMS | 51 | Bulbar |
| Pocock | F | 49 | Left leg and arm | PPMS | 49 | Limb |
| Pocock | M | UNK | UNK | SPMS | 63 | Bulbar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiondella, L.; Cavallieri, F.; Canali, E.; Cabboi, M.P.; Marti, A.; Sireci, F.; Fiocchi, A.; Montanari, G.; Montepietra, S.; Valzania, F. Co-Occurrence of Multiple Sclerosis and Amyotrophic Lateral Sclerosis in an FUS-Mutated Patient: A Case Report. Brain Sci. 2022, 12, 531. https://doi.org/10.3390/brainsci12050531
Fiondella L, Cavallieri F, Canali E, Cabboi MP, Marti A, Sireci F, Fiocchi A, Montanari G, Montepietra S, Valzania F. Co-Occurrence of Multiple Sclerosis and Amyotrophic Lateral Sclerosis in an FUS-Mutated Patient: A Case Report. Brain Sciences. 2022; 12(5):531. https://doi.org/10.3390/brainsci12050531
Chicago/Turabian StyleFiondella, Luigi, Francesco Cavallieri, Elena Canali, Maria Paola Cabboi, Alessandro Marti, Francesca Sireci, Alena Fiocchi, Gloria Montanari, Sara Montepietra, and Franco Valzania. 2022. "Co-Occurrence of Multiple Sclerosis and Amyotrophic Lateral Sclerosis in an FUS-Mutated Patient: A Case Report" Brain Sciences 12, no. 5: 531. https://doi.org/10.3390/brainsci12050531
APA StyleFiondella, L., Cavallieri, F., Canali, E., Cabboi, M. P., Marti, A., Sireci, F., Fiocchi, A., Montanari, G., Montepietra, S., & Valzania, F. (2022). Co-Occurrence of Multiple Sclerosis and Amyotrophic Lateral Sclerosis in an FUS-Mutated Patient: A Case Report. Brain Sciences, 12(5), 531. https://doi.org/10.3390/brainsci12050531







