Protective Effects of Nuciferine in Middle Cerebral Artery Occlusion Rats Based on Transcriptomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Animal Models and Experimental Protocol
2.4. Assessment of Neurological Defects
2.5. Evaluation of Cerebral Infarct Volume
2.6. Evaluation of Cerebral Edema
2.7. Histological Observation
2.8. Nissl Staining
2.9. RNA Extraction
2.10. Library Preparation, and Illumina Novaseq 6000 Sequencing
2.11. Read Mapping
2.12. Differential Expression Analysis and Functional Enrichment
2.13. Statistical Analysis
3. Results
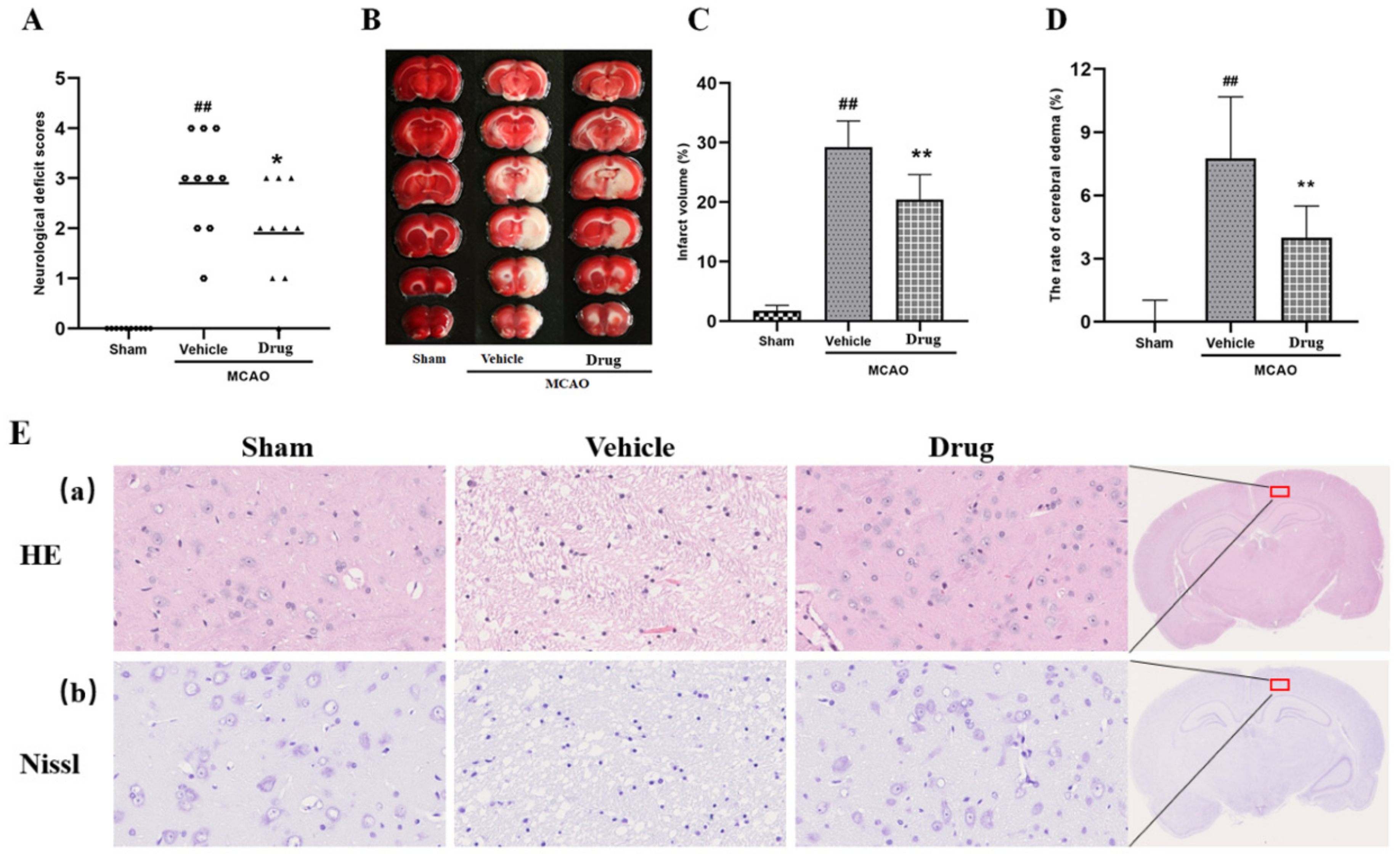
3.1. Effects of Nuciferine on Anti-Cerebral Ischemia
3.2. HE Staining and Nissl Staining
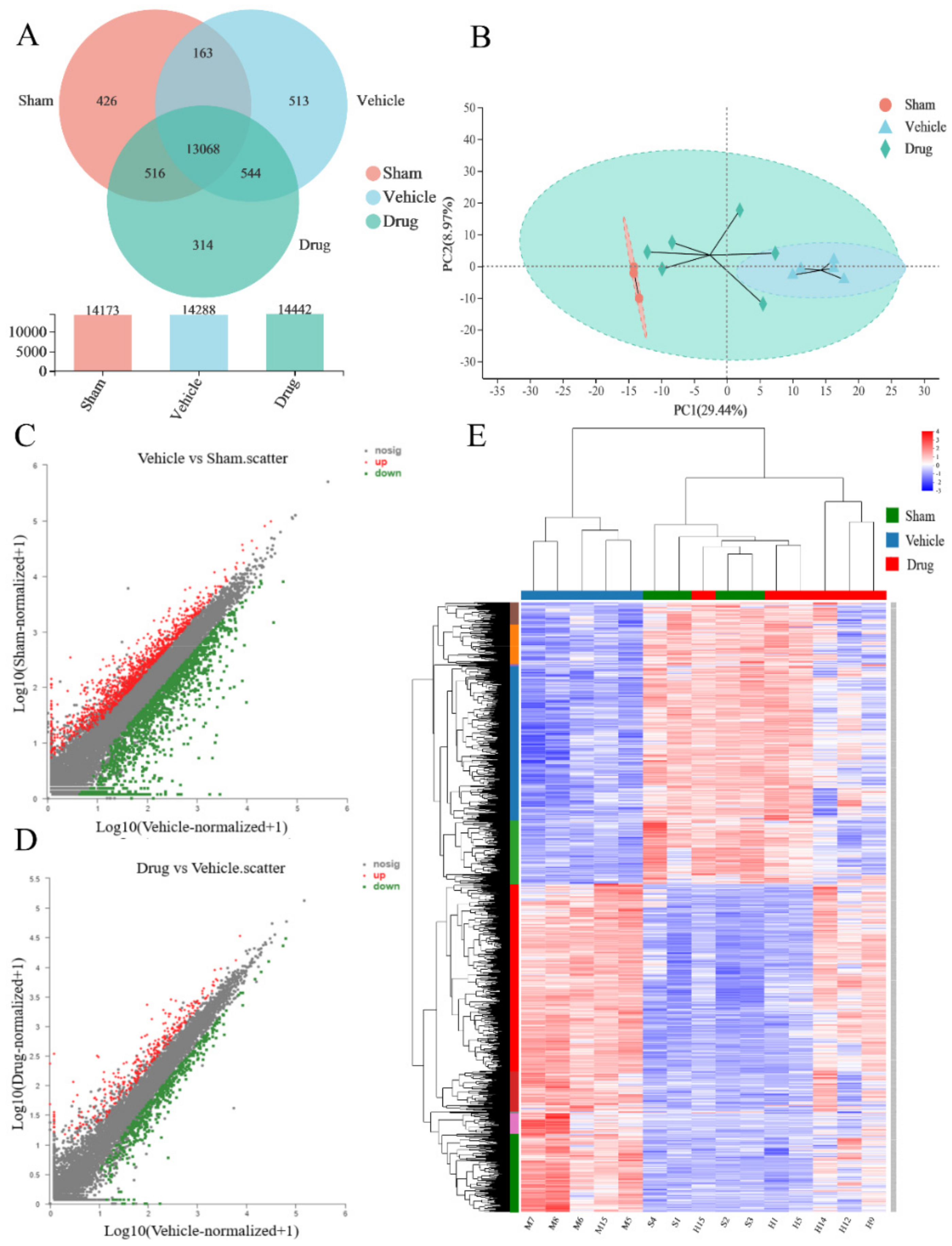
3.3. Transcriptomic Analysis Results
3.3.1. Sample Relationship Analysis
3.3.2. Analysis of Sample Expression Differences
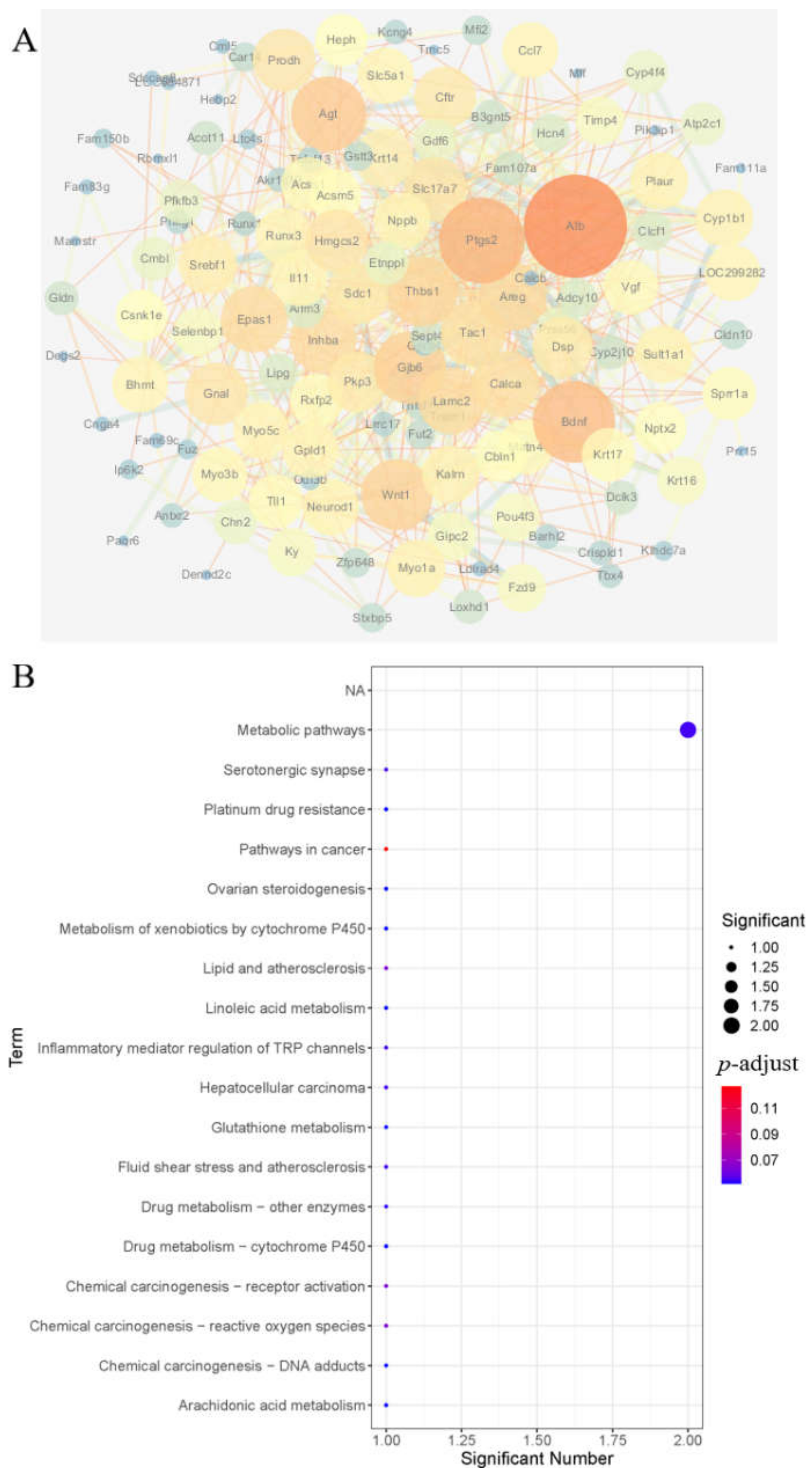
3.3.3. Functional Enrichment Analysis of Genes Regulated by Nuciferine
3.3.4. Analysis of a Differential Gene Interaction Network Regulated by Nuciferine and Enrichment of KEGG Pathway
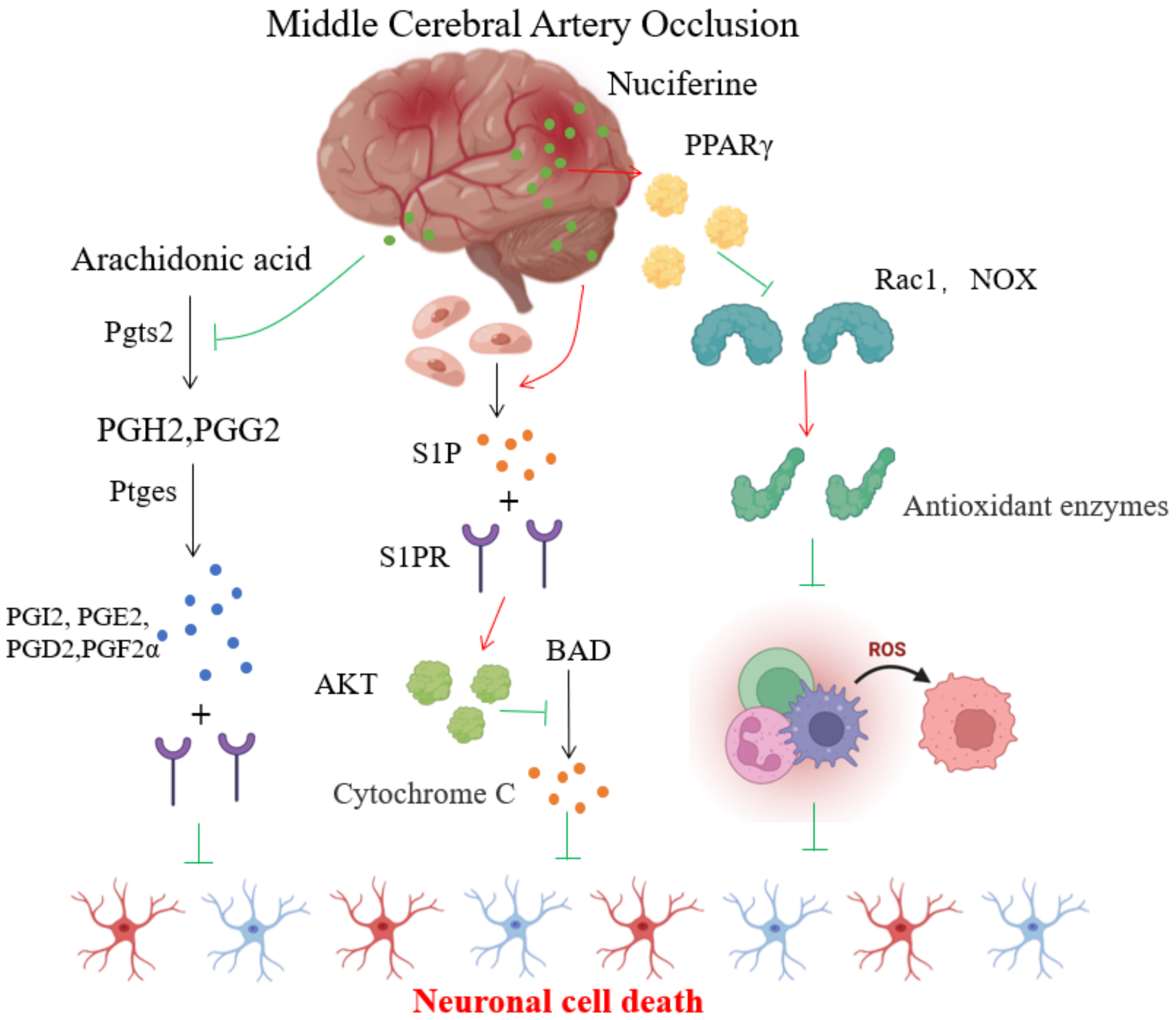
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berretta, A.; Tzeng, Y.; Clarkson, A.N. Post-stroke recovery: The role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev. Neurother. 2014, 14, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-L.; Ding, L.-S.; He, W.; Tian, X.-Y.; Cao, H.; Song, Y.Q.; Yu, L.; Sun, X.-Y. Systolic hypertension related single nucleotide polymorphism is associated with susceptibility of ischemic stroke. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2901–2906. [Google Scholar] [PubMed]
- Xie, W.; Zhou, P.; Sun, Y.; Meng, X.; Dai, Z.; Sun, G.; Sun, X. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A comprehensive overview of experimental studies. Cells 2018, 7, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wańkowicz, P.; Nowacki, P.; Gołąb-Janowska, M. Atrial fibrillation risk factors in patients with ischemic stroke. Arch. Med. Sci. AMS 2021, 17, 19–24. [Google Scholar] [CrossRef]
- Wong, C.-L.; Tam, H.-K.V.; Fok, C.-K.V.; Lam, P.-K.E.; Fung, L.-M. Thyrotoxic atrial fibrillation: Factors associated with persistence and risk of ischemic stroke. J. Thyroid Res. 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouriou, Y.; Demaurex, N.; Bijlenga, P.; De Marchi, U. Mitochondrial calcium handling during is chemia-induced cell death in neurons. Biochimie 2011, 93, 2060–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, M.J.; Connell, B.J.; Khan, B.V.; Saleh, T.M. Cellular mechanisms by which lipoic acid confers protection during the early stages of cerebral ischemia: A possible role for calcium. Neurosci. Res. 2011, 69, 299–307. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.A.; Pei, D.S.; Xu, T.J. Calcium/calmodulin-dependent kinase II facilitated GluR6 subunit serine phosphorylation through GluR6-PSD95-CaMKII signaling module assembly in cerebral ischemia injury. Brain Res. 2010, 1366, 197–203. [Google Scholar] [CrossRef]
- Nielsen, T.H.; Bindslev, T.T.; Pedersen, S.M.; Toft, P.; Olsen, N.V.; Nordström, C.H. Cerebralenergy metabolism during induced mitochondrial dysfunction. Acta Anaesthesiol. Scand. 2013, 57, 229–235. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Morales, C.R.; Pedrozo, Z.; Lavandero, S.; Hill, J.A. Oxidative stress and autophagy in cardiovascular homeostasis. Antioxid. Redox Signal. 2014, 20, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Zhang, L.; Sun, S.; Yi, Z.; Jiang, X.; Jia, D. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int. J. Mol. Med. 2016, 38, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Ahadpour, M.; Eskandari, M.R.; Mashayekhi, V.; Haj Mohammad Ebrahim Tehrani, K.; Jafarian, I.; Naserzadeh, P.; Hosseini, M.J. Mitochondrial oxidative stress and dysfunction induced by isoniazid: Study on isolated rat liver and brain mitochondria. Drug Chem. Toxicol. 2016, 39, 224–232. [Google Scholar] [CrossRef]
- Tao, R.R.; Ji, Y.L.; Lu, Y.M.; Fukunaga, K.; Han, F. Targeting nitrosative stress for neurovascular protection: New implications in brain diseases. Curr. Drug Targets 2012, 13, 272–284. [Google Scholar] [CrossRef]
- Wang, L.; Deng, S.; Lu, Y.; Zhang, Y.; Yang, L.; Guan, Y.; Jiang, H.; Li, H. Increased inflammation and brain injury after transient focal cerebra ischemia in activating transcription factor 3 knockout mice. Neuroscience 2012, 220, 100–108. [Google Scholar] [CrossRef]
- Kleinschnitz, C.; Kraft, P.; Dreykluft, A.; Hagedorn, I.; Göbel, K.; Schuhmann, M.K.; Langhauser, F.; Helluy, X.; Schwarz, T.; Bittner, S.; et al. Regulatory T cells are strongpromoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013, 121, 679–691. [Google Scholar] [CrossRef]
- Arranz, A.M.; Gottlieb, M.; Pérez-Cerdá, F.; Matute, C. Increased expression of glutamate transporters in subcortical white matter after transient focal cerebral ischemia. Neurobiol. Dis. 2010, 37, 156–165. [Google Scholar] [CrossRef]
- Ashraf, M.I.; Ebner, M.; Wallner, C.; Haller, M.; Khalid, S.; Schwelberger, H.; Koziel, K.; Enthammer, M.; Hermann, M.; Sickinger, S.; et al. A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and isehemia/reperfusion injury. Cell Commun. Signal. 2014, 12, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Xia, J.; Xu, J.-F.; Chen, L.; Yang, Y.; Wu, J.-J.; Tang, F.; Ao, H.; Peng, C. Nuciferine, an active ingredient derived from lotus leaf, lights up the way for the potential treatment of obesity and obesity-related diseases. Pharmacol. Res. 2022, 175, 106002. [Google Scholar] [CrossRef]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Turoń-Skrzypińska, A.; Rotter, I. Pre-stroke statin therapy improves in-hospital prognosis following acute ischemic stroke associated with well-controlled nonvalvular atrial fibrillation. J. Clin. Med. 2021, 10, 3036. [Google Scholar] [CrossRef]
- Wu, L.; Chen, C.; Li, Y.; Guo, C.; Fan, Y.; Yu, D.; Zhang, T.; Wen, B.; Yan, Z.; Liu, A. UPLC-Q-TOF/MS-Based Serum Metabolomics Reveals the Anti-Ischemic Stroke Mechanism of Nuciferine in MCAO Rats. ACS Omega 2020, 5, 33433–33444. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, S.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Hamann, G.F. Acute cerebral infarct: Physiopathology and modern therapeutic concepts. Radiologe 1997, 37, 843–852. [Google Scholar] [CrossRef]
- Montaner, J. Treatment with statins in the acute phase of ischemic stroke. Expert Rev. Neurother. 2005, 5, 211–221. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Wang, Y.; Crawford, M.A.; Cunnane, S.C.; Parkington, J.E.; Schmidt, W.F. Brain-specific lipids from marine, lacustrine, or terrestrial food resources: Potential impact on early African Homo sapiens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 653–673. [Google Scholar] [CrossRef]
- Han, X. Neurolipidomics: Challenges and developments. Front. Biosci. J. Virtual Libr. 2007, 12, 2601–2622. [Google Scholar] [CrossRef] [Green Version]
- Edsall, L.C.; Spiegel, S. Enzymatic measurement of sphingosine 1-phosphate. Anal. Biochem. 1999, 272, 80–86. [Google Scholar] [CrossRef]
- Tosaka, M.; Okajima, F.; Hashiba, Y.; Saito, N.; Nagano, T.; Watanabe, T.; Kimura, T.; Sasaki, T. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: Possible role in pathogenesis of cerebral vasospasm. Stroke 2001, 32, 2913–2919. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, Y.; Suzuki, H.; Sozen, T.; Rolland, W.; Zhang, J.H. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 2010, 41, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Aruga, J. Sphingosine 1-Phosphate Signaling Is Involved in Impaired Blood–Brain Barrier Function in Ischemia–Reperfusion Injury. Mol. Neurobiol. 2020, 57, 1594–1606. [Google Scholar] [CrossRef]
- Blondeau, N.; Lai, Y.; Tyndall, S.; Popolo, M.; Topalkara, K.; Pru, J.K.; Zhang, L.; Kim, H.; Liao, J.K.; Ding, K.; et al. Distribution of sphingosine kinase activity and mRNA in rodent brain. J. Neurochem. 2007, 103, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Villapol, S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell. Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Han, L.; Cai, W.; Mao, L.; Liu, J.; Li, P.; Leak, R.K.; Xu, Y.; Hu, X.; Chen, J. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke 2015, 46, 2628–2636. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Tsai, H.D.; Cheung, W.M.; Hsu, C.Y.; Lin, T.N. PPAR-γ ameliorates neuronal apoptosis and ischemic brain injury via suppressing NF-κB-driven p22phox transcription. Mol. Neurobiol. 2016, 53, 3626–3645. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Chen, G. Aggravation of cerebral ischemia/reperfusion injury by peroxisome proliferator-activated receptor-gamma deficiency via endoplasmic reticulum stress. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7518. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Z.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Li, J.; Xie, X.; Pang, X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 66, 309–316. [Google Scholar] [CrossRef]
- Wu, J.-S.; Cheung, W.-M.; Tsai, Y.-S.; Chen, Y.-T.; Fong, W.-H.; Tsai, H.-D.; Chen, Y.-C.; Liou, J.-Y.; Shyue, S.-K.; Chen, J.-J.; et al. Ligand-Activated Peroxisome Proliferator–Activated Receptor-γ Protects Against Ischemic Cerebral Infarction and Neuronal Apoptosis by 14-3-3ε Upregulation. Circulation 2009, 119, 1124–1134. [Google Scholar] [CrossRef]

| Transcript ID | Gene ID | Gene Name | Vehicle vs. Sham | Drug vs. Vehicle | Fold Change |
|---|---|---|---|---|---|
| ENSRNOT00000045087 | ENSRNOG00000013305 | Atp2c1 | down | up | 1,682,348.77 |
| ENSRNOT00000040559 | ENSRNOG00000013351 | Stxbp5 | down | up | 169.688 |
| ENSRNOT00000081050 | ENSRNOG00000018716 | Dennd2c | down | up | 168.795 |
| ENSRNOT00000076780 | ENSRNOG00000011160 | AC126641.1 | down | up | 153.802 |
| ENSRNOT00000025172 | ENSRNOG00000010440 | Gnal | down | up | 101.410 |
| ENSRNOT00000016184 | ENSRNOG00000012138 | Rbmxl1 | down | up | 100.251 |
| ENSRNOT00000025465 | ENSRNOG00000018842 | Pou4f3 | down | up | 39.891 |
| ENSRNOT00000005382 | ENSRNOG00000026371 | Krt17 | down | up | 35.125 |
| ENSRNOT00000019477 | ENSRNOG00000014441 | Krt16 | down | up | 26.506 |
| ENSRNOT00000002887 | ENSRNOG00000002117 | Barhl2 | down | up | 26.208 |
| ENSRNOT00000088424 | ENSRNOG00000013076 | Csnk1e | up | down | 0.000 |
| ENSRNOT00000092103 | ENSRNOG00000012294 | Heph | up | down | 0.003 |
| ENSRNOT00000089142 | ENSRNOG00000015428 | Mff | up | down | 0.008 |
| ENSRNOT00000083336 | ENSRNOG00000053084 | Fuz | up | down | 0.010 |
| ENSRNOT00000012655 | ENSRNOG00000009411 | Chn2 | up | down | 0.024 |
| ENSRNOT00000011742 | ENSRNOG00000022116 | Gjb6 | up | down | 0.065 |
| ENSRNOT00000001222 | ENSRNOG00000000920 | Phkg1 | up | down | 0.073 |
| ENSRNOT00000048453 | ENSRNOG00000028837 | AC128207.1 | up | down | 0.086 |
| ENSRNOT00000015855 | ENSRNOG00000011716 | Degs2 | up | down | 0.105 |
| ENSRNOT00000021120 | ENSRNOG00000015768 | Nat8f5 | up | down | 0.111 |
| Transcript ID | Gene ID | Gene Name | Degree | Betweenness Centrality | Closeness Centrality |
|---|---|---|---|---|---|
| ENSRNOT00000003921 | ENSRNOG00000002911 | Alb | 48 | 0.1530 | 0.5829 |
| ENSRNOT00000003567 | ENSRNOG00000002525 | Ptgs2 | 35 | 0.0669 | 0.5256 |
| ENSRNOT00000083542 | ENSRNOG00000047466 | Bdnf | 32 | 0.0757 | 0.5279 |
| ENSRNOT00000024917 | ENSRNOG00000018445 | Agt | 27 | 0.0360 | 0.5062 |
| ENSRNOT00000011742 | ENSRNOG00000022116 | Gjb6 | 25 | 0.0361 | 0.4862 |
| ENSRNOT00000090156 | ENSRNOG00000061818 | Wnt1 | 24 | 0.0450 | 0.5000 |
| ENSRNOT00000070912 | ENSRNOG00000045829 | Thbs1 | 23 | 0.0142 | 0.4843 |
| ENSRNOT00000003703 | ENSRNOG00000002754 | Areg | 22 | 0.0312 | 0.4767 |
| ENSRNOT00000036947 | ENSRNOG00000002667 | Lamc2 | 21 | 0.0226 | 0.4659 |
| ENSRNOT00000019272 | ENSRNOG00000014320 | Inhba | 21 | 0.0390 | 0.4862 |
| ENSRNOT00000014948 | ENSRNOG00000011130 | Calca | 20 | 0.0218 | 0.4862 |
| ENSRNOT00000034719 | ENSRNOG00000007374 | Tac1 | 20 | 0.0331 | 0.4731 |
| ENSRNOT00000026121 | ENSRNOG00000019120 | Hmgcs2 | 18 | 0.0211 | 0.4556 |
| ENSRNOT00000025172 | ENSRNOG00000010440 | Gnal | 18 | 0.0657 | 0.4731 |
| ENSRNOT00000092245 | ENSRNOG00000020650 | Slc17a7 | 18 | 0.0239 | 0.4731 |
| ENSRNOT00000085043 | ENSRNOG00000055103 | Cftr | 17 | 0.0239 | 0.4624 |
| ENSRNOT00000086633 | ENSRNOG00000059947 | Sdc1 | 17 | 0.0132 | 0.4624 |
| ENSRNOT00000018649 | ENSRNOG00000013928 | Dsp | 16 | 0.0082 | 0.4539 |
| ENSRNOT00000020864 | ENSRNOG00000015152 | Pkp3 | 16 | 0.0262 | 0.4362 |
| ENSRNOT00000004753 | ENSRNOG00000003463 | Srebf1 | 15 | 0.0450 | 0.4659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Ma, Q.; Jiang, J.; Wang, T.; Qiu, L.; Liu, A. Protective Effects of Nuciferine in Middle Cerebral Artery Occlusion Rats Based on Transcriptomics. Brain Sci. 2022, 12, 572. https://doi.org/10.3390/brainsci12050572
Chen C, Ma Q, Jiang J, Wang T, Qiu L, Liu A. Protective Effects of Nuciferine in Middle Cerebral Artery Occlusion Rats Based on Transcriptomics. Brain Sciences. 2022; 12(5):572. https://doi.org/10.3390/brainsci12050572
Chicago/Turabian StyleChen, Chang, Quantao Ma, Jinzhu Jiang, Tieshan Wang, Linghui Qiu, and An Liu. 2022. "Protective Effects of Nuciferine in Middle Cerebral Artery Occlusion Rats Based on Transcriptomics" Brain Sciences 12, no. 5: 572. https://doi.org/10.3390/brainsci12050572
APA StyleChen, C., Ma, Q., Jiang, J., Wang, T., Qiu, L., & Liu, A. (2022). Protective Effects of Nuciferine in Middle Cerebral Artery Occlusion Rats Based on Transcriptomics. Brain Sciences, 12(5), 572. https://doi.org/10.3390/brainsci12050572







