Grape-Seed-Derived Procyanidin Attenuates Chemotherapy-Induced Cognitive Impairment by Suppressing MMP-9 Activity and Related Blood–Brain-Barrier Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug and Chemicals
2.3. Cisplatin and Procyanidin Administration
2.4. Fear Conditioning
2.5. The Object Recognition Test (ORT)
2.6. Gelatin Zymography
2.7. An In Vivo Evans Blue Assay to Test Brain–Blood Vessel Permeability
2.8. Cell Preparation and Stimulation
2.9. Western Blot
2.10. Data Analysis
3. Results
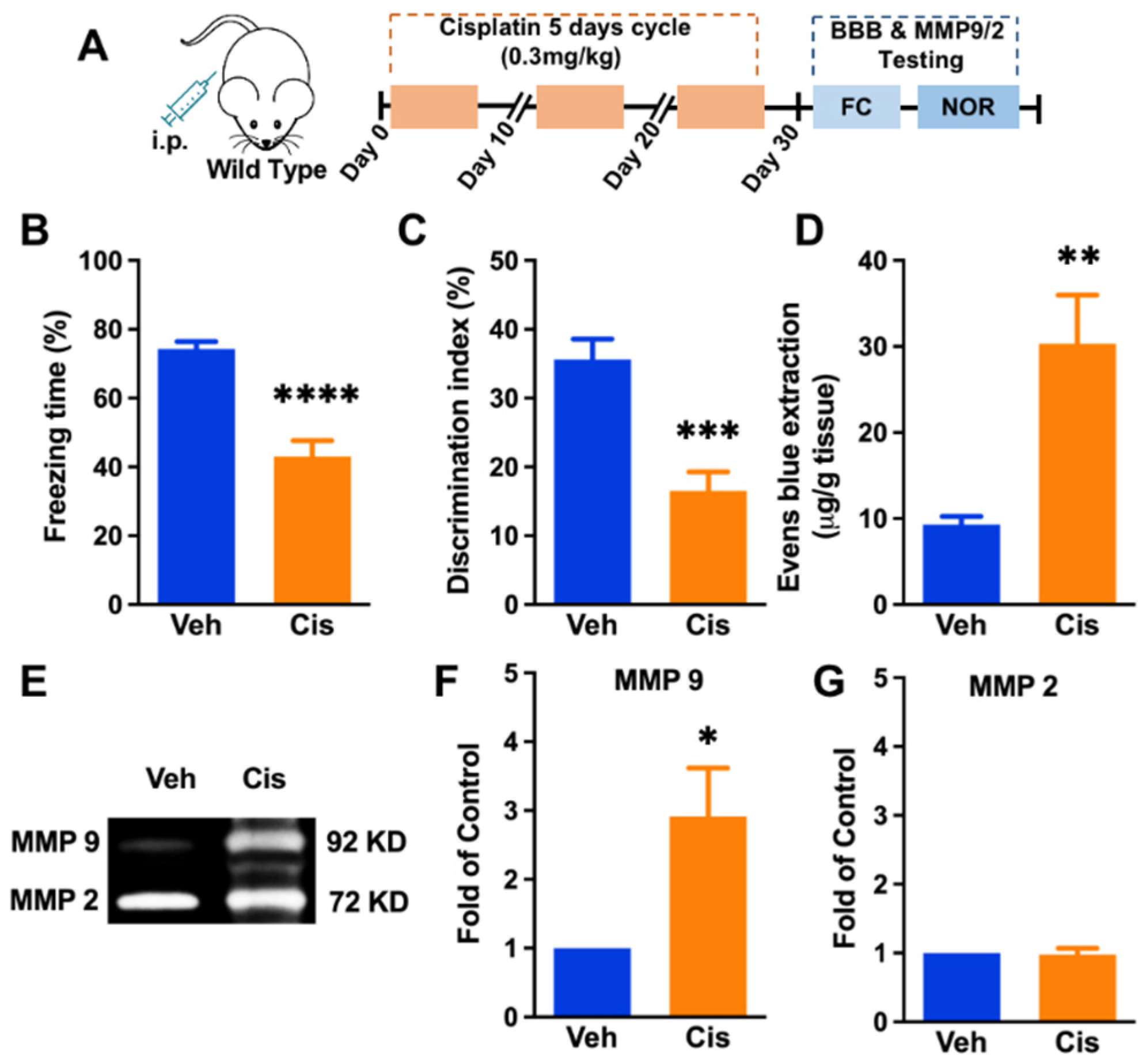
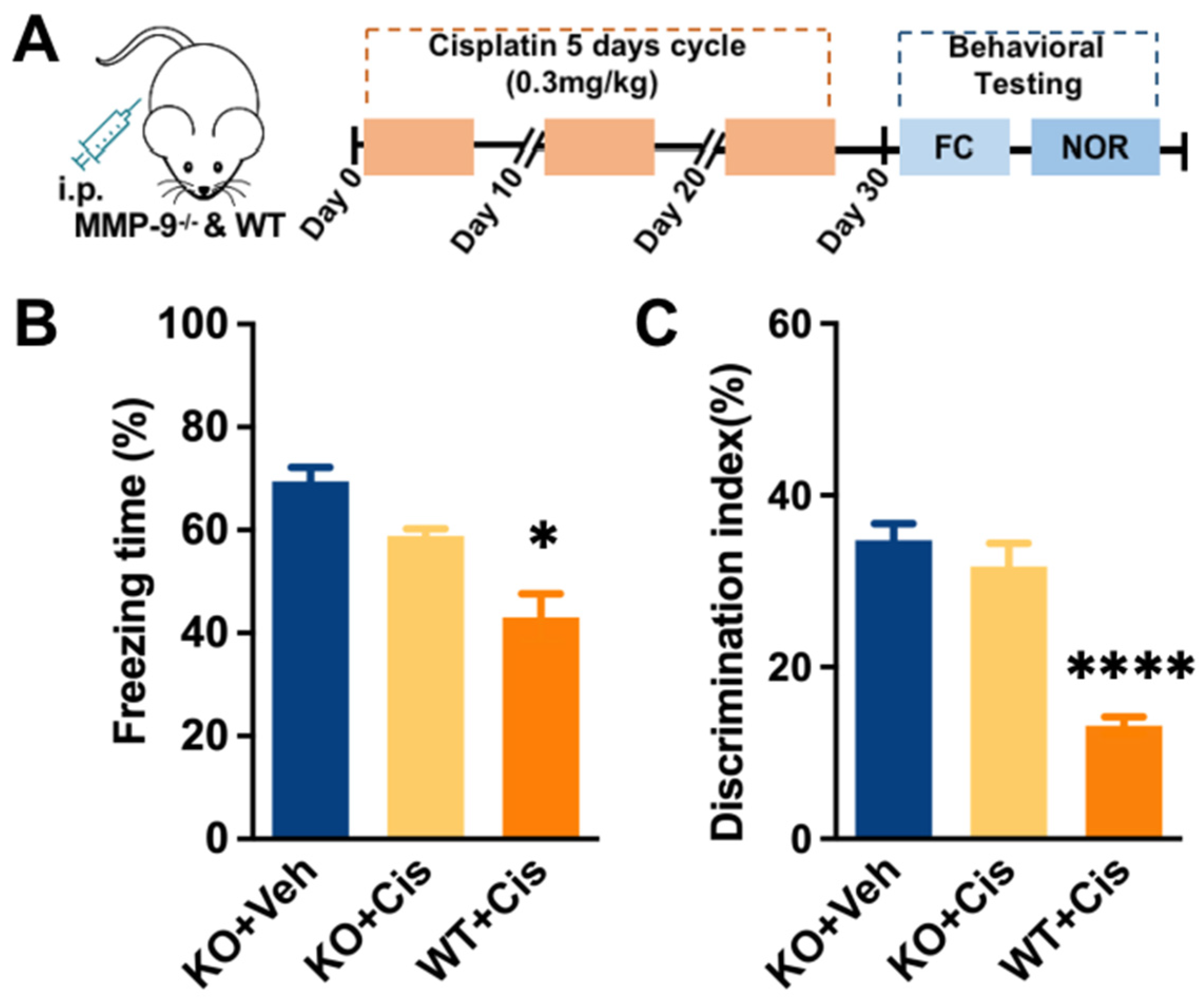
3.1. Repeated Treatment of Cisplatin-Induced Cognitive Impairment, Blood–Brain-Barrier Impairment, and Upregulation of MMP-9 Activity in the Hippocampus
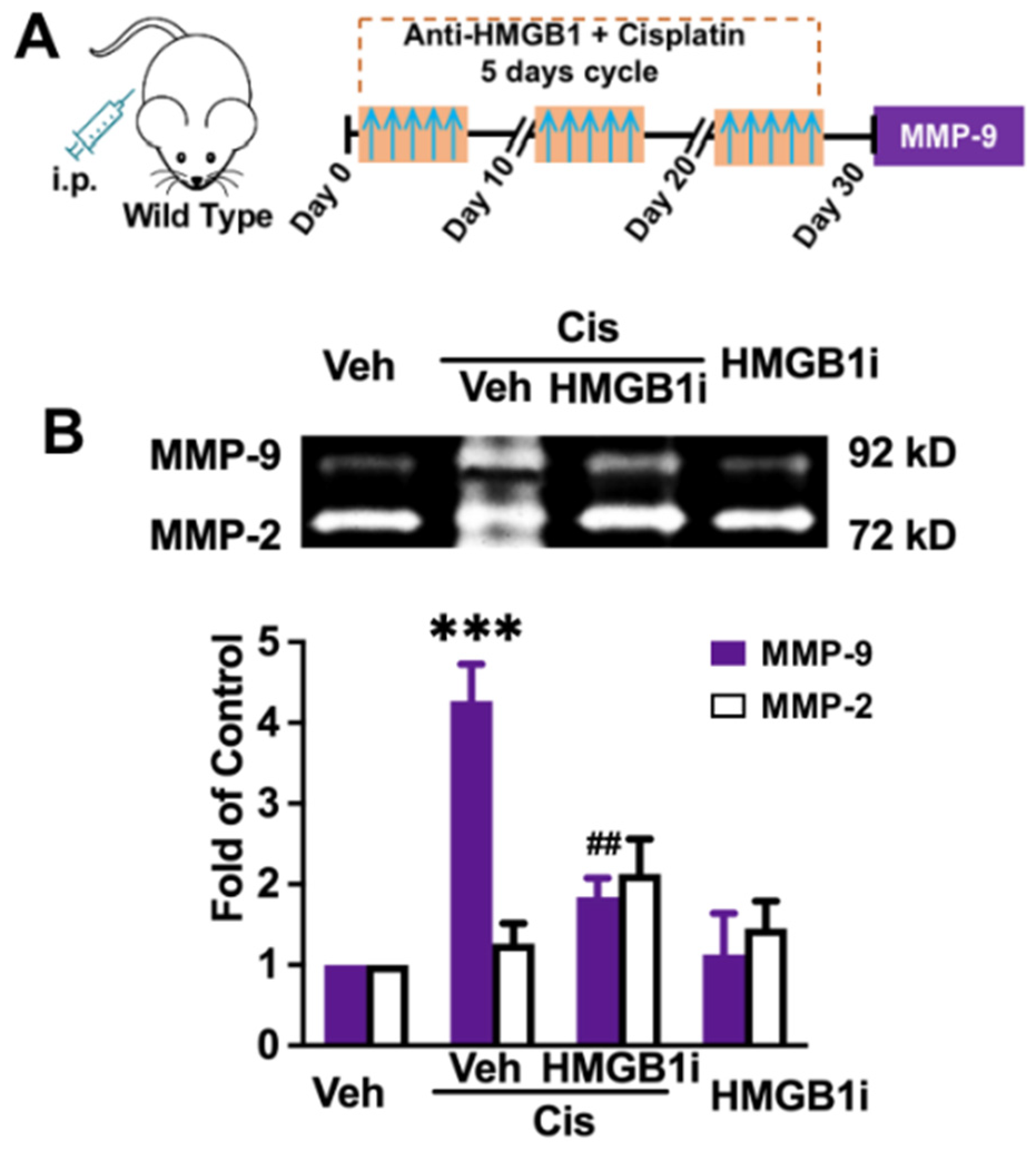
3.2. Peripheral Inhibition of HMGB1 Significantly Prevents MMP-9 Increase
3.3. The HMGB1/TLR4/PI3K/Akt Axis Is Involved in Activating MMP-9 in Raw 264.7 Cells In Vitro
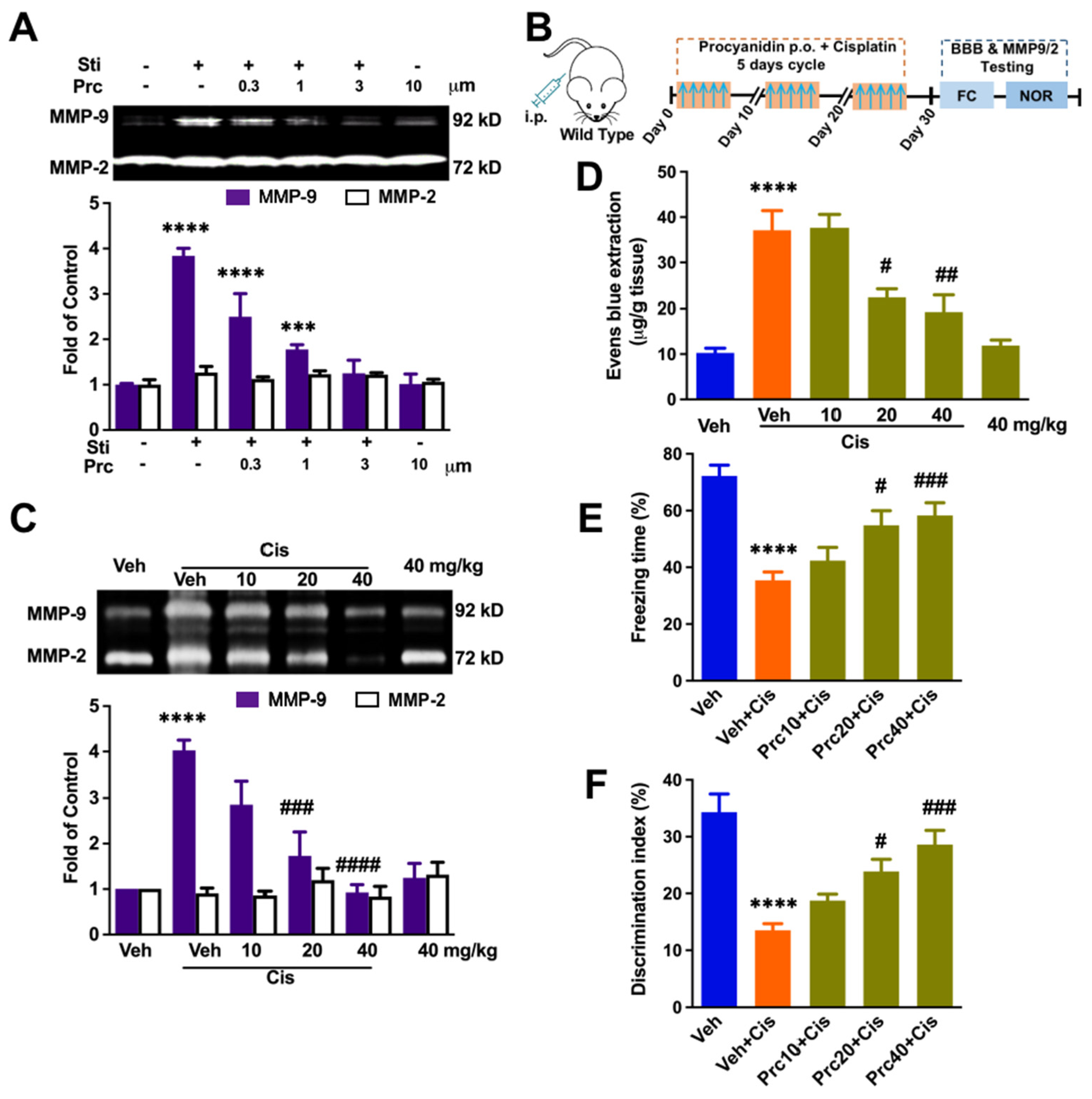
3.4. Procyanidin Suppressed MMP-9 Activity, the BBB Interruption, and Cisplatin-Induced Cognitive Decline in a Dose-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias-Carvalho, A.; Ferreira, M.; Ferreira, R.; Bastos, M.L.; Sa, S.I.; Capela, J.P.; Carvalho, F.; Costa, V.M. Four decades of chemotherapy-induced cognitive dysfunction: Comprehensive review of clinical, animal and in vitro studies, and insights of key initiating events. Arch. Toxicol. 2022, 96, 11–78. [Google Scholar] [CrossRef] [PubMed]
- Vichaya, E.G.; Chiu, G.S.; Krukowski, K.; Lacourt, T.E.; Kavelaars, A.; Dantzer, R.; Heijnen, C.J.; Walker, A.K. Mechanisms of chemotherapy-induced behavioral toxicities. Front. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simo, M.; Rifa-Ros, X.; Rodriguez-Fornells, A.; Bruna, J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neurosci. Biobehav. Rev. 2013, 37, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J.; McDonald, B.C.; Li, Y.; Furstenberg, C.T.; Hanscom, B.S.; Mulrooney, T.J.; Schwartz, G.N.; Kaufman, P.A. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J. Clin. Oncol. 2010, 28, 4434–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuertes, M.A.; Castilla, J.; Alonso, C.; Perez, J.M. Cisplatin biochemical mechanism of action: From cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr. Med. Chem. 2003, 10, 257–266. [Google Scholar] [CrossRef]
- George, R.P.; Semendric, I.; Hutchinson, M.R.; Whittaker, A.L. Neuroimmune reactivity marker expression in rodent models of chemotherapy-induced cognitive impairment: A systematic scoping review. Brain. Behav. Immun. 2021, 94, 392–409. [Google Scholar] [CrossRef]
- Ongnok, B.; Chattipakorn, N.; Chattipakorn, S.C. Doxorubicin and cisplatin induced cognitive impairment: The possible mechanisms and interventions. Exp. Neurol. 2020, 324, 113118. [Google Scholar] [CrossRef]
- Oppegaard, K.; Harris, C.S.; Shin, J.; Paul, S.M.; Cooper, B.A.; Chan, A.; Anguera, J.A.; Levine, J.; Conley, Y.; Hammer, M.; et al. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine 2021, 148, 155653. [Google Scholar] [CrossRef]
- Simic, G.; Spanic, E.; Langer Horvat, L.; Hof, P.R. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2019, 168, 99–145. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139 (Suppl. 2), 91–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandooren, J.; Van Damme, J.; Opdenakker, G. On the structure and functions of gelatinase B/matrix metalloproteinase-9 in neuroinflammation. Prog. Brain Res. 2014, 214, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Krekoski, C.A.; Forsyth, P.A.; Bell, R.; Edwards, D.R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998, 21, 75–80. [Google Scholar] [CrossRef]
- Gu, H.; Wang, C.; Li, J.; Yang, Y.; Sun, W.; Jiang, C.; Li, Y.; Ni, M.; Liu, W.T.; Cheng, Z.; et al. High mobility group box-1-toll-like receptor 4-phosphatidylinositol 3-kinase/protein kinase B-mediated generation of matrix metalloproteinase-9 in the dorsal root ganglion promotes chemotherapy-induced peripheral neuropathy. Int. J. Cancer 2020, 146, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Cong, J.; Yang, B.; Sun, Y. Association analysis of high-mobility group box-1 protein 1 (HMGB1)/toll-like receptor (TLR) 4 with nasal interleukins in allergic rhinitis patients. Cytokine 2020, 126, 154880. [Google Scholar] [CrossRef]
- Yang, H.; Hreggvidsdottir, H.S.; Palmblad, K.; Wang, H.; Ochani, M.; Li, J.; Lu, B.; Chavan, S.; Rosas-Ballina, M.; Al-Abed, Y.; et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 2010, 107, 11942–11947. [Google Scholar] [CrossRef] [Green Version]
- Lomeli, N.; Di, K.; Czerniawski, J.; Guzowski, J.F.; Bota, D.A. Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic. Biol. Med. 2017, 102, 274–286. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.D.; Huang, Y.H.; Lai, C.S.W.; Dong, C.M.; Ho, L.C.; Li, X.Y.; Wu, E.X.; Li, Q.; Wang, X.M.; Chen, Y.J.; et al. Ginsenoside Rg1 Prevents Chemotherapy-Induced Cognitive Impairment: Associations with Microglia-Mediated Cytokines, Neuroinflammation, and Neuroplasticity. Mol. Neurobiol. 2019, 56, 5626–5642. [Google Scholar] [CrossRef]
- Yi, L.T.; Dong, S.Q.; Wang, S.S.; Chen, M.; Li, C.F.; Geng, D.; Zhu, J.X.; Liu, Q.; Cheng, J. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol. Dis. 2020, 136, 104715. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Deng, X.; Jiang, C.; Pan, C.; Chen, L.; Han, Y.; Dai, W.; Hu, L.; Zhang, G.; et al. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 2016, 157, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; You, Q.; Hu, L.; Gao, J.; Meng, Q.; Liu, W.; Wu, X.; Xu, Q. The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice. Front. Immunol. 2017, 8, 1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Eliseo, D.; Pannucci, E.; Bernini, R.; Campo, M.; Romani, A.; Santi, L.; Velotti, F. In vitro studies on anti-inflammatory activities of kiwifruit peel extract in human THP-1 monocytes. J. Ethnopharmacol. 2019, 233, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Kavelaars, A.; Heijnen, C.J. Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLoS ONE 2016, 11, e0151890. [Google Scholar] [CrossRef]
- Gao, C.; Frausto, S.F.; Guedea, A.L.; Tronson, N.C.; Jovasevic, V.; Leaderbrand, K.; Corcoran, K.A.; Guzman, Y.F.; Swanson, G.T.; Radulovic, J. IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. J. Neurosci. 2011, 31, 8533–8542. [Google Scholar] [CrossRef]
- Ahuja, M.; Buabeid, M.; Abdel-Rahman, E.; Majrashi, M.; Parameshwaran, K.; Amin, R.; Ramesh, S.; Thiruchelvan, K.; Pondugula, S.; Suppiramaniam, V.; et al. Immunological alteration & toxic molecular inductions leading to cognitive impairment & neurotoxicity in transgenic mouse model of Alzheimer’s disease. Life Sci. 2017, 177, 49–59. [Google Scholar] [CrossRef]
- Manaenko, A.; Chen, H.; Kammer, J.; Zhang, J.H.; Tang, J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J. Neurosci. Methods 2011, 195, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Andres, A.L.; Gong, X.; Di, K.; Bota, D.A. Low-doses of cisplatin injure hippocampal synapses: A mechanism for ‘chemo’ brain? Exp. Neurol. 2014, 255, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Dominguez, R.L.; Perez-Medina, M.A.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Matias-Florentino, M.; Avila-Rios, S.; Rumbo-Nava, U.; Salgado-Aguayo, A.; Gonzalez-Gonzalez, C.; Aguilar-Cazares, D. Role of HMGB1 in Cisplatin-Persistent Lung Adenocarcinoma Cell Lines. Front. Oncol. 2021, 11, 750677. [Google Scholar] [CrossRef]
- Degos, V.; Vacas, S.; Han, Z.; van Rooijen, N.; Gressens, P.; Su, H.; Young, W.L.; Maze, M. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology 2013, 118, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrando, N.; Eriksson, L.I.; Ryu, J.K.; Yang, T.; Monaco, C.; Feldmann, M.; Jonsson Fagerlund, M.; Charo, I.F.; Akassoglou, K.; Maze, M. Resolving postoperative neuroinflammation and cognitive decline. Ann. Neurol. 2011, 70, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Maique, J.; Shepard, S.; Li, P.; Seli, O.; Moe, O.W.; Hu, M.C. In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 2022, 101, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Goodwani, S.; Acton, P.J.; Buggia-Prevot, V.; Kesler, S.R.; Jamal, I.; Mahant, I.D.; Liu, Z.; Mseeh, F.; Roth, B.L.; et al. Inhibition of dual leucine zipper kinase prevents chemotherapy-induced peripheral neuropathy and cognitive impairments. Pain 2021, 162, 2599–2612. [Google Scholar] [CrossRef]
- Geraghty, A.C.; Gibson, E.M.; Ghanem, R.A.; Greene, J.J.; Ocampo, A.; Goldstein, A.K.; Ni, L.; Yang, T.; Marton, R.M.; Pasca, S.P.; et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 2019, 103, 250–265.e258. [Google Scholar] [CrossRef]
- Morrison, C.; Dadar, M.; Shafiee, N.; Villeneuve, S.; Louis Collins, D.; for Alzheimer’s Disease Neuroimaging Initiative. Regional brain atrophy and cognitive decline depend on definition of subjective cognitive decline. Neuroimage Clin. 2022, 33, 102923. [Google Scholar] [CrossRef]
- Pereira, A.C.; Lambert, H.K.; Grossman, Y.S.; Dumitriu, D.; Waldman, R.; Jannetty, S.K.; Calakos, K.; Janssen, W.G.; McEwen, B.S.; Morrison, J.H. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc. Natl. Acad. Sci. USA 2014, 111, 18733–18738. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.K.L.; Chau, T.W.; Lim, E.J.; Ahmed, I.; Chang, R.C.; Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K.B. Hippocampal CA2 Lewy pathology is associated with cholinergic degeneration in Parkinson’s disease with cognitive decline. Acta Neuropathol. Commun. 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Piccolini, V.M.; Cerri, S.; Romanelli, E.; Bernocchi, G. Interactions of neurotransmitter systems during postnatal development of the rat hippocampal formation: Effects of cisplatin. Exp. Neurol. 2012, 234, 239–252. [Google Scholar] [CrossRef]
- Sun, Y.X.; Yang, J.; Wang, P.Y.; Li, Y.J.; Xie, S.Y.; Sun, R.P. Cisplatin regulates SH-SY5Y cell growth through downregulation of BDNF via miR-16. Oncol. Rep. 2013, 30, 2343–2349. [Google Scholar] [CrossRef] [Green Version]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gardella, S.; Andrei, C.; Ferrera, D.; Lotti, L.V.; Torrisi, M.R.; Bianchi, M.E.; Rubartelli, A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002, 3, 995–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yang, H.; Czura, C.J.; Sama, A.E.; Tracey, K.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, 1768–1773. [Google Scholar] [CrossRef] [PubMed]
- Toure, F.; Zahm, J.M.; Garnotel, R.; Lambert, E.; Bonnet, N.; Schmidt, A.M.; Vitry, F.; Chanard, J.; Gillery, P.; Rieu, P. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem. J. 2008, 416, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, J.C.; Lee, M.H.; Yang, C.E.; Lee, J.H.; Lee, W.J. High-Mobility Group Box 1 Mediates Fibroblast Activity via RAGE-MAPK and NF-kappaB Signaling in Keloid Scar Formation. Int. J. Mol. Sci. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuccolo, E.; Di Buduo, C.; Lodola, F.; Orecchioni, S.; Scarpellino, G.; Kheder, D.A.; Poletto, V.; Guerra, G.; Bertolini, F.; Balduini, A.; et al. Stromal Cell-Derived Factor-1alpha Promotes Endothelial Colony-Forming Cell Migration Through the Ca(2+)-Dependent Activation of the Extracellular Signal-Regulated Kinase 1/2 and Phosphoinositide 3-Kinase/AKT Pathways. Stem Cells Dev. 2018, 27, 23–34. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Wang, X.W.; Chen, J.F.; Ren, Q.L.; Wang, J.; Gao, B.W.; Shi, Z.H.; Zhang, Z.J.; Bai, X.X.; Xing, B.S. Grape Seed Procyanidin B2 Protects Porcine Ovarian Granulosa Cells against Oxidative Stress-Induced Apoptosis by Upregulating let-7a Expression. Oxid. Med. Cell. Longev. 2019, 2019, 1076512. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.C.; Huang, C.C.; Chen, C.H.; Chiang, H.C.; Chen, K.B.; Chen, Y.J.; Liu, C.L.; Chuang, L.T.; Liu, M.; Hsu, C.P. Grape-seed procyanidins inhibit the in vitro growth and invasion of pancreatic carcinoma cells. Pancreas 2012, 41, 447–454. [Google Scholar] [CrossRef]
- Mounier, N.M.; Abdel-Maged, A.E.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Chemotherapy-induced cognitive impairment (CICI): An overview of etiology and pathogenesis. Life Sci. 2020, 258, 118071. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Gao, C.; Wang, Z. Grape-Seed-Derived Procyanidin Attenuates Chemotherapy-Induced Cognitive Impairment by Suppressing MMP-9 Activity and Related Blood–Brain-Barrier Damage. Brain Sci. 2022, 12, 571. https://doi.org/10.3390/brainsci12050571
Song C, Gao C, Wang Z. Grape-Seed-Derived Procyanidin Attenuates Chemotherapy-Induced Cognitive Impairment by Suppressing MMP-9 Activity and Related Blood–Brain-Barrier Damage. Brain Sciences. 2022; 12(5):571. https://doi.org/10.3390/brainsci12050571
Chicago/Turabian StyleSong, Chao, Chao Gao, and Zhenxin Wang. 2022. "Grape-Seed-Derived Procyanidin Attenuates Chemotherapy-Induced Cognitive Impairment by Suppressing MMP-9 Activity and Related Blood–Brain-Barrier Damage" Brain Sciences 12, no. 5: 571. https://doi.org/10.3390/brainsci12050571
APA StyleSong, C., Gao, C., & Wang, Z. (2022). Grape-Seed-Derived Procyanidin Attenuates Chemotherapy-Induced Cognitive Impairment by Suppressing MMP-9 Activity and Related Blood–Brain-Barrier Damage. Brain Sciences, 12(5), 571. https://doi.org/10.3390/brainsci12050571




