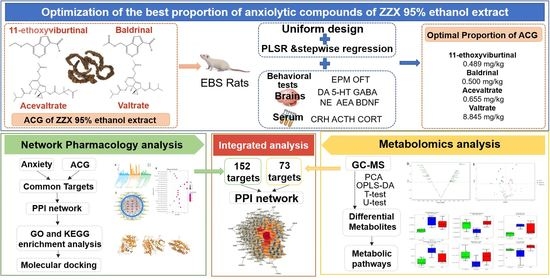
Dose Optimization of Anxiolytic Compounds Group in Valeriana jatamansi Jones and Mechanism Exploration by Integrating Network Pharmacology and Metabolomics Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optimization of Anxiolytic Dose Ratio and Efficacy Evaluation
2.1.1. Chemicals and Materials
2.1.2. Animals
2.1.3. Establishment of the Rat EBS Model
2.1.4. Uniform Design Experiment
Animal Grouping and Drug Administration
Elevated Plus-Maze Test (EPM)
Open Field Test (OFT)
Sample Collection and Detection
Determination of Optimal Proportion of ACGs
Verification Test
Statistical Analysis of the Pharmacodynamic Experiment
2.2. Network Pharmacology Analysis
2.2.1. Acquisition of Information and Targets of ACG Compounds
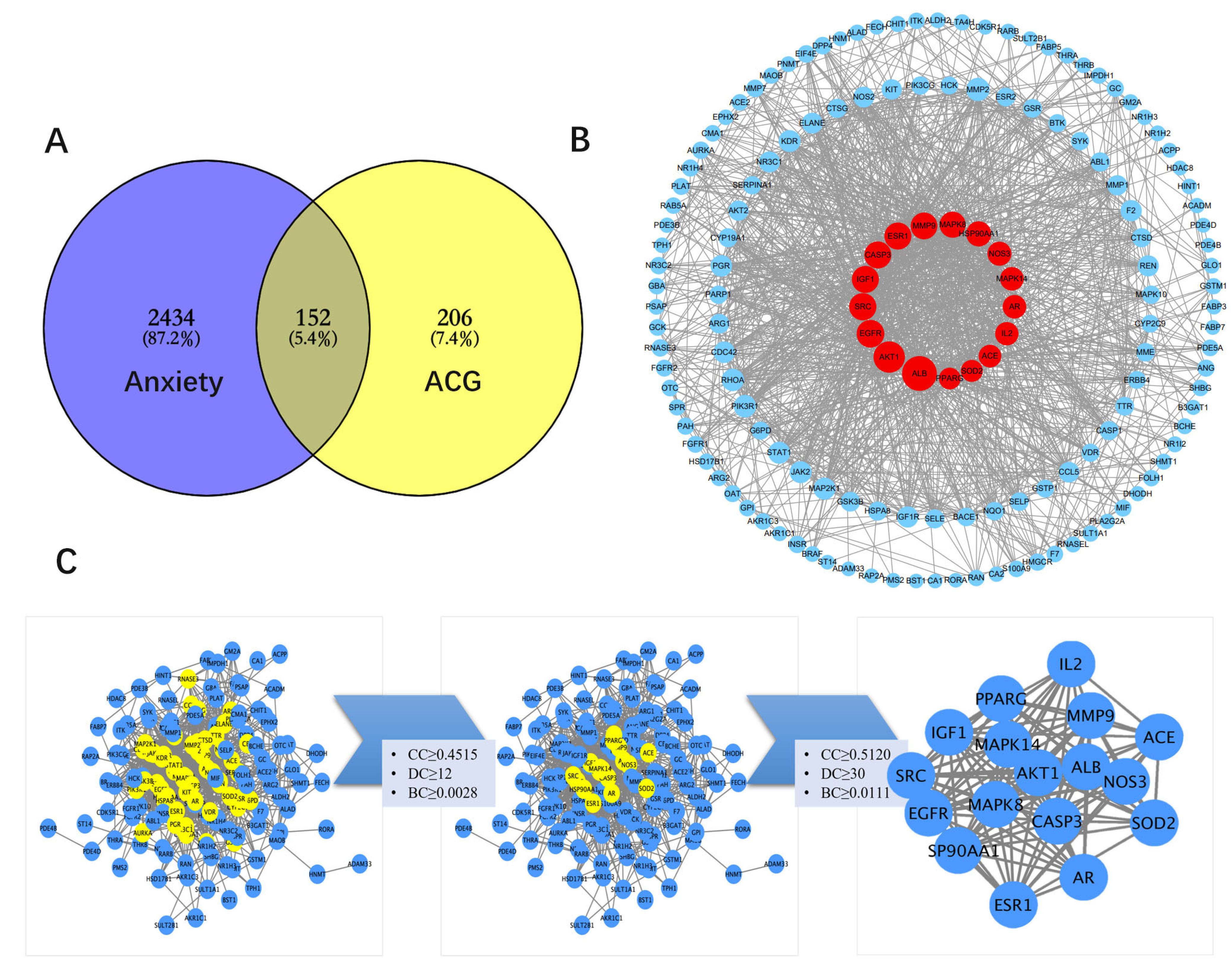
2.2.2. Screening of Anxiety-Associated Targets
2.2.3. Construction of the PPI Network and Enrichment of Biological Function and Pathway
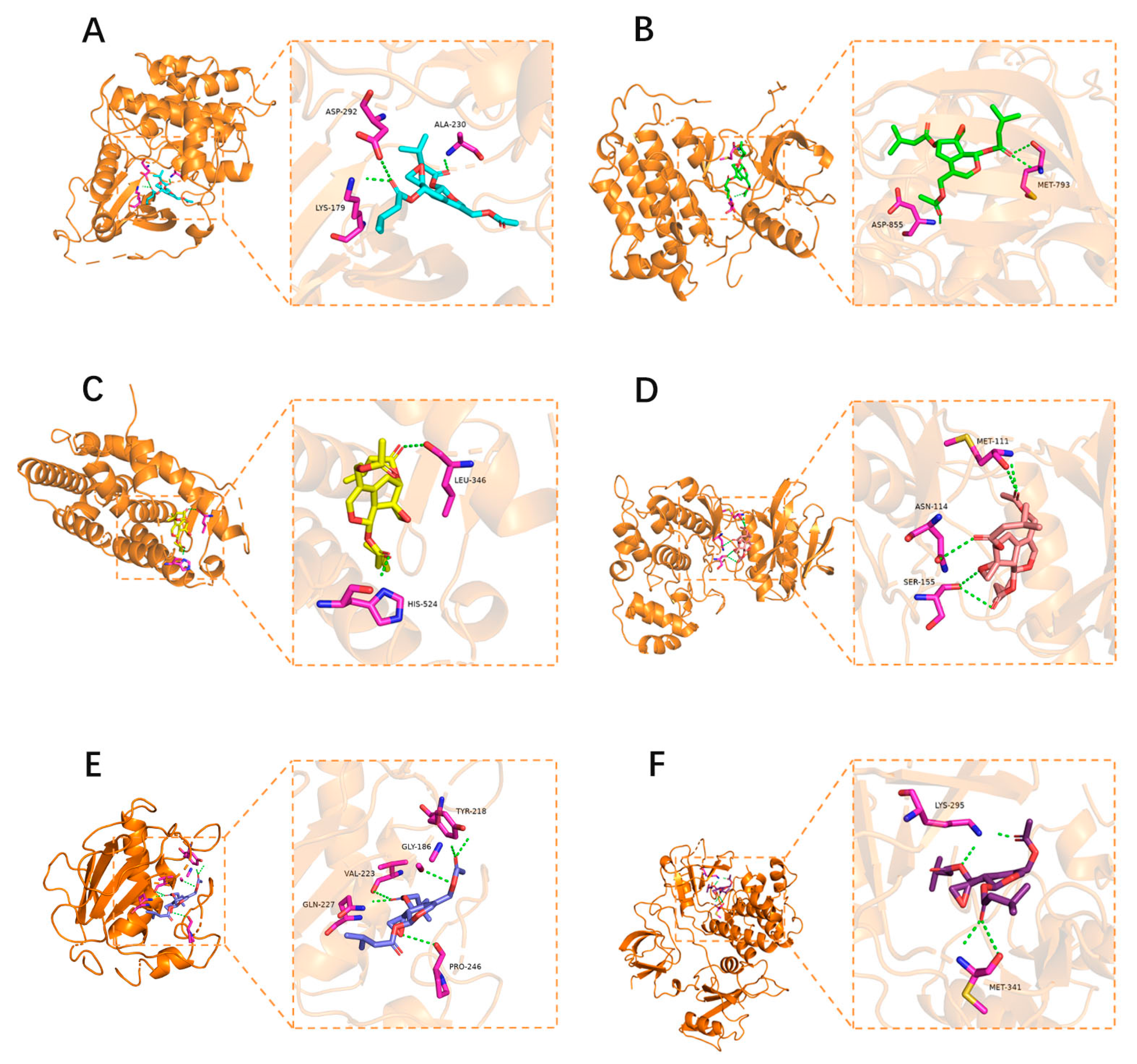
2.2.4. Molecular Docking
2.3. Metabolomics Analysis
2.3.1. Sample Preparation
2.3.2. GC-MS Detection Conditions
2.3.3. Screening of Differential Metabolites and Analysis of In Vivo Metabolic Pathway
2.3.4. Integration of Network Pharmacology and Metabolomics
3. Results
3.1. Elevated Plus-Maze Test (EPM)
3.2. Open Field Test (OFT)
3.3. Biochemical Indicators in Serum and Brain Samples
3.4. Determination of the Best Proportion of Anxiolytic Components of 95% Ethanol Extract of ZZX
3.5. Verification Test
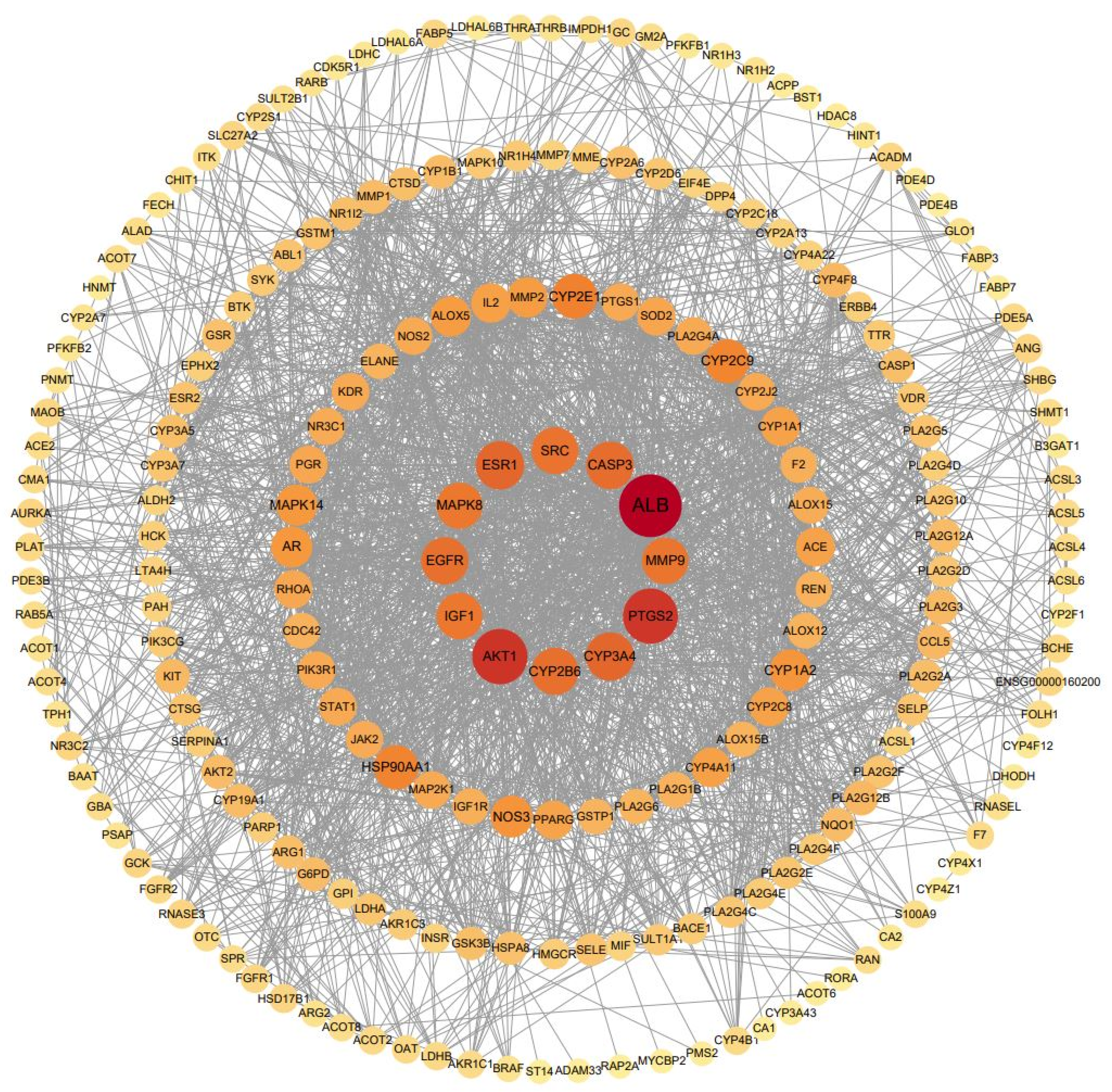
3.6. Construction and Topology Analysis of PPI Network of Compounds–Anxiety Disease Target Genes
3.7. GO and KEGG Analysis
3.8. Molecular Docking Verification
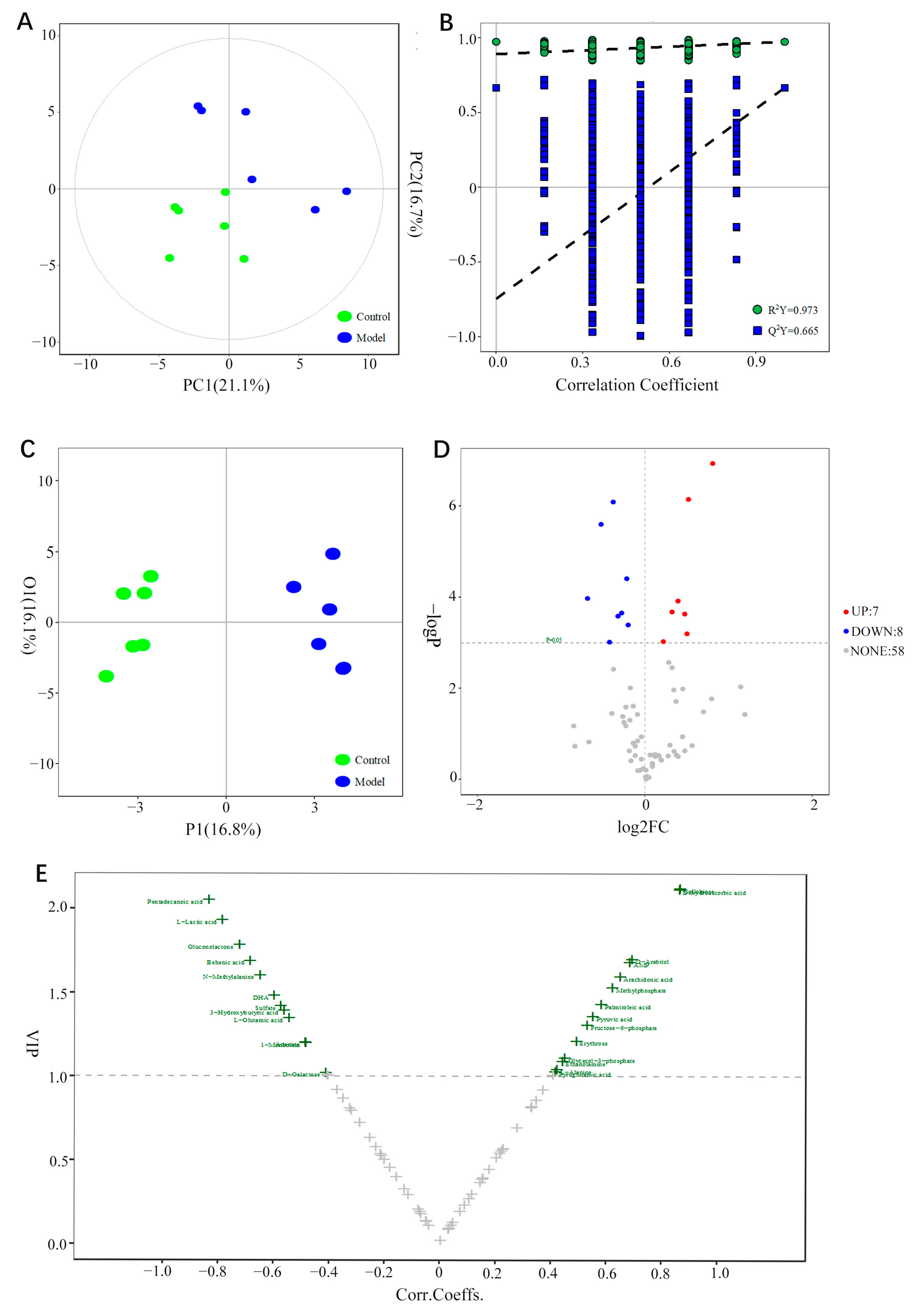
3.9. Screening of Potential Biomarkers
3.10. Enrichment of Metabolic Pathways
3.11. Analysis of the Integration Mechanism of Metabolomics and Network Pharmacology
4. Discussion
4.1. Dose Optimization of Anxiolytic Compounds Group
4.2. Mechanism Prediction and Verification by Network Pharmacology and Molecular Docking
4.3. Mechanism Exploration by Metabolomics
4.4. Mechanism Exploration by Integrating Network Pharmacology and Metabolomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Griebel, G.; Holmes, A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013, 12, 667–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twenge, J.M.; McAllister, C.; Joiner, T.E. Anxiety and depressive symptoms in U.S. Census Bureau assessments of adults: Trends from 2019 to fall 2020 across demographic groups. J. Anxiety Disord. 2021, 83, 102455. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.E.; Dee, N.; Bernard, A.; Lerchner, W.; Heintz, N.; Anderson, D.J. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 2014, 156, 522–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatziv, T.; Vancor, E.A.; Bunderson, M.; Rutherford, H.J.V. Maternal perinatal anxiety and neural responding to infant affective signals: Insights, challenges, and a road map for neuroimaging research. Neurosci. Biobehav. Rev. 2021, 131, 387–399. [Google Scholar] [CrossRef]
- De las Cuevas, C.; Sanz, E.J.; de la Fuente, J.A.; Padilla, J.; Berenguer, J.C. The Severity of Dependence Scale (SDS) as screening test for benzodiazepine dependence: SDS validation study. Addiction 2000, 95, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Jugran, A.K.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Valeriana jatamansi: An herbaceous plant with multiple medicinal uses. Phytother. Res. 2019, 33, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.N.; Shi, J.L.; Liu, Y.; Wang, Y.L.; Wang, C.G.; Hou, W.H.; Guo, J.Y. The anxiolytic effects of valtrate in rats involves changes of corticosterone levels. Evid.-Based Complement. Alternat Med. 2014, 2014, 325948. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhang, T.E.; Xiao, T.; Pan, L.Z.; Qin, J.Z.; Zhang, Z.P.; Zuo, C.Y. Anti-anxiety effect of Valeriana jatamansi Jones extract via regulation of the hypothalamus-pituitary-adrenal axis. Neural Regen. Res. 2010, 5, 1071–1075. [Google Scholar]
- Zhang, X.M.; Zhu, J.L.; Sun, Y.; Dai, Y.L.; Chen, X.; Cao, J.H.; Yang, R.C.; Chen, C.; Ma, C.Y.; Zhang, T.E.; et al. Anxiolytic potency of iridoid fraction extracted from Valeriana jatamansi Jones and its mechanism: A preliminary study. Nat. Prod. Res. 2018, 32, 2071–2075. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhang, T.E.; Pan, L.Z.; Zuo, C.Y. Action of Valeriana jatamansi Jones on the Apoptosis-related Genes Expression in the Anxiety Model of Rat. Procedia Environ. Sci. 2011, 8, 744–750. [Google Scholar]
- Wang, S.N.; Ding, Y.S.; Ma, X.J.; Zhao, C.B.; Lin, M.X.; Luo, J.; Jiang, Y.N.; He, S.; Guo, J.Y.; Shi, J.L. Identification of Bioactive Chemical Markers in Zhi zhu xiang Improving Anxiety in Rat by Fingerprint-Efficacy Study. Molecules 2018, 23, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.N.; Yao, Z.W.; Zhao, C.B.; Ding, Y.S.; Jing, L.; Bian, L.H.; Li, Q.Y.; Wang, X.M.; Shi, J.L.; Guo, J.Y.; et al. Discovery and proteomics analysis of effective compounds in Valeriana jatamansi jones for the treatment of anxiety. J. Ethnopharmacol. 2021, 265, 113452. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.J.; Huang, Y.X.; Feng, L.M.; Yue, S.J.; Chen, Y.Y.; Fu, R.J.; Xu, D.Q.; Tang, Y.P. Network pharmacology-based study on immunomodulatory mechanism of danggui-yimucao herb pair for the treatment of RU486-induced abortion. J. Ethnopharmacol. 2022, 282, 114609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, M.; Zhang, B.; Liu, C.; Guo, Q.; Sun, Y.; Wang, D.; Wang, C.; Jiang, Y.; Lin, N.; et al. Uncovering pharmacological mechanisms of Wu-tou decoction acting on rheumatoid arthritis through systems approaches: Drug-target prediction, network analysis and experimental validation. Sci. Rep. 2015, 5, 9463. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Hong, B.; Li, Q.; Li, Z.; Bi, K. An integrated serum and urinary metabonomic research of Rhizoma Curcumae-Rhizoma Sparganii drug pair in hysteromyoma rats based on UPLC-Q-TOF-MS analysis. J. Ethnopharmacol. 2019, 231, 374–385. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Li, J.; Zhou, X.J.; Wu, M.H.; Li, L.; Pei, G.; Chen, N.H.; Liu, K.L.; Xie, M.Z.; Huang, H.Y. HS-GC-IMS-Based metabonomics study of Baihe Jizihuang Tang in a rat model of chronic unpredictable mild stress. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1148, 122143. [Google Scholar] [CrossRef]
- He, Y.; Ouyang, J.; Hu, Z.; Yang, J.; Chu, Y.; Huang, S.; Yang, Y.; Liu, C. Intervention mechanism of repeated oral GABA administration on anxiety-like behaviors induced by emotional stress in rats. Psychiat. Res.-Neuroim. 2019, 271, 649–657. [Google Scholar] [CrossRef]
- Shoji, H.; Miyakawa, T. Effects of test experience, closed-arm wall color, and illumination level on behavior and plasma corticosterone response in an elevated plus maze in male C57BL/6J mice: A challenge against conventional interpretation of the test. Mol. Brain 2021, 14, 34. [Google Scholar] [CrossRef]
- Snyder, C.N.; Brown, A.R.; Buffalari, D. Similar tests of anxiety-like behavior yield different results: Comparison of the open field and free exploratory rodent procedures. Physiol. Behav. 2021, 230, 113246. [Google Scholar] [CrossRef]
- Hill, M.N.; McLaughlin, R.J.; Morrish, A.C.; Viau, V.; Floresco, S.B.; Hillard, C.J.; Gorzalka, B.B. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 2009, 34, 2733–2745. [Google Scholar] [CrossRef]
- Lang, U.E.; Hellweg, R.; Kalus, P.; Bajbouj, M.; Lenzen, K.P.; Sander, T.; Kunz, D.; Gallinat, J. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology 2005, 180, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.M.; Ji, Y.B.; Zhu, D.N. An Integrated Approach Based on Experimental Designs for Fingerprint Development of the Complex Herbal Prescription Sheng-Mai-San by MEKC. Chromatographia 2010, 71, 667–677. [Google Scholar] [CrossRef]
- Wang, L.; Liu, L.F.; Wang, J.Y.; Shi, Z.Q.; Chang, W.Q.; Chen, M.L.; Yin, Y.H.; Jiang, Y.; Li, H.J.; Li, P.; et al. A strategy to identify and quantify closely related adulterant herbal materials by mass spectrometry-based partial least squares regression. Anal. Chim. Acta 2017, 977, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L. Stepwise regression prediction model of multiple regression analysis. Exam. Wkly. 2009, 26, 93. [Google Scholar]
- Fan, B.; Li, Y.; Bi, Y.; Tong, L.; Li, D.; Wang, Y. Design of multi-drug combinations for poly-pharmacological effects using composition-activity relationship modeling and multi-objective optimization approach: Application in traditional Chinese medicine. Chem. Biol. Drug Des. 2019, 93, 1073–1082. [Google Scholar] [CrossRef]
- Wan, S.; Zhang, J.; Hou, R.; Zheng, M.; Liu, L.; Zhang, M.; Li, Z.; Huang, X. A strategy for component-based Chinese medicines design approach of Polygonum orientale L. against hypoxia/reoxygenation based on uniform design-stepwise regression-simulated annealing. Biomed. Pharmacother. 2021, 135, 111177. [Google Scholar] [CrossRef]
- Ye, H.; Wei, J.; Tang, K.; Feuers, R.; Hong, H. Drug Repositioning Through Network Pharmacology. Curr. Top. Med. Chem. 2016, 16, 3646–3656. [Google Scholar] [CrossRef]
- Scariot, R.; Tomaz, C.O.; Calixto, R.D.; Gerber, J.T.; Pivetta Petinati, M.F.; Cavalcante, R.C.; Küchler, E.C.; João da Costa, D. Association between gender, estrogen receptors genes and anxiety levels in patients undergoing orthognathic surgery. J. Craniomaxillofac. Surg. 2019, 47, 1300–1305. [Google Scholar] [CrossRef]
- Ward, K.R.; Featherstone, R.E.; Naschek, M.J.; Melnychenko, O.; Banerjee, A.; Yi, J.; Gifford, R.L.; Borgmann-Winter, K.E.; Salter, M.W.; Hahn, C.G.; et al. Src deficient mice demonstrate behavioral and electrophysiological alterations relevant to psychiatric and developmental disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 84–92. [Google Scholar] [CrossRef]
- Delawary, M.; Tezuka, T.; Kiyama, Y.; Yokoyama, K.; Inoue, T.; Hattori, S.; Hashimoto, R.; Umemori, H.; Manabe, T.; Yamamoto, T.; et al. NMDAR2B tyrosine phosphorylation regulates anxiety-like behavior and CRF expression in the amygdala. Mol. Brain 2010, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, H.; Murata, Y.; Okazawa, H.; Matozaki, T. Src family kinases: Modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011, 34, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhang, G.; Hou, S.; Sha, L.G. MAPK8 mediates resistance to temozolomide and apoptosis of glioblastoma cells through MAPK signaling pathway. Biomed. Pharmacother. 2018, 106, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Jungke, P.; Ostrow, G.; Li, J.L.; Norton, S.; Nieber, K.; Kelber, O.; Butterweck, V. Profiling of hypothalamic and hippocampal gene expression in chronically stressed rats treated with St. John’s wort extract (STW 3-VI) and fluoxetine. Psychopharmacology 2011, 213, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Fenart, L.; Landry, V.; Coisne, C.; Cecchelli, R.; Dehouck, M.P.; Buée-Scherrer, V. The MAP kinase pathway mediates transcytosis induced by TNF-alpha in an in vitro blood-brain barrier model. Eur. J. Neurosci. 2005, 22, 835–844. [Google Scholar] [CrossRef] [PubMed]
- McGregor, N.W.; Dimatelis, J.J.; Van Zyl, P.J.; Hemmings, S.M.J.; Kinnear, C.; Russell, V.A.; Stein, D.J.; Lochner, C. A translational approach to the genetics of anxiety disorders. Behav. Brain Res. 2018, 341, 91–97. [Google Scholar] [CrossRef]
- Blume, A.; Torner, L.; Liu, Y.; Subburaju, S.; Aguilera, G.; Neumann, I.D. Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology 2009, 150, 1841–1849. [Google Scholar] [CrossRef] [Green Version]
- Hökfelt, T.; Fuxe, K. Effects of prolactin and ergot alkaloids on the tubero-infundibular dopamine (DA) neurons. Neuroendocrinology 1972, 9, 100–122. [Google Scholar] [CrossRef]
- Borrow, A.P.; Handa, R.J. Estrogen Receptors Modulation of Anxiety-Like Behavior. Vitam. Horm. 2017, 103, 27–52. [Google Scholar]
- Walf, A.A.; Frye, C.A. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology 2006, 31, 1097–1111. [Google Scholar] [CrossRef]
- Kastenberger, I.; Schwarzer, C. GPER1 (GPR30) knockout mice display reduced anxiety and altered stress response in a sex and paradigm dependent manner. Horm. Behav. 2014, 66, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Krezel, W.; Dupont, S.; Krust, A.; Chambon, P.; Chapman, P.F. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 12278–12282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, C.; Ivanova, T.; Karolczak, M.; Küppers, E. Cell type-specificity of nonclassical estrogen signaling in the developing midbrain. J. Steroid. Biochem. Mol. Biol. 2002, 81, 319–325. [Google Scholar] [CrossRef]
- Filardo, E.J.; Quinn, J.A.; Frackelton, A.R., Jr.; Bland, K.I. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002, 16, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Frasor, J.; Gibori, G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol. Metab. 2003, 14, 118–123. [Google Scholar] [CrossRef]
- Altemus, M.; Sarvaiya, N.; Neill Epperson, C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, A.; Horrobin, D.F.; Welin, C.; Rosengren, A.; Wilhelmsen, L. 14-34—Trait anxiety is associated with high arachidonic acid levels in plasma—A study of men born in 1933. Biol. Psychiat. 1997, 42, 27S. [Google Scholar] [CrossRef]
- Santos-Soto, I.J.; Chorna, N.; Carballeira, N.M.; Vélez-Bartolomei, J.G.; Méndez-Merced, A.T.; Chornyy, A.P.; Peña de Ortiz, S. Voluntary running in young adult mice reduces anxiety-like behavior and increases the accumulation of bioactive lipids in the cerebral cortex. PLoS ONE 2013, 8, e81459. [Google Scholar] [CrossRef] [Green Version]
- Cadas, H.; di Tomaso, E.; Piomelli, D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. 1997, 17, 1226–1242. [Google Scholar] [CrossRef] [Green Version]
- Andrade, A.K.; Renda, B.; Murray, J.E. Cannabinoids, interoception, and anxiety. Pharmacol. Biochem. Behav. 2019, 180, 60–73. [Google Scholar] [CrossRef]
- Jefsen, O.H.; Elfving, B.; Wegener, G.; Müller, H.K. Transcriptional regulation in the rat prefrontal cortex and hippocampus after a single administration of psilocybin. J. Psychopharmacol. 2021, 35, 483–493. [Google Scholar] [CrossRef]
- Nishikawa, H.; Inoue, T.; Masui, T.; Izumi, T.; Koyama, T. Effects of cytochrome P450 (CYP) 3A4 inhibitors on the anxiolytic action of tandospirone in rat contextual conditioned fear. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 926–931. [Google Scholar] [CrossRef]
- Thompson, J.L.; Butterfield, G.E.; Gylfadottir, U.K.; Yesavage, J.; Marcus, R.; Hintz, R.L.; Pearman, A.; Hoffman, A.R. Effects of human growth hormone, insulin-like growth factor I, and diet and exercise on body composition of obese postmenopausal women. J. Clin. Endocrinol. Metab. 1998, 83, 1477–1484. [Google Scholar]




| Groups | 11-Ethoxyviburtinal | Baldrinal | Acevaltrate | Valtrate |
|---|---|---|---|---|
| UDG-1 | 0.0210 | 0.0843 | 6.1004 | 8.8450 |
| UDG-2 | 0.0879 | 0.2229 | 13.3610 | 7.6340 |
| UDG-3 | 0.1547 | 0.3614 | 4.2853 | 6.4230 |
| UDG-4 | 0.2216 | 0.5000 | 11.5459 | 5.2120 |
| UDG-5 | 0.2884 | 0.0150 | 2.4701 | 4.0010 |
| UDG-6 | 0.3553 | 0.1536 | 9.7307 | 2.7900 |
| UDG-7 | 0.4221 | 0.2921 | 0.6550 | 1.5790 |
| UDG-8 | 0.4890 | 0.4307 | 7.9156 | 0.3680 |
| Groups | CRH (pg/mL) | ACTH (pg/mL) | CORT (ng/mL) |
|---|---|---|---|
| Control | 154.40 ± 14.86 | 39.59 ± 2.53 | 6.98 ± 0.54 |
| EBS | 390.17 ± 9.21 ## | 81.41 ± 2.69 ## | 18.58 ± 0.64 ## |
| DZP | 221.20 ± 10.18 ** | 48.57 ± 2.00 ** | 10.57 ± 0.56 ** |
| UDG-1 | 337.93 ± 10.77 ** | 74.48 ± 2.68 * | 16.73 ± 0.69 * |
| UDG-2 | 317.92 ± 12.64 ** | 66.23 ± 2.83 ** | 13.21 ± 0.56 ** |
| UDG-3 | 272.24 ± 11.12 ** | 53.96 ± 2.24 ** | 12.60 ± 0.57 ** |
| UDG-4 | 318.58 ± 13.93 ** | 68.93 ± 1.93 ** | 14.59 ± 0.71 ** |
| UDG-5 | 219.45 ± 15.28 ** | 49.35 ± 1.94 ** | 9.03 ± 0.31 ** |
| UDG-6 | 281.65 ± 12.29 ** | 55.17 ± 2.57 ** | 11.89 ± 0.45 ** |
| UDG-7 | 227.29 ± 11.99 ** | 57.30 ± 1.84 ** | 10.68 ± 0.43 ** |
| UDG-8 | 249.70 ± 9.46 ** | 51.46 ± 2.25 ** | 11.28 ± 0.38 ** |
| Groups | DA (pg/mL) | NE (ng/mL) | 5-HT (ng/mL) | AEA (pg/mL) | GABA (μmol/L) | BDNF (pg/mL) |
|---|---|---|---|---|---|---|
| Control | 2236.63 ± 511.43 | 41.57 ± 1.23 | 116.07 ± 8.00 | 858.43 ± 35.57 | 0.08 ± 0.00 | 6909.32 ± 271.64 |
| EBS | 9088.27 ± 687.71 ## | 78.59 ± 2.11 ## | 248.17 ± 11.28 ## | 305.42 ± 17.56 ## | 0.04 ± 0.00 ## | 2340.67 ± 125.69 ## |
| DZP | 3439.27 ± 353.16 ** | 46.33 ± 2.45 ** | 135.78 ± 4.69 ** | 759.61 ± 24.88 * | 0.07 ± 0.00 ** | 5259.82 ± 319.53 ** |
| UDG-1 | 7450.23 ± 613.73 * | 66.51 ± 1.97 ** | 228.36 ± 5.59 * | 376.09 ± 19.89 ** | 0.05 ± 0.00 * | 3229.53 ± 221.48 ** |
| UDG-2 | 5273.24 ± 560.59 ** | 50.10 ± 2.34 ** | 158.03 ± 5.76 ** | 505.42 ± 33.21 ** | 0.06 ± 0.00 ** | 3378.58 ± 218.25 ** |
| UDG-3 | 6115.79 ± 713.41 ** | 53.56 ± 3.01 ** | 173.86 ± 8.18 ** | 650.88 ± 31.15 ** | 0.06 ± 0.00 ** | 4989.29 ± 272.07 ** |
| UDG-4 | 7310.78 ± 417.66 * | 62.17 ± 2.84 ** | 214.11 ± 5.96 ** | 669.44 ± 33.61 ** | 0.06 ± 0.00 ** | 4690.22 ± 284.78 ** |
| UDG-5 | 5798.07 ± 34.47 ** | 54.96 ± 0.24 ** | 176.35 ± 0.39 ** | 592.23 ± 2.24 ** | 0.06 ± 0.00 ** | 4234.74 ± 19.98 ** |
| UDG-6 | 5512.12 ± 576.85 ** | 56.78 ± 2.59 ** | 188.06 ± 6.09 ** | 594.20 ± 3.09 ** | 0.06 ± 0.00 ** | 3949.78 ± 263.03 ** |
| UDG-7 | 6962.47 ± 420.10 ** | 62.98 ± 0.99 ** | 184.72 ± 4.59 ** | 596.73 ± 1.57 ** | 0.06 ± 0.00 ** | 4861.39 ± 265.60 ** |
| UDG-8 | 5806.90 ± 44.41** | 54.53 ± 0.23 ** | 176.19 ± 0.25 ** | 591.40 ± 2.70 ** | 0.06 ± 0.00 ** | 4177.36 ± 26.76 ** |
| No. | Gene Symbol | Closeness Centrality | Degree Centrality | Betweenness Centrality |
|---|---|---|---|---|
| 1 | ALB | 0.68981481 | 89 | 0.18587974 |
| 2 | AKT1 | 0.64224138 | 74 | 0.08939001 |
| 3 | EGFR | 0.58893281 | 58 | 0.04228520 |
| 4 | SRC | 0.57088123 | 56 | 0.05227749 |
| 5 | IGF1 | 0.57976654 | 55 | 0.02219982 |
| 6 | ESR1 | 0.58431373 | 55 | 0.05721287 |
| 7 | CASP3 | 0.59126984 | 55 | 0.03857080 |
| 8 | MMP9 | 0.58203125 | 54 | 0.03623026 |
| 9 | MAPK8 | 0.57528958 | 51 | 0.01905757 |
| 10 | HSP90AA1 | 0.56439394 | 47 | 0.03408826 |
| 11 | NOS3 | 0.55805243 | 42 | 0.02089920 |
| 12 | AR | 0.54779412 | 40 | 0.02311410 |
| 13 | MAPK14 | 0.54578755 | 40 | 0.01428905 |
| 14 | IL2 | 0.53985507 | 39 | 0.02361781 |
| 15 | ACE | 0.53024911 | 36 | 0.01298736 |
| 16 | SOD2 | 0.53214286 | 33 | 0.04665146 |
| 17 | PPARG | 0.52836879 | 32 | 0.02068498 |
| Protein | PDB ID | Grid Size | Docking Score (kcal/mol) | |||
|---|---|---|---|---|---|---|
| Acevaltrate | Valtrate | Baldrinal | 11-Ethoxyviburtinal | |||
| ESR1 | 6VJ1 | 42 × 44 × 44 | −7.1 | −7.8 | −7.0 | −6.4 |
| SRC | 2PTK | 62 × 58 × 66 | −8.3 | −8.3 | −7.1 | −6.6 |
| AKT1 | 4EKL | 40 × 40 × 56 | −8.1 | −8.0 | −7.0 | −6.7 |
| MAPK8 | 3VUD | 58 × 58 × 62 | −7.6 | −7.1 | −6.6 | −6.4 |
| EGFR | 6S9C | 46 × 48 × 66 | −7.3 | −8.2 | −6.7 | −6.1 |
| MMP9 | 6ESM | 42 × 34 × 40 | −8.2 | −8.2 | −8.1 | −7.4 |
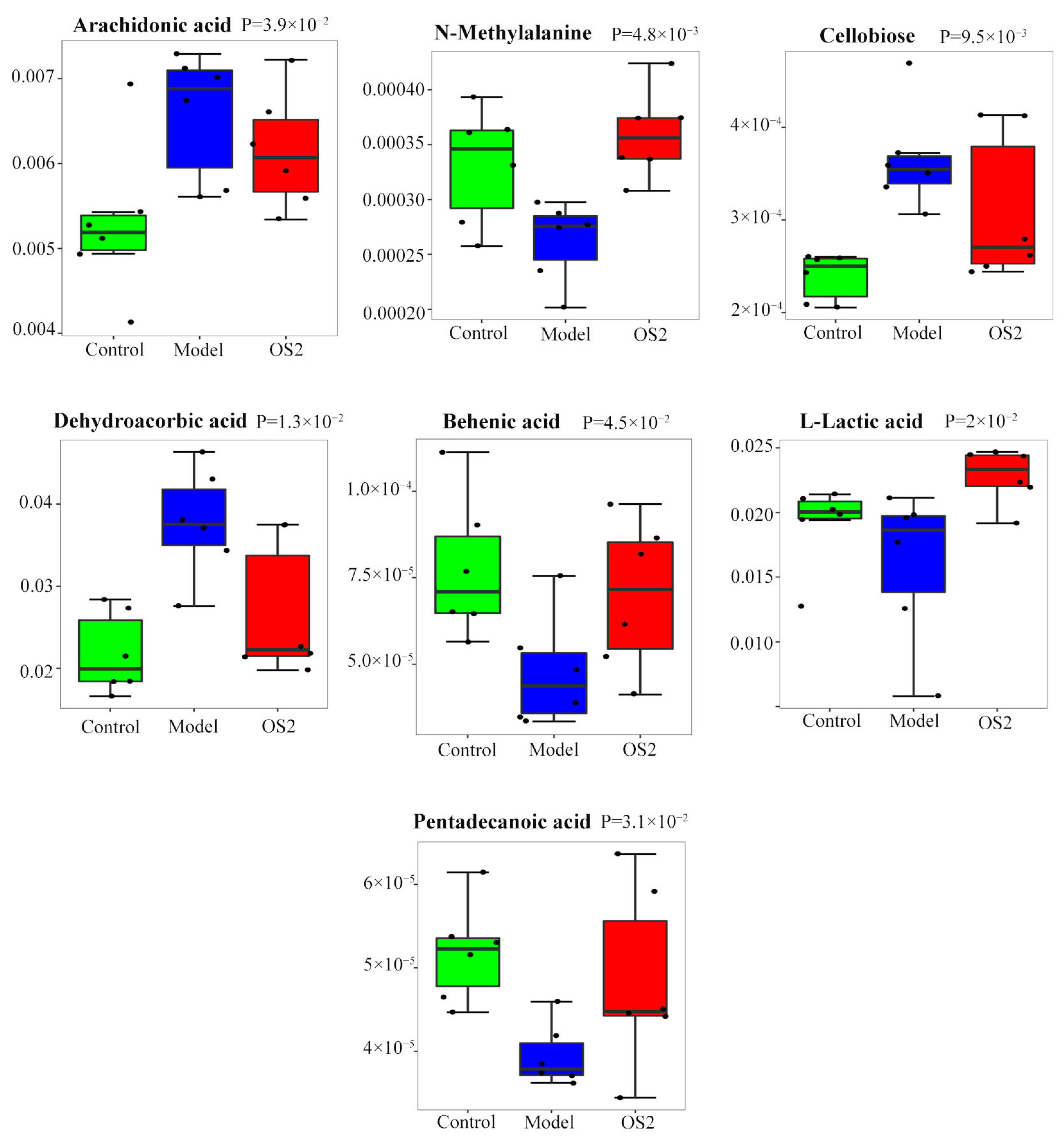
| No. | Metabolites | p-Value | Trend |
|---|---|---|---|
| 1 | L-Lactic acid | 0.019994847 | ↑ |
| 2 | N-Methylalanine | 0.004791963 | ↑ |
| 3 | Behenic acid | 0.045431849 | ↑ |
| 4 | Pentadecanoic acid | 0.030987525 | ↑ |
| 5 | Cellobiose | 0.009514191 | ↓ |
| 6 | Arachidonic acid | 0.039333617 | ↓ |
| 7 | Dehydroascorbic acid | 0.013123729 | ↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Wei, X.; Guo, J.; Ding, Y.; Luo, J.; Yang, X.; Li, J.; Wan, G.; Yu, J.; Shi, J. Dose Optimization of Anxiolytic Compounds Group in Valeriana jatamansi Jones and Mechanism Exploration by Integrating Network Pharmacology and Metabolomics Analysis. Brain Sci. 2022, 12, 589. https://doi.org/10.3390/brainsci12050589
Zhao C, Wei X, Guo J, Ding Y, Luo J, Yang X, Li J, Wan G, Yu J, Shi J. Dose Optimization of Anxiolytic Compounds Group in Valeriana jatamansi Jones and Mechanism Exploration by Integrating Network Pharmacology and Metabolomics Analysis. Brain Sciences. 2022; 12(5):589. https://doi.org/10.3390/brainsci12050589
Chicago/Turabian StyleZhao, Chengbowen, Xiaojia Wei, Jianyou Guo, Yongsheng Ding, Jing Luo, Xue Yang, Jiayuan Li, Guohui Wan, Jiahe Yu, and Jinli Shi. 2022. "Dose Optimization of Anxiolytic Compounds Group in Valeriana jatamansi Jones and Mechanism Exploration by Integrating Network Pharmacology and Metabolomics Analysis" Brain Sciences 12, no. 5: 589. https://doi.org/10.3390/brainsci12050589
APA StyleZhao, C., Wei, X., Guo, J., Ding, Y., Luo, J., Yang, X., Li, J., Wan, G., Yu, J., & Shi, J. (2022). Dose Optimization of Anxiolytic Compounds Group in Valeriana jatamansi Jones and Mechanism Exploration by Integrating Network Pharmacology and Metabolomics Analysis. Brain Sciences, 12(5), 589. https://doi.org/10.3390/brainsci12050589