Changes in Tyrosine Hydroxylase Activity and Dopamine Synthesis in the Nigrostriatal System of Mice in an Acute Model of Parkinson’s Disease as a Manifestation of Neurodegeneration and Neuroplasticity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Procedures
2.2. Modeling of the Early Clinical Stage of Parkinson’s Disease
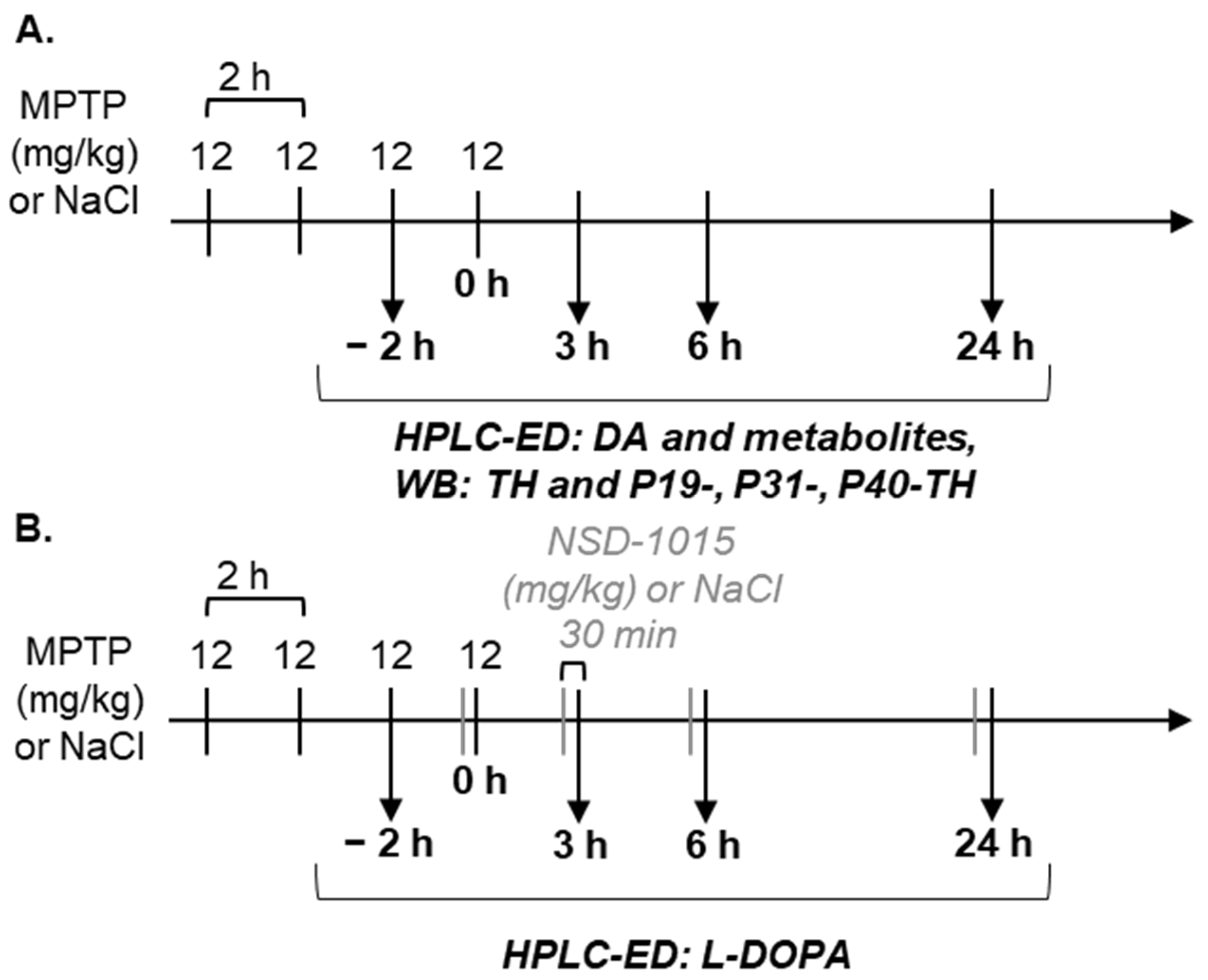
2.3. Design of Experiments
2.4. Sample Analysis
2.4.1. High-Performance Liquid Chromatography with Electrochemical Detection
2.4.2. Western Blot
2.5. Statistical Analysis
3. Results
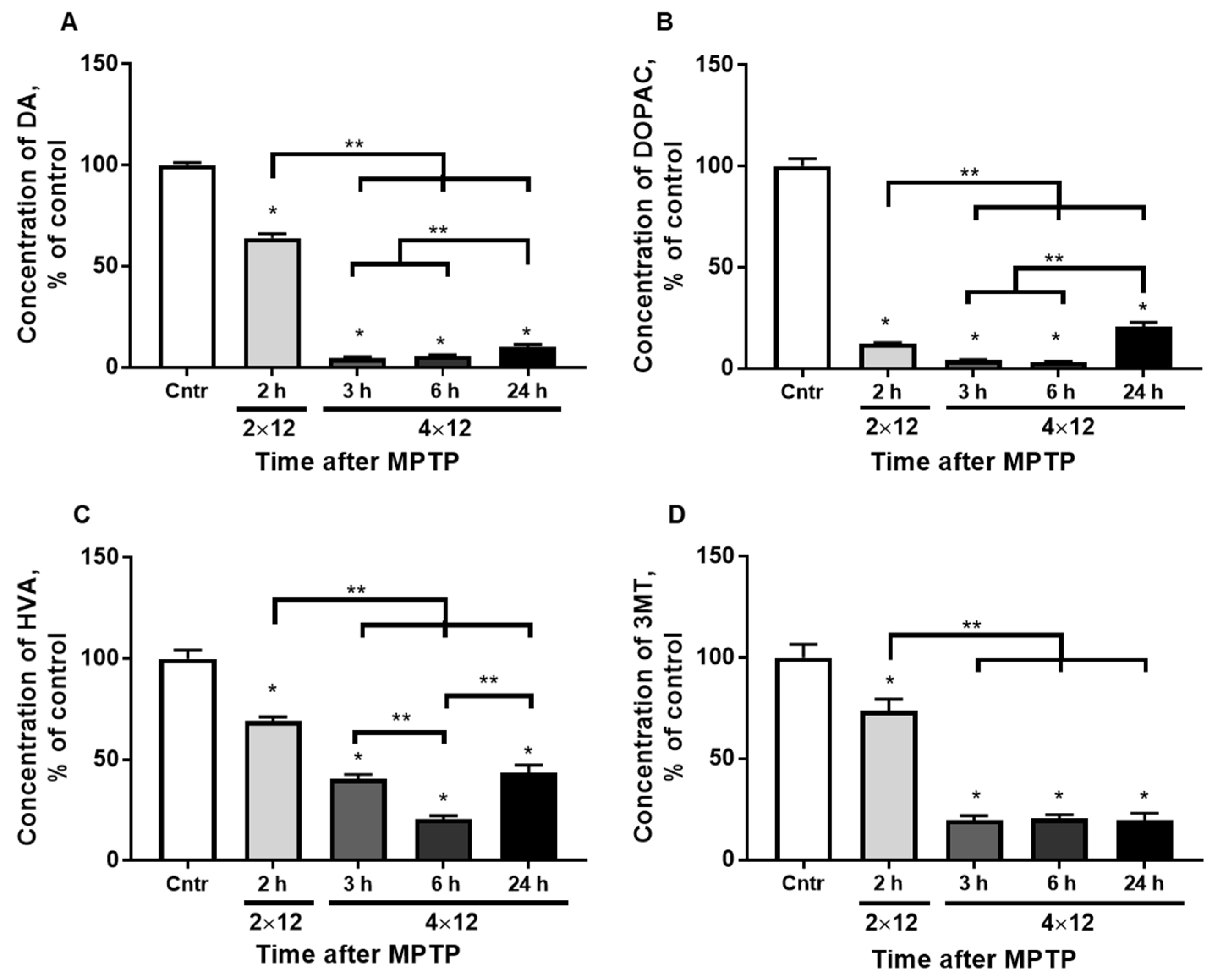
3.1. The Concentration of Dopamine and Its Metabolites in the Striatum
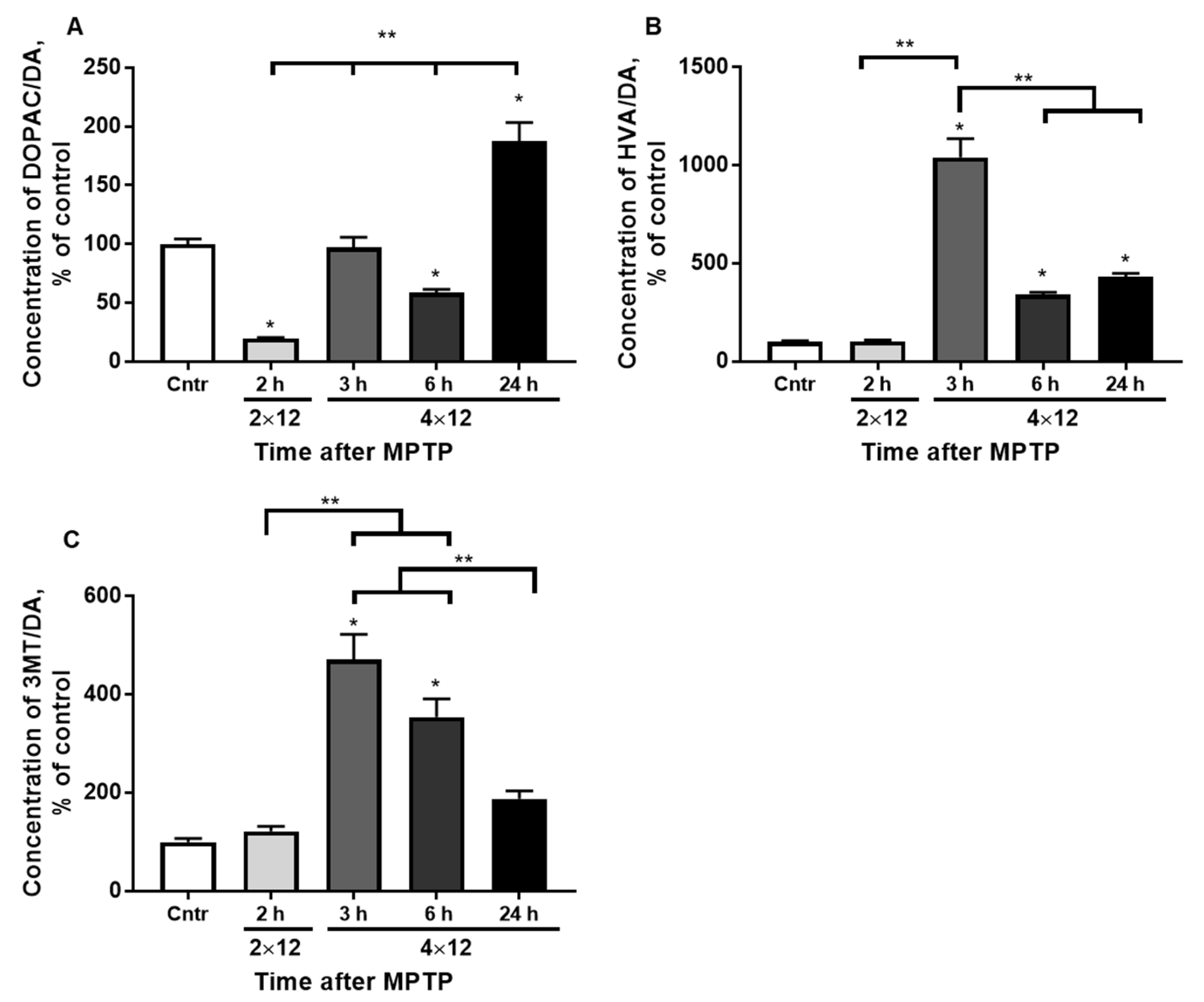
3.2. Dopamine Turnover in the Striatum
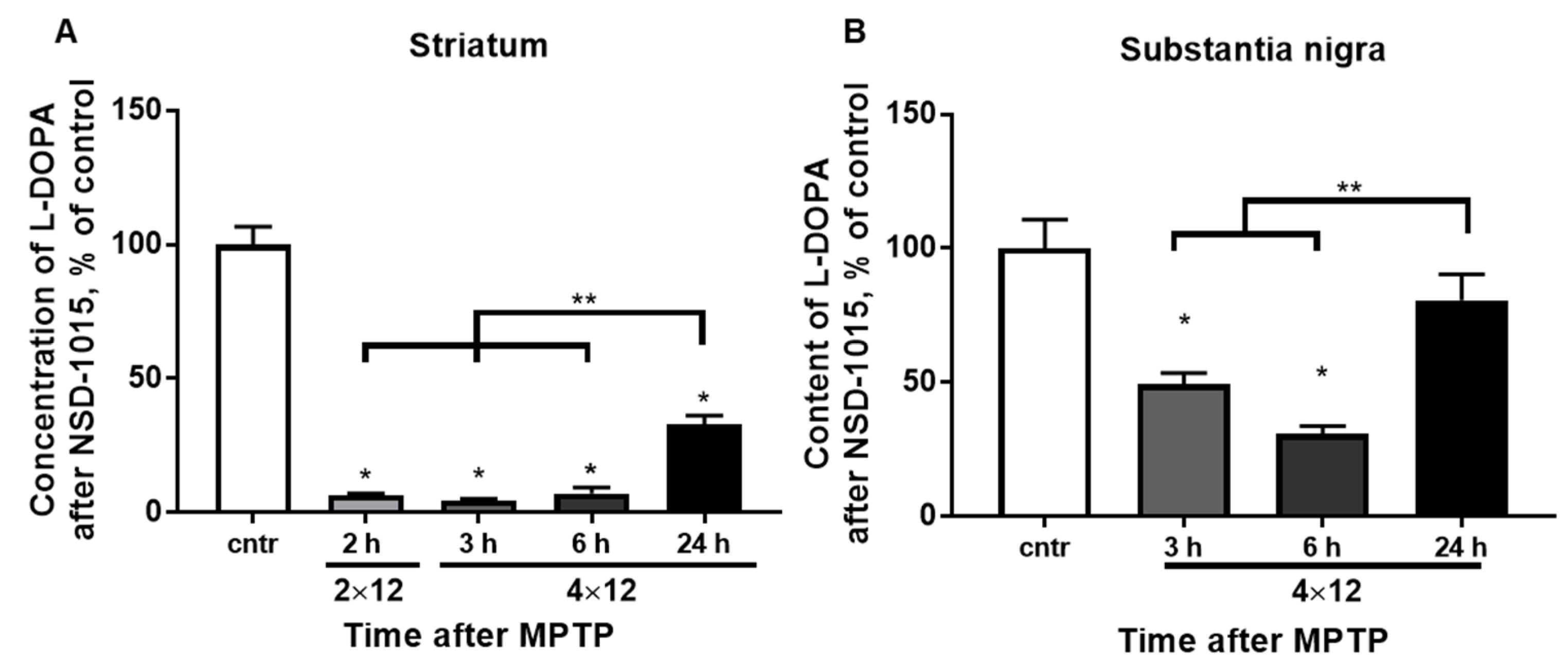
3.3. The Concentration of L-DOPA in the Striatum and SN after Inhibition of AADC with NSD-1015
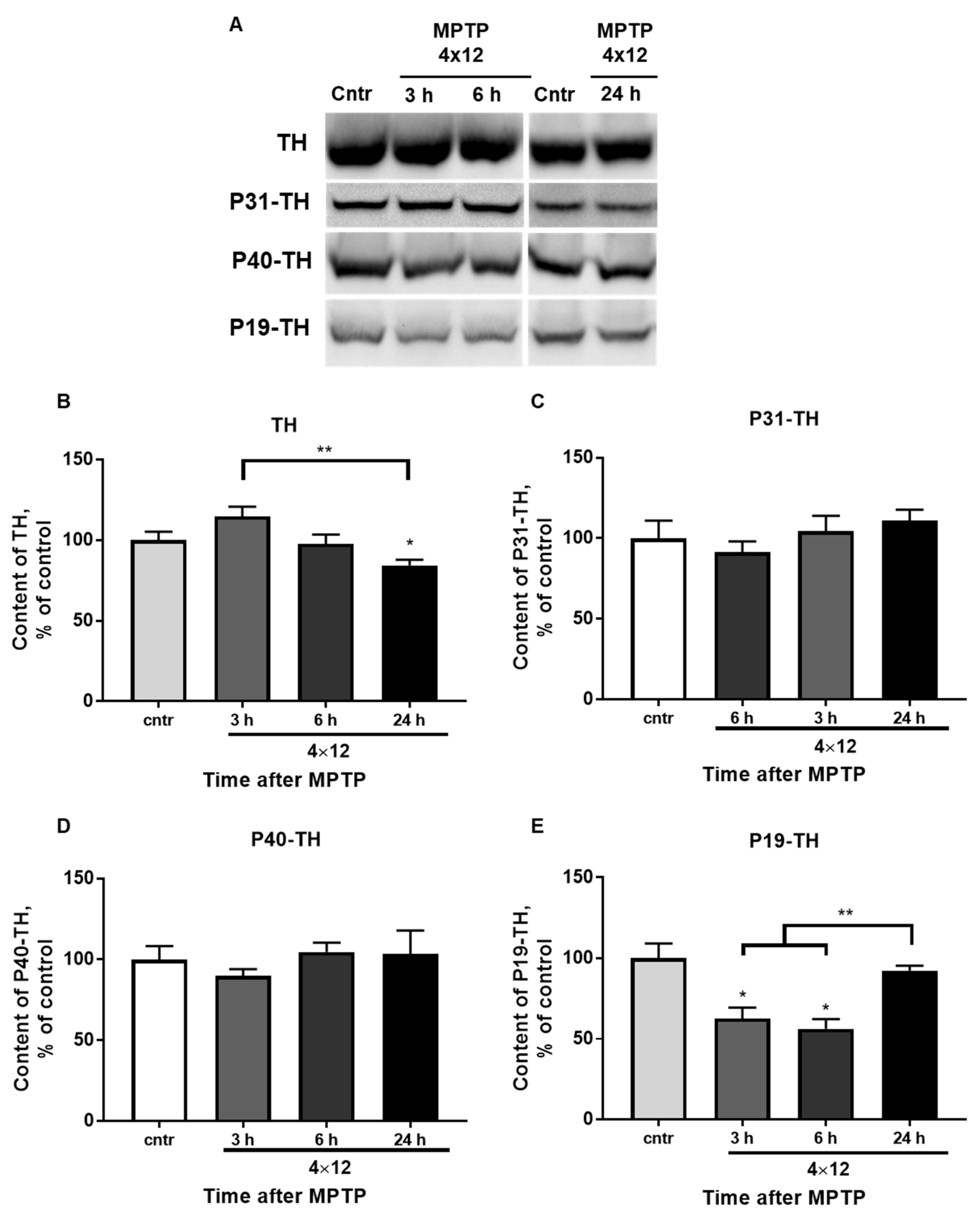
3.4. The Content of TH and Its Phosphorylated Forms in the Striatum
3.5. The Content of TH and Its Phosphorylated Forms in the SN
4. Discussion
4.1. Dopamine Synthesis in the Substantia Nigra
4.2. Dopamine Synthesis in the Striatum
5. Conclusions
- DA content in the SN and the striatum did not depend on TH content but correlated with enzyme activity.
- TH activity did not depend on the total protein content either in the SN or in the striatum.
- TH activity in the SN was determined by the content of P19-TH; TH activity in the striatum was determined by P31-TH and P40-TH (to a lesser extent).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaufman, S. The structure of the phenylalanine-hydroxylation cofactor. Proc. Natl. Acad. Sci. USA 1963, 50, 1085–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagatsu, T.; Levitt, M.; Udenfriend, S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J. Biol. Chem. 1964, 239, 2910–2917. [Google Scholar] [CrossRef]
- Matsuura, S.; Sugimoto, T.; Murata, S.; Sugawara, Y.; Iwasaki, H. Stereochemistry of biopterin cofactor and facile methods for the determination of the stereochemistry of a biologically active 5,6,7,8-tetrahydropterin. J. Biochem. 1985, 98, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Kaakkola, S.; Männistö, P.T.; Nissinen, E. Striatal membrane-bound and soluble catechol-O-methyl-transferase after selective neuronal lesions in the rat. J. Neural Transm. 1987, 69, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Akiguchi, I.; Kimura, J. Topographic distributions of monoamine oxidase-B-containing neurons in the mouse striatum. Neurosci. Lett. 1995, 184, 29–31. [Google Scholar] [CrossRef]
- Francis, A.; Pearce, L.B.; Roth, J.A. Cellular localization of MAO A and B in brain: Evidence from kainic acid lesions in striatum. Brain Res. 1985, 334, 59–64. [Google Scholar] [CrossRef]
- Yavich, L.; Forsberg, M.M.; Karayiorgou, M.; Gogos, J.A.; Männistö, P.T. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 2007, 27, 10196–10209. [Google Scholar] [CrossRef]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [Green Version]
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef]
- Agid, Y. Parkinson’s disease: Pathophysiology. Lancet 1991, 337, 1321–1324. [Google Scholar] [CrossRef]
- Bezard, E.; Dovero, S.; Prunier, C.; Ravenscroft, P.; Chalon, S.; Guilloteau, D.; Crossman, A.R.; Bioulac, B.; Brotchie, J.M.; Gross, C.E. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J. Neurosci. 2001, 21, 6853–6861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136 Pt 8, 2419–2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagatsu, T.; Yamaguchi, T.; Rahman, M.K.; Trocewicz, J.; Oka, K.; Hirata, Y.; Nagatsu, I.; Narabayashi, H.; Kondo, T.; Iizuka, R. Catecholamine-related enzymes and the biopterin cofactor in Parkinson’s disease and related extrapyramidal diseases. Adv. Neurol. 1984, 40, 467–473. [Google Scholar]
- Mogi, M.; Harada, M.; Kiuchi, K.; Kojima, K.; Kondo, T.; Narabayashi, H.; Rausch, D.; Riederer, P.; Jellinger, K.; Nagatsu, T. Homospecific activity (activity per enzyme protein) of tyrosine hydroxylase increases in parkinsonian brain. J. Neural Transm. 1988, 72, 77–82. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Bobrovskaya, L.; Graham, M.E.; von Nagy-Felsobuki, E.I.; Dickson, P.W. Tyrosine hydroxylase phosphorylation: Regulation and consequences. J. Neurochem. 2004, 91, 1025–1043. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Dickson, P.W. Tyrosine hydroxylase phosphorylation in vivo. J. Neurochem. 2019, 149, 706–728. [Google Scholar] [CrossRef] [Green Version]
- Daubner, S.C.; Lauriano, C.; Haycock, J.W.; Fitzpatrick, P.F. Site-directed mutagenesis of serine 40 of rat tyrosine hydroxylase. Effects of dopamine and cAMP-dependent phosphorylation on enzyme activity. J. Biol. Chem. 1992, 267, 12639–12646. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W.; Wakade, A.R. Activation and multiple-site phosphorylation of tyrosine hydroxylase in perfused rat adrenal glands. J. Neurochem. 1992, 58, 57–64. [Google Scholar] [CrossRef]
- Haycock, J.W.; Lew, J.Y.; Garcia-Espana, A.; Lee, K.Y.; Harada, K.; Meller, E.; Goldstein, M. Role of serine-19 phosphorylation in regulating tyrosine hydroxylase studied with site- and phosphospecific antibodies and site-directed mutagenesis. J. Neurochem. 1998, 71, 1670–1675. [Google Scholar] [CrossRef]
- Sutherland, C.; Alterio, J.; Campbell, D.G.; le Bourdellès, B.; Mallet, J.; Haavik, J.; Cohen, P. Phosphorylation and activation of human tyrosine hydroxylase in vitro by mitogen-activated protein (MAP) kinase and MAP-kinase-activated kinases 1 and 2. Eur. J. Biochem. 1993, 217, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskaya, L.; Dunkley, P.R.; Dickson, P.W. Phosphorylation of Ser19 increases both Ser40 phosphorylation and enzyme activity of tyrosine hydroxylase in intact cells. J. Neurochem. 2004, 90, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Ohnuma, S.; Kodani, Y.; Kaneko, Y.S.; Nagasaki, H.; Nagatsu, T.; Ota, A. Inhibition of deubiquitinating activity of USP14 decreases tyrosine hydroxylase phosphorylated at Ser19 in PC12D cells. Biochem. Biophys. Res. Commun. 2016, 472, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Prabhakar, V.; Singh, R.; Dutta, D.; Mohanakumar, K.P. Melatonin enhances L-DOPA therapeutic effects, helps to reduce its dose, and protects dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. J. Pineal Res. 2015, 58, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hunter, R.; Nguyen, X.V.; Kim, H.C.; Bing, G. Microsomal epoxide hydrolase deletion enhances tyrosine hydroxylase phosphorylation in mice after MPTP treatment. J. Neurosci. Res. 2008, 86, 2792–2801. [Google Scholar] [CrossRef]
- Boukhzar, L.; Hamieh, A.; Cartier, D.; Tanguy, Y.; Alsharif, I.; Castex, M.; Arabo, A.; el Hajji, S.; Bonnet, J.J.; Errami, M.; et al. Selenoprotein T Exerts an Essential Oxidoreductase Activity That Protects Dopaminergic Neurons in Mouse Models of Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 557–574. [Google Scholar] [CrossRef]
- Salvatore, M.F. ser31 Tyrosine hydroxylase phosphorylation parallels differences in dopamine recovery in nigrostriatal pathway following 6-OHDA lesion. J. Neurochem. 2014, 129, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Ugrumov, M.V.; Khaindrava, V.G.; Kozina, E.A.; Kucheryanu, V.G.; Bocharov, E.V.; Kryzhanovsky, G.N.; Kudrin, V.S.; Narkevich, V.B.; Klodt, P.M.; Rayevsky, K.S.; et al. Modeling of presymptomatic and symptomatic stages of parkinsonism in mice. Neuroscience 2011, 181, 175–188. [Google Scholar] [CrossRef]
- Kolacheva, A.A.; Kozina, E.A.; Volina, E.V.; Ugryumov, M.V. Time course of degeneration of dopaminergic neurons and respective compensatory processes in the nigrostriatal system in mice. Dokl. Biol. Sci. 2014, 456, 160–164. [Google Scholar] [CrossRef]
- Kolacheva, A.A.; Ugrumov, M.V. A Mouse Model of Nigrostriatal Dopaminergic Axonal Degeneration as a Tool for Testing Neuroprotectors. Acta Naturae 2021, 13, 110–113. [Google Scholar] [CrossRef]
- Kozina, E.A.; Khakimova, G.R.; Khaindrava, V.G.; Kucheryanu, V.G.; Vorobyeva, N.E.; Krasnov, A.N.; Georgieva, S.G.; Goff, L.K.; Ugrumov, M.V. Tyrosine hydroxylase expression and activity in nigrostriatal dopaminergic neurons of MPTP-treated mice at the presymptomatic and symptomatic stages of parkinsonism. J. Neurol. Sci. 2014, 340, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Lindqvist, M. In-vivo measurements of tryptophan and tyrosine hydroxylase activities in mouse brain. J. Neural Transm. 1973, 34, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Aldridge, G.M.; Podrebarac, D.M.; Greenough, W.T.; Weiler, I.J. The use of total protein stains as loading controls: An alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods 2008, 172, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef]
- Eaton, S.L.; Roche, S.L.; Hurtado, M.L.; Oldknow, K.J.; Farquharson, C.; Gillingwater, T.H.; Wishar, T.M. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting. PLoS ONE 2013, 8, e72457. [Google Scholar] [CrossRef]
- de Iuliis, A.; Grigoletto, J.; Recchia, A.; Giusti, P.; Arslan, P. A proteomic approach in the study of an animal model of Parkinson’s disease. Clin. Chim. Acta 2005, 357, 202–209. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Kozina, E.A.; Kim, A.R.; Kurina, A.Y.; Ugrumov, M.V. Cooperative synthesis of dopamine by non-dopaminergic neurons as a compensatory mechanism in the striatum of mice with MPTP-induced Parkinsonism. Neurobiol. Dis. 2017, 98, 108–121. [Google Scholar] [CrossRef]
- Mingazov, E.R.; Khakimova, G.R.; Kozina, E.A.; Medvedev, A.E.; Buneeva, O.A.; Bazyan, A.S.; Ugrumov, M.V. MPTP Mouse Model of Preclinical and Clinical Parkinson’s Disease as an Instrument for Translational Medicine. Mol. Neurobiol. 2018, 55, 2991–3006. [Google Scholar] [CrossRef]
- Kühn, K.; Wellen, J.; Link, N.; Maskri, L.; Lübbert, H.; Stichel, C.C. The mouse MPTP model: Gene expression changes in dopaminergic neurons. Eur. J. Neurosci. 2003, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Schlüter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G.; et al. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morfini, G.; Pigino, G.; Opalach, K.; Serulle, Y.; Moreira, J.E.; Sugimori, M.; Llinás, R.R.; Brady, S.T. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc. Natl. Acad. Sci. USA 2007, 104, 2442–2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartelli, D.; Casagrande, F.; Busceti, C.L.; Bucci, D.; Molinaro, G.; Traficante, A.; Passarella, D.; Giavini, E.; Pezzoli, G.; Battaglia, G.; et al. Microtubule alterations occur early in experimental parkinsonism and the microtubule stabilizer epothilone D is neuroprotective. Sci. Rep. 2013, 3, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitayama, S.; Wang, J.B.; Uhl, G.R. Dopamine transporter mutants selectively enhance MPP+ transport. Synapse 1993, 15, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Fumagalli, F.; Jones, S.R.; Caron, M.G. Dopamine transporter is required for in vivo MPTP neurotoxicity: Evidence from mice lacking the transporter. J. Neurochem. 1997, 69, 1322–1325. [Google Scholar] [CrossRef]
- del Zompo, M.; Piccardi, M.P.; Ruiu, S.; Quartu, M.; Gessa, G.L.; Vaccari, A. Selective MPP+ uptake into synaptic dopamine vesicles: Possible involvement in MPTP neurotoxicity. Br. J. Pharmacol. 1993, 109, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Miner, L.L.; Sora, I.; Ujike, H.; Revay, R.S.; Kostic, V.; Jackson-Lewis, V.; Przedborski, S.; Uhl, G.R. VMAT2 knockout mice: Heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc. Natl. Acad. Sci. USA 1997, 94, 9938–9943. [Google Scholar] [CrossRef] [Green Version]
- Staal, R.G.; Sonsalla, P.K. Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata. J. Pharmacol. Exp. Ther. 2000, 293, 336–342. [Google Scholar]
- Goldstein, D.S.; Sullivan, P.; Holmes, C.; Miller, G.W.; Alter, S.; Strong, R.; Mash, D.C.; Kopin, I.J.; Sharabi, Y. Determinants of buildup of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J. Neurochem. 2013, 126, 591–603. [Google Scholar] [CrossRef]
- Choi, S.J.; Panhelainen, A.; Schmitz, Y.; Larsen, K.E.; Kanter, E.; Wu, M.; Sulzer, D.; Mosharov, E.V. Changes in neuronal dopamine homeostasis following 1-methyl-4-phenylpyridinium (MPP+) exposure. J. Biol. Chem. 2015, 290, 6799–6809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.L.; Quinsey, N.S.; Dunkley, P.R.; Dickson, P.W. Tyrosine hydroxylase activity is regulated by two distinct dopamine-binding sites. J. Neurochem. 2008, 106, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Briggs, G.D.; Gordon, S.L.; Dickson, P.W. Mutational analysis of catecholamine binding in tyrosine hydroxylase. Biochemistry 2011, 50, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Montoya, S.E.; Alerte, T.N.; Wang, J.; Wu, J.; Peng, X.; Hong, C.S.; Friedrich, E.E.; Mader, S.A.; Pedersen, C.J.; et al. Serine 129 phosphorylation reduces the ability of alpha-synuclein to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J. Biol. Chem. 2010, 285, 17648–17661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgenaidi, I.S.; Spiers, J.P. Regulation of the phosphoprotein phosphatase 2A system and its modulation during oxidative stress: A potential therapeutic target? Pharmacol. Ther. 2019, 198, 68–89. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Bao, R.; Xia, C.; Zhang, Y.; Zhu, Z.; Lv, Q.; Qi, Y.; Xue, J. Mitochondrial dysfunction and apoptosis are attenuated through activation of AMPK/GSK-3β/PP2A pathway in Parkinson’s disease. Eur. J. Pharmacol. 2021, 907, 174202. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Li, X.; Chen, M.; Liu, C.; Li, J.; Yu, S. Alpha-synuclein oligomerization and dopaminergic degeneration occur synchronously in the brain and colon of MPTP-intoxicated parkinsonian monkeys. Neurosci. Lett. 2020, 716, 134640. [Google Scholar] [CrossRef]
- Choi, H.K.; Won, L.A.; Kontur, P.J.; Hammond, D.N.; Fox, A.P.; Wainer, B.H.; Hoffmann, P.C.; Heller, A. Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res. 1991, 552, 67–76. [Google Scholar] [CrossRef]
- Choi, H.K.; Won, L.; Roback, J.D.; Wainer, B.H.; Heller, A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc. Natl. Acad. Sci. USA 1992, 89, 8943–8947. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lou, H.; Pedersen, C.J.; Smith, A.D.; Perez, R.G. 14-3-3zeta contributes to tyrosine hydroxylase activity in MN9D cells: Localization of dopamine regulatory proteins to mitochondria. J. Biol. Chem. 2009, 284, 14011–14019. [Google Scholar] [CrossRef] [Green Version]
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002, 22, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Rudenok, M.M.; Alieva, A.K.; Starovatykh, J.S.; Nesterov, M.S.; Stanishevskaya, V.A.; Kolacheva, A.A.; Ugryumov, M.V.; Slominsky, P.A.; Shadrina, M.I. Expression analysis of genes involved in mitochondrial biogenesis in mice with MPTP-induced model of Parkinson’s disease. Mol. Genet. Metab. Rep. 2020, 23, 100584. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Vukosavic, S.; Jackson-Lewis, V.; Neystat, M.; Jakowec, M.; Przedborski, S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J. Neurochem. 2000, 74, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cawthon, D.; McCastlain, K.A.; Slikker, W., Jr.; Ali, S.F. Selective alterations of gene expression in mice induced by MPTP. Synapse 2005, 55, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F.; Garcia-Espana, A.; Goldstein, M.; Deutch, A.Y.; Haycock, J.W. Stoichiometry of tyrosine hydroxylase phosphorylation in the nigrostriatal and mesolimbic systems in vivo: Effects of acute haloperidol and related compounds. J. Neurochem. 2000, 75, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, M.F.; Pruett, B.S. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PLoS ONE 2012, 7, e29867. [Google Scholar] [CrossRef] [Green Version]
- Fornai, F.; Alessandrì, M.G.; Torracca, M.T.; Bassi, L.; Corsini, G.U. Effects of noradrenergic lesions on MPTP/MPP+ kinetics and MPTP-induced nigrostriatal dopamine depletions. J. Pharmacol. Exp. Ther. 1997, 283, 100–107. [Google Scholar]
- Ara, J.; Przedborski, S.; Naini, A.B.; Jackson-Lewis, V.; Trifiletti, R.R.; Horwitz, J.; Ischiropoulos, H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Proc. Natl. Acad. Sci. USA 1998, 95, 7659–7663. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolacheva, A.; Alekperova, L.; Pavlova, E.; Bannikova, A.; Ugrumov, M.V. Changes in Tyrosine Hydroxylase Activity and Dopamine Synthesis in the Nigrostriatal System of Mice in an Acute Model of Parkinson’s Disease as a Manifestation of Neurodegeneration and Neuroplasticity. Brain Sci. 2022, 12, 779. https://doi.org/10.3390/brainsci12060779
Kolacheva A, Alekperova L, Pavlova E, Bannikova A, Ugrumov MV. Changes in Tyrosine Hydroxylase Activity and Dopamine Synthesis in the Nigrostriatal System of Mice in an Acute Model of Parkinson’s Disease as a Manifestation of Neurodegeneration and Neuroplasticity. Brain Sciences. 2022; 12(6):779. https://doi.org/10.3390/brainsci12060779
Chicago/Turabian StyleKolacheva, Anna, Leyla Alekperova, Ekaterina Pavlova, Alyona Bannikova, and Michael V. Ugrumov. 2022. "Changes in Tyrosine Hydroxylase Activity and Dopamine Synthesis in the Nigrostriatal System of Mice in an Acute Model of Parkinson’s Disease as a Manifestation of Neurodegeneration and Neuroplasticity" Brain Sciences 12, no. 6: 779. https://doi.org/10.3390/brainsci12060779
APA StyleKolacheva, A., Alekperova, L., Pavlova, E., Bannikova, A., & Ugrumov, M. V. (2022). Changes in Tyrosine Hydroxylase Activity and Dopamine Synthesis in the Nigrostriatal System of Mice in an Acute Model of Parkinson’s Disease as a Manifestation of Neurodegeneration and Neuroplasticity. Brain Sciences, 12(6), 779. https://doi.org/10.3390/brainsci12060779






